ZnO@ZIF-8 core-shell structure nanorods superhydrophobic coating on magnesium alloy with corrosion resistance and self-cleaning
2023-12-27ShiqunJingWeidongLiJinyuLiuJiqiongJingZheZhngWeiShngNingPengYuqingWen
Shiqun Jing ,Weidong Li ,Jinyu Liu ,Jiqiong Jing ,Zhe Zhng ,Wei Shng,* ,Ning Peng,*,Yuqing Wen,*
a Guangxi Key Laboratory of Electrochemical and Magnetochemical Function Materials, College of Chemistry and Bioengineering, Guilin University of Technology, Guilin, 541004, China
Abstract A longstanding quest in material science has been the development of superhydrophobic coating based on a single material,without the requirement of fluorination or silane treatment.In this work,the micro-arc oxidation (MAO) coating as transition layer can effectively enhance the bonding force of the superhydrophobic coating.The semiconductor@metal organic frameworks (MOFs) core-shell structure was synthesized by a simple self-templating method,and obtained ZnO@2-methylimidazole zinc salt (ZIF-8) nanorods array on magnesium (Mg)alloy.ZnO nanorods not only act as the template but also provide Zn2+ for ZIF-8.In addition,we proved that the ligand concentration,synthesis time and temperature are the keys to the preparation of ZnO@ZIF-8 nanorods.As we expect,the ZnO@ZIF-8 nanorods array can trap air in the gaps to form an air layer,and the coating exhibits superhydrophobic properties (154.81°).Excitingly,ZnO@ZIF-8 nanorods array shown a superhydrophobic property,without the requirement of fluorination or silane treatment.The results shown that the coating has good chemical stability and self-cleaning performance.Meanwhile,the corrosion resistance has been significantly improved, Rct was increased from 1.044×103 to 1.414×106 Ω/cm2 and Icorr was reduced from 4.275×10-5 to 5.611×10-9 A/cm2.Therefore,the semiconductor@MOFs core-shell structure has broad application prospects in anti-corrosion.
Keywords: Mg alloy;ZnO@ZIF-8;Coatings;Corrosion resistance;Superhydrophobic.
1.Introduction
With the overall upgrade of the world automotive industry and the continuous development and progress of 3C products,light-weighting is an urgent technical problem in related industries.Among the known metals,Mg alloys has the minimum density,which can well solve the problem of lightening of automobiles and 3C products [1-3].At the same time,Mg alloys with good seismic performance,excellent biocompatibility,excellent hydrogen storage properties and high theoretical capacity [4-5].Therefore,the application research of Mg alloys has attracted the continuous attention of researchers,especially the development of bio-magnesium materials,Mg batteries and hydrogen storage Mg materials in recent years,which has further widen the application market of Mg alloys[2].However,the actual application of Mg alloys still needs to overcome many difficulties.The most serious problem is poor corrosion resistance,and the faster degradation rate severely limits the application range of Mg alloys [6].Even facing an enormous challenge,researchers still spared no effort to carry out extensive studies,including alloy design optimization and surface treatment.Surface modification of Mg alloys was a simple and effective treatment method to improve corrosion resistance [7].At present,the multifunctional application of superhydrophobic coatings has gradually become a research hotspot [8].
Generally,superhydrophobic surfaces were defined as having water contact angles greater than 150°,which first originated from the "lotus effect" in nature.The superhydrophobic coatings can prevent the water droplets from penetrating the rough surface,effectively reducing the contact area between the corrosive medium and the substrate,thus achieve the purpose of anti-corrosion [9].The superhydrophobic surfaces endow Mg alloy substrate more functions,such as selfcleaning,anti-icing,oil-water separation,and resistance reduction [10-12].Traditional superhydrophobic surfaces first requires the construction of micro-nano structures,and then modification with low surface energy substances.To date,the construction of micro-nano structures includes hydrothermal[13],electrodeposition [14],etching [15]and chemical deposition [16].The common low surface energy materials mainly include organosilicon compounds,fluorine-containing compounds and other long-chain alkyl compounds [17-18].For example,the surface micro-nano structure of Mg alloy was constructed by a combination of hydrothermal and electroless plating,and the surface is modified with n-dodecyl mercaptan to stimulate excellent superhydrophobicity and corrosion resistance [13].The SiO2is modified by fluorosilane,and then the SiO2suspension is sprayed onto the surface of Mg alloy to obtain a superhydrophobic coating with a high contact angle [19].The nanograss textures are prepared by reactive ion etching,and then nanograss textures are hydrophobized with fluorosilane chemicals [20].A superhydrophobic coating was prepared on the surface of AZ31 magnesium alloy by a combination of one-step electrodeposition method and low surface energy modification.The coating showed improved corrosion performance [21].However,these compounds are very unfriendly to the environment and may introduce highly toxic substances into the natural environment [22].Therefore,the development of superhydrophobic surfaces that without the requirement of fluorination or silane treatment is particularly important.
MOFs are a new class of two-dimensional materials,which has flourished in the past ten years due to its wide variety,adjustable morphology,high stability and non-toxicity [23].Consequently,much attention has been paid to potential applications of MOFs in sensors,catalysis,gas separation,and biomedicine [24-26].Recently,MOFs materials have been tried to be used in superhydrophobic coatings and have shown excellent anti-corrosion effects [27].The zeolitic imidazolate framework (ZIF) materials have zeolite-like topologies.Yin et al.synthesized ZIF-8 particles and then used to fabricate a super-hydrophobic ZIF-8/PVDF/LDH double-layered coating with excellent corrosion resistance via a dip-coating process [28].Zhang et al.ZIF-8-based anti-corrosion coatings with diverse microstructures were prepared by the facile hydrothermal growth of ZnAl-NO3LDH buffer layers followed by solvothermal treatment with ZIF-8 precursor solutions with different recipes [29].However,reports of magnetic core–shell architectures on the basis of ZIF-type materials have been very limited in the literatures[30].Compared with single MOFs,the core-shell structure based on MOFs materials can show better advantages.For example,the Fe3O4@MOFs,SiO2@MOFs,and metal@MOFs shown satisfactory results[31-33].However,In the field of anti-corrosion,the study of core–shell architectures on the basis of MOFs materials have been to be explored.Therefore,we reasonably constructed the semiconductor@MOFs heterostructure.
In this work,compared with traditional superhydrophobic coatings,a new coating with a superhydrophobic state without the requirement of fluorination or silane treatment,the superhydrophobic coating base on ZnO@ZIF-8 core-shell heterostructure on the Mg alloy prepared by a simple synthesis process.In the process of synthesizing the core-shell structure of ZnO@ZIF-8,two difficulties must be overcome:(1) controlling the growth of ZIF-8 on the ZnO surface;(2)constructing the shape of a core-shell structure.According to our work,the metal oxides self-template was an effective strategy to control the structure [34].In this regard,ZnO nanorods regarded as a sacrificial template,Zn2+is etched by the ligand solution to induce ZIF-8 to growth on its surface,and ZnO@ZIF-8 nanorods with core-shell structure were successfully prepared (shown in Scheme 1).The ZnO@ZIF-8 heterostructure array exhibits good corrosion resistance and excellent superhydrophobicity.
2.Experimental section
2.1. Chemicals
Mg alloys (AZ91D,30 × 20 × 2 mm3,wt.%: Al 8.50,Mn 0.30,Zn 0.90,Cu 0.03,Si 0.13,Mg balance) and Sandpaper were bought from the local market.Zn(NO3)2·6H2O,2-methylimidazole and Methanol were purchased from Shanghai Aladdin Biochemical Technology Co.,Ltd.Other reagents used in the experiment (such as NaOH,Na2SiO3,Na2CO3,Anhydrous ethanol,Na3PO4,NaF,CH3COOH) were purchased from Sinopharm Chemical Reagent Co.,Ltd.All reagents are of analytical grade and can be used directly without purification.
2.2. Pretreatment of AZ91D
Before synthesizing the superhydrophobic coating,the AZ91D needs to be cleaned.First,polished with sandpaper of 180 #,600 #,1000 # and 1500 #.And AZ91D was degreasing with alkaline solution (Na2CO330 g/L,Na3PO420 g/L,NaOH 20 g/L) at 60°C for 60 s.After that,washed with ethanol and deionized water for 10 min,and dried at 50°C.The pretreated AZ91D was immersed in the MAO electrolyte as anode and stainless steel as cathode,using silicate system electrolyte (11 g/L NaOH,5 g/L Na2SiO3,8 g/L NaF,4 g/L Na2B4O7,1 g/L Na2WO4,4 mL/L C3H8O3and 4 mL/L C6H15NO3),frequency 50 Hz and duty cycle 30%treatment the sample for 40 min,and the termination voltage was around 220 V.
2.3. Syntheses of ZnO@ZIF-8 array
The synthesis of superhydrophobic ZnO@ZIF-8 array was divided into three steps.The first was to prepare a ZnO seed layer on the AZ91D.A seed solution was prepared according to Zn(NO3)2·6H2O: water: NaOH=1:222:6 (molar ratio),then the seed solution and AZ91D were transferred to the reactor at 120°C for 2 h;then place it and a ZnO nanorods growth solution (0.25 mol/L Zn(NO3)2·6H2O,1.5 mol/L NaOH) in the reactor at 150°C for 4 h.After the reactor was cooled to room temperature naturally,take out the AZ91D covered with the ZnO nanorods,flush and dry.Finally,growth of ZIF-8 on the surface of ZnO nanorods,prepared a 0.5 mol/L 2-methylimidazole methanol solution,then place it and AZ91D carrying ZnO nanorods in a reactor at different temperature (70,100,120,150,180°C) for different time (1,3,5 h) to obtain ZnO@ZIF-8 nanorods,take out and dry overnight.
2.4. Characterizations
The microscopic morphology of the samples was characterized by field emission electron scanning microscope(SEM,SU5000,Japan) and field emission transmission electron microscope (TEM,JEM-2100F,Japan) with an acceleration voltage of 200 kV and which was equipped with X-ray energy spectrometer (XFlash5030T,Germany).An Xray diffractometer (XRD,X,perPRO,Holland) equipped with Cu Kα(1.54 ˚A) radiation to understand the phase structure of the sample.X-ray photoelectron spectroscopy (XPS,KAlpha,h=1486.6 eV) was used to investigate the bonding states.The wettability of the samples was inspected by XGCAM (TT260,China) type testing device,and the average value of five positions was taken.To evaluate the stability of the superhydrophobic coating,solution with different pH(add CH3COOH or NaOH in the water.) and different NaCl concentrations as the testing media.High and low temperature resistance measurement was performed by determining the water contact angle of coating after treated at-40°C to 100°C for 24 h In addition,the stability of coating evaluated by determining the water contact angle of coating after immersion in ethanol for different time.All water contact angle were performed at room temperature.To demonstrate the self-cleaning property of the superhydrophobic coating,the chalk dust was used as the contaminant.The chalk dust was distributed on the surface and subsequently cleaned by the water droplets using a syringe.A Zennium (Zahner,Germany) electrochemical workstation was used to analyze the electrochemical performance of the samples in a 3.5 wt.%NaCl solution.A typical three-electrode electrolytic cell was used for test: a platinum electrode as a counter electrode,a saturated calomel electrode as an auxiliary electrode,and exposed specimen 1 cm2was as a working electrode.Before the electrochemical test,immerse the sample in a 3.5 wt.%NaCl solution for 10 min to avoid large fluctuations in the open circuit potential.The frequency range of electrochemical impedance spectrum (EIS) was 105to 10-2Hz,and the amplitude of the sinusoidal signal of 10 mV.The polarization curves were measured at a scan rate of 5 mV·s-1.The Mott-Schottky curves was performed with a constant frequency of 1 kHz and a sine wave modulated signal of 10 mV.
3.Results and discussions
3.1. Characterization of ZnO@ZIF-8 coating
The growth process of ZnO@ZIF-8 core-shell structure prepared on the surface of AZ91D was observed by SEM,and the dependence of ZnO@ZIF-8 on ligand concentration,reaction time and reaction temperature were further studied.Fig.1 shows the SEM images and contact angles of different ligand concentrations of ZnO@ZIF-8.The concentration of ligand has a great influence on the growth and wettability of ZnO@ZIF-8.The MAO coating was rougher,and the surface has many micropores (Fig.1a).The reason for the formation of micropores was that at higher voltage,the dense oxide film was breakdown [35].MAO coating as transition layer could provide good adhesion to ZnO@ZIF-8 coating,and bring better corrosion resistance to AZ91D Fig.1b shows a flower-like ZnO array.These flower-like structures were composed of many ZnO nanorods,providing the micro-nano structures necessary for superhydrophobicity.The ZnO@ZIF-8 core-shell structure was synthesized in situ by flower-like ZnO array as a sacrificial template.With a low concentration of ligand,the formation of ZIF-8 crystals was almost invisible on the surface of ZnO array (Fig.1c).This may be due to insufficient activity of the ZnO,and ZIF-8 crystals cannot be induced in a lower concentration of ligand.As the ligand concentration increases,scattered ZIF-8 crystals began to appear on the surface of ZnO,with a grain size of 1 to 2 μm(Fig.1d).When the concentration increased to 0.5 mol/L,the surface of ZnO nanorods was covered by ZIF-8 crystals,and the flower-like structure was well preserved (Fig.1e and f).Since ZnO nanorods induce the growth of ZIF-8,they will also partially dissolve themselves,so there is a balance between the dissolution rate of ZnO and the rate of formation of ZIF-8.When the ligand concentration increases and the crystal growth rate is greater than the dissolution rate,ZIF-8 crystals can be formed on the surface of the ZnO nanorods.In addition,we also characterized the wettability of different coatings.It can be seen from Fig.1 that all coatings except Fig.1f exhibit typical hydrophilicity,and the contact angle of ZnO coating has dropped to 18.49°,which is also related to the hydrophilic property of ZnO itself [36].It is worth noting that after treatment with the ligand solution,the contact angles had a rising trend,and when the ligand concentration was 0.5 mol/L,the contact angle reach 154.81° without modification of low surface energy substances,achieving superhydrophobic state.This is rarely reported in previous superhydrophobic coatings.
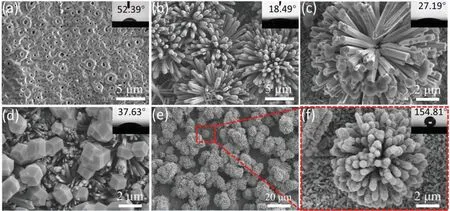
Fig.1.SEM images of (a) MAO coating,(b) ZnO array,and samples with different ligand concentrations in (c) 0.1 mol/L,(d) 0.2 mol/L,(e-f) 0.5 mol/L.The insets of the images of water drop on the surface.
The growth process of ZnO@ZIF-8 core-shell structure in different periods was studied.As shown in Fig.2a,at 1 h,scattered particles began to appear on the surface of ZnO nanorods,indicating that ZnO reacted with the ligand and produces crystals,but the number of crystals produced in a short time was limited.When the time was extended to 3 h,the particles on the surface of the nanorods increased significantly,and the cross-linked intergrowth of the particles form a thin layer around the surface of ZnO nanorods(Fig.2b) Fig.2c shows the surface morphology of 5 h Many crystals are accumulated on the surface of the carrier,and the morphology of ZnO nanorods disappears completely.Besides,the wettability of the coating was analyzed (Fig.2d),and the coating achieved superhydrophobic properties at 3 h The reason was that the inherent hydrophobicity of ZIF-8 and ZnO@ZIF-8 array can capture a large amount of air [28].However,the accumulation of many crystals on the surface would cause a decrease in the contact angle,but still maintains a high hydrophobicity.These results indicated that a more accurate structures were required for a superhydrophobic surface make only by specific structures.Fig.3 shows the effect of synthesis temperature on morphology and wettability.At 70 °C,there were a few particles on the surface of ZnO (Fig.3a);the particles on the surface of ZnO nanorods increase with increasing temperature and were smaller in size(Fig.3b–c);when reaches 150 °C,the ZnO nanorods were completely covered by the particles,and there are wide gaps between the ZnO@ZIF-8 nanorods (Fig.3d).When the temperature reaches 180 °C,the dissolution rate of ZnO accelerates,resulting in the disappearance of the original structure and an amorphous structure on the surface (Fig.3e);Fig.3f shows the contact angles at different synthesis temperatures.The contact angles increase with the increase of temperature,at 150 °C,it reached a superhydrophobic state with a contact angle of 154.94°.However,after the temperature reaches 180 °C,the contact angles dropped sharply due to the change of the surface structure.This was attributed to the destruction of ZnO structure caused the coating cannot capture air,resulting in a drop in contact angle.These results indicate that the micro-nano structures were a key factor in the construction of superhydrophobic surfaces,because the micro-nano structure affects the volume of trapped air.

Fig.3.SEM images of different ZIF-8 growth temperatures (a) 70 °C,(b) 100 °C,(c) 120 °C,(d) 150 °C,(e) 180 °C and (f) the contact angles.
Figure 4 a shown a typical TEM image and element mapping of ZnO@ZIF-8 nanorods.The TEM image shown that the core ZnO nanorods with dark-contrast,and the outer shell with light-contrast was ZIF-8.Element maps demonstrate that C,N,and Zn elements were uniformly distributed on the nanorods.Zn is the common element of ZnO nanorods and ZIF-8 crystals,while C and N are the two main elements of 2-methylimidazole which only as a ligand exists in the ZIF-8 crystal.The results shown that the ZIF-8 crystals have good symbiosis on the surface of ZnO nanorods.The cross-sectional composition line profiles of ZnO@ZIF-8 nanorods also confirmed the same result.The content of Zn element in the core area increased suddenly due to ZnO as the core,while the content of C and N elements remained unchanged (Fig.4b).The cross-sectional view of ZnO@ZIF-8 nanorods could visually see the ZnO as the core and the ZIF-8 as the outer shell.The formation of ZIF-8 not cause damage to the ZnO morphology,and ZIF-8 can also grow tightly and continuously on the surface (Fig.4c) Fig.4d reveals the growth mechanism of ZnO@ZIF-8 nanorods.2-Methylimidazole acted as an etchant to seize Zn2+from ZnO nanorods,and combine with Zn2+to form ZIF-8 crystals on the surface of ZnO nanorods.The high temperature can stimulate the activity of ZnO nanorods,which is conducive to the etching of metal ions by the ligand.Therefore,to promote achieve a balance between the dissolution rate of ZnO nanorods and the growth rate of ZIF-8,which is beneficial to the growth of ZnO@ZIF-8 core-shell structure.The crosssection morphology of ZnO@ZIF-8 nanorods are exhibited in Fig.4e,the interface between the coating and substrate had good connections.In order to further determine the crystal structure of the coating,via XRD analyze (as shown in Fig.4f and g).The XRD pattern shows that the coating was mainly composed of ZnO and ZIF-8.Except for the diffraction peaks at 31.78°,34.42°,36.26° and 47.54°,which are attributed to the hexagonal wurtzite type ZnO (JCPDS no.70–2551),the other diffraction peaks correspond to ZIF-8,and were consistent with the reported crystal structure data of ZIF-8 [37].These results prove the successful preparation of ZnO@ZIF-8 nanorods with a core-shell structure on the surface of AZ91.
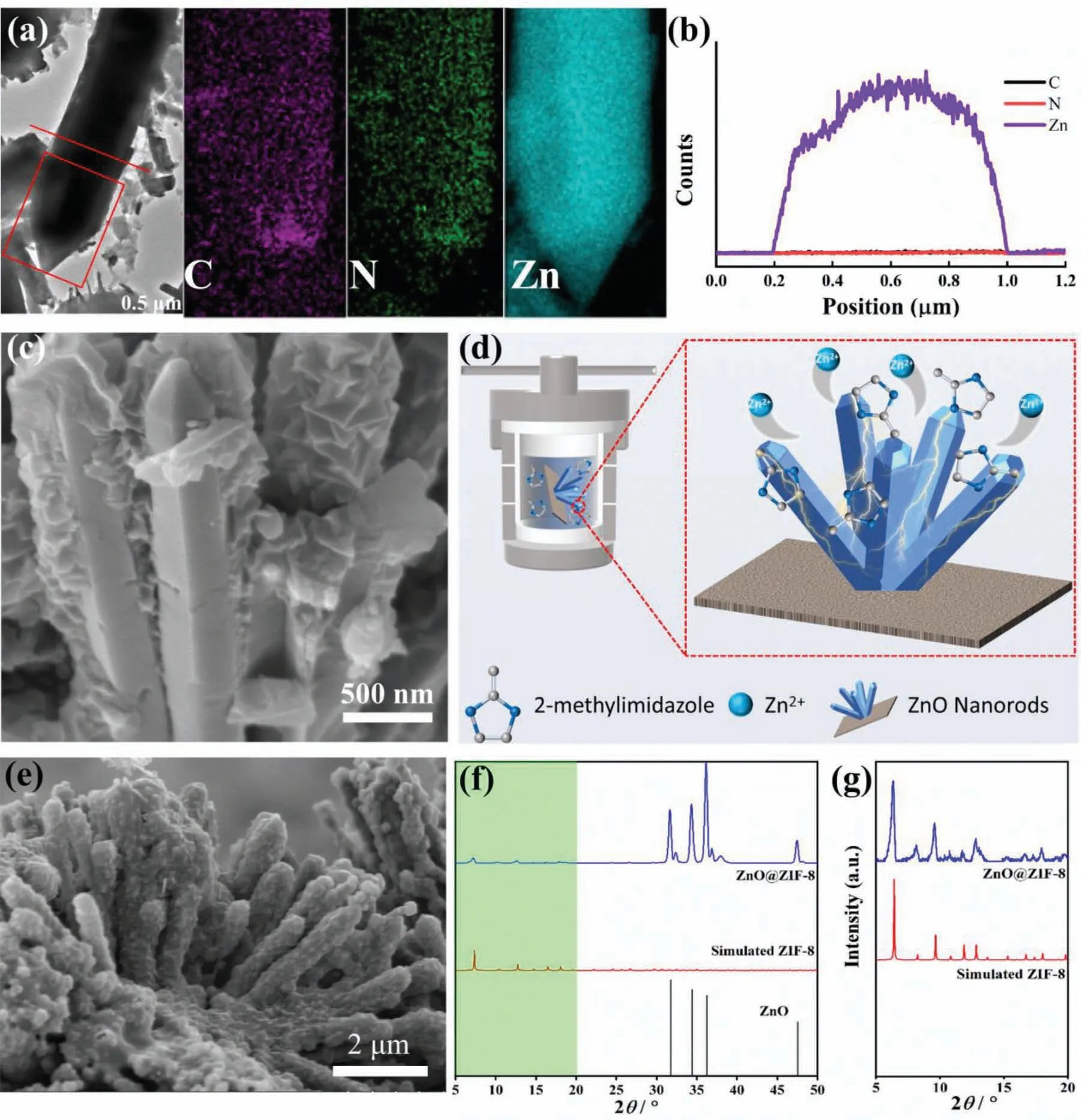
Fig.4.(a) TEM image of ZnO@ZIF-8 nanorods and C,N,Zn element maps (the zone marked with a rectangle in the TEM image),(b) cross-section compositional line profiles of ZnO@ZIF-8 nanorods (the line marked in the TEM image),(c) sectional view of ZnO@ZIF-8 nanorods,(d) growth mechanism of ZnO@ZIF-8 nanorods,(e) cross-section morphology of ZnO@ZIF-8 nanorods,(f) XRD pattern of ZnO@ZIF-8 superhydrophobic coating,(g) the shadow region in the (f).
The chemical composition of ZnO@ZIF-8 nanorods was characterized by XPS.The surface valence state of each element was analyzed on the high-resolution spectra of Zn 2p,C 1s,N 1s,O 1s Fig.5.a shows the high-resolution spectrum of Zn 2p.The characteristic peaks at 1021.7 eV and 1044.7 eV correspond to Zn 2p3/2and Zn 2p1/2,respectively,indicating that the Zn element had a divalent state in ZnO@ZIF-8 nanorods.The C 1s high-resolution spectrum of ZnO@ZIF-8 nanorods showed two characteristic peaks at 284.6 eV and 285.9 eV,which were mainly attributed to 2-methylimidazole and adventitious hydrocarbon (Fig.5b) [38].It can be observed in Fig.5c that the high-resolution spectrum of N 1s can be divided into two characteristic peaks.The peaks at 398.39 and 398.95 eV correspond to the C=N-Zn and N=C between the ZnO and ZIF-8 interfaces,respectively.In Fig.5d,the binding energies of the O 1s spectrum of ZnO@ZIF-8 nanorods were concentrated at 530.7 eV and 531.9 eV,respectively.The peak of 530.7 eV corresponds to the lattice oxygen(O2-) in ZnO@ZIF-8.The peak of 531.9 eV corresponds to the chemisorbed oxygen or hydroxyls in the oxygen-deficient regions on the surface of the material.
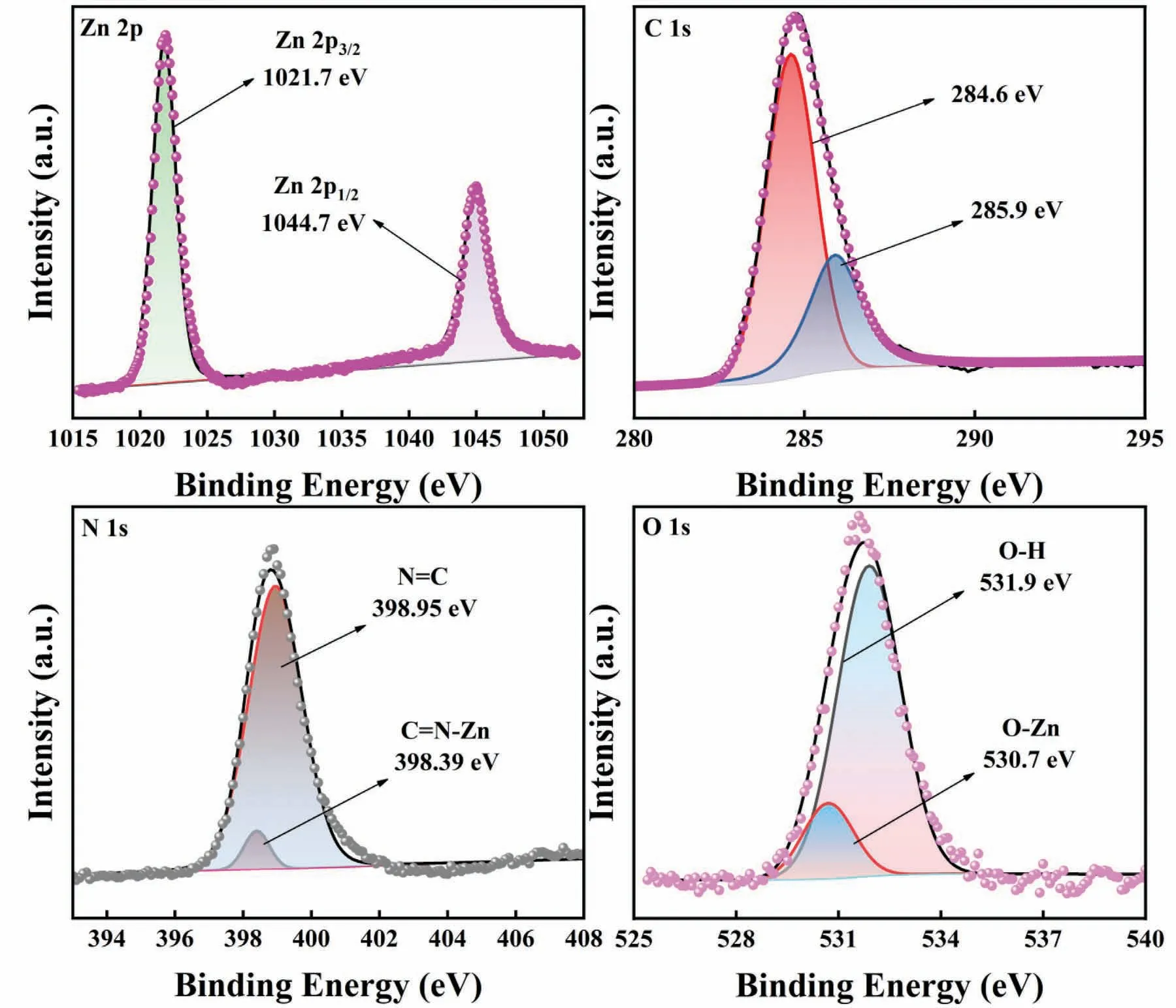
Fig.5.High-resolution XPS spectra of ZnO@ZIF-8 nanorods.
3.2. Stability and self-cleaning
The industrial application of superhydrophobic coatings is mainly limited by chemical stability.In order to investigate the stability of the superhydrophobic coating,Fig.6a shows the wettability of different concentrations of NaCl solution in the superhydrophobic surface.The superhydrophobicity has not changed and maintained at a high contact angle.The results shown that the ionic strength had little effect on the superhydrophobic coating,and exhibit good hydrophobicity even in a corrosive environment Fig.6b studies the effect of pH on the hydrophobicity of the coating.When the coating was contact with acidic droplets,the contact angle fluctuates slightly but still exceed 150°,which indicates that the superhydrophobic coating has an excellent stability.As the alkalinity increases,the contact angle increases slightly and tends to be stabilize.The air layer retained by the flower-like structure of superhydrophobic coating could reduce the contact area between droplets and solid surface.At the same time,the superhydrophobic coating was insensitive to acid and alkali condition and chemically stable.In fact,superhydrophobic coatings would face harsh external environments for a long time in applications,so long-term stability was particularly important Fig.6c shows the change of contact angles after different temperature treatments.When the coating was placed at low-temperature for 24 h,the superhydrophobicity of coating without change significantly,and maintained at a higher contact angle.This result demonstrates the coating have excellent hydrophobicity and stability in low-temperature environment.With the temperature increased to 100 °C,the contact angles have little fluctuation and showed a downward trend,the contact angle still 151.93°,indicating that temperature not damage microstructure of the coating.The contact angle changes of superhydrophobic coatings immersed in ethanol for different time were shown in Fig.6d.When immersed for 7 days,the wettability of the coating was not affected,and still in a superhydrophobic state.During the immersion process,ethanol with lower surface tension would infiltrate the coating surface.However,ethanol would not destroy the rough structure that traps the air layer,so that the contact angle of the superhydrophobic coating unchanged.The results indicate that the prepared coating has good stability and long-term performance,which brings important significance to the practical application of superhydrophobic coatings.
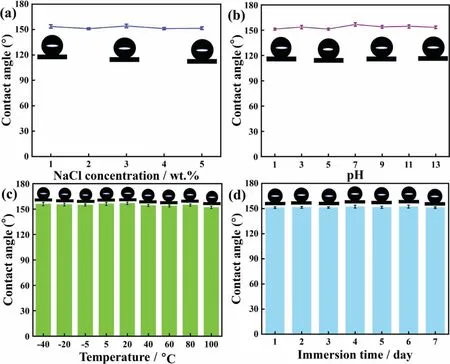
Fig.6.Stability evaluation of ZnO@ZIF-8 superhydrophobic coating by the changes of contact angle: (a) contact with different NaCl concentrations,(b)contact with different pH solutions,(c) different temperature treatment for 24 h,(d) immersion in ethanol for different time.
In order to further prove the superhydrophobicity of the coating,the deformation process of water droplets between two superhydrophobic coatings was recorded.In this work,a water droplet (5 μL) was dropped on the surface of the superhydrophobic coating,and another superhydrophobic coating was used to squeeze the water droplet into deformation to ensure sufficient contact,and then the coating was pulled away (Fig.7a).The superhydrophobic coating without adhere to the water droplets during the extraction process,and it could stand on the surface of the coating perfectly.This indicates that the ZnO@ZIF-8 coating has excellent superhydrophobicity and low adhesion.This phenomenon could be explained by the Cassie-Baxter model.According to this model,the flower-like ZnO@ZIF-8 array can capture a large amount of air to form an air cushion,reduced the contact area between liquid and solid surface,thus exhibit strong water repellency.The contact angle could be calculated according to the Cassie-Baxter Eq.(1) [39]:
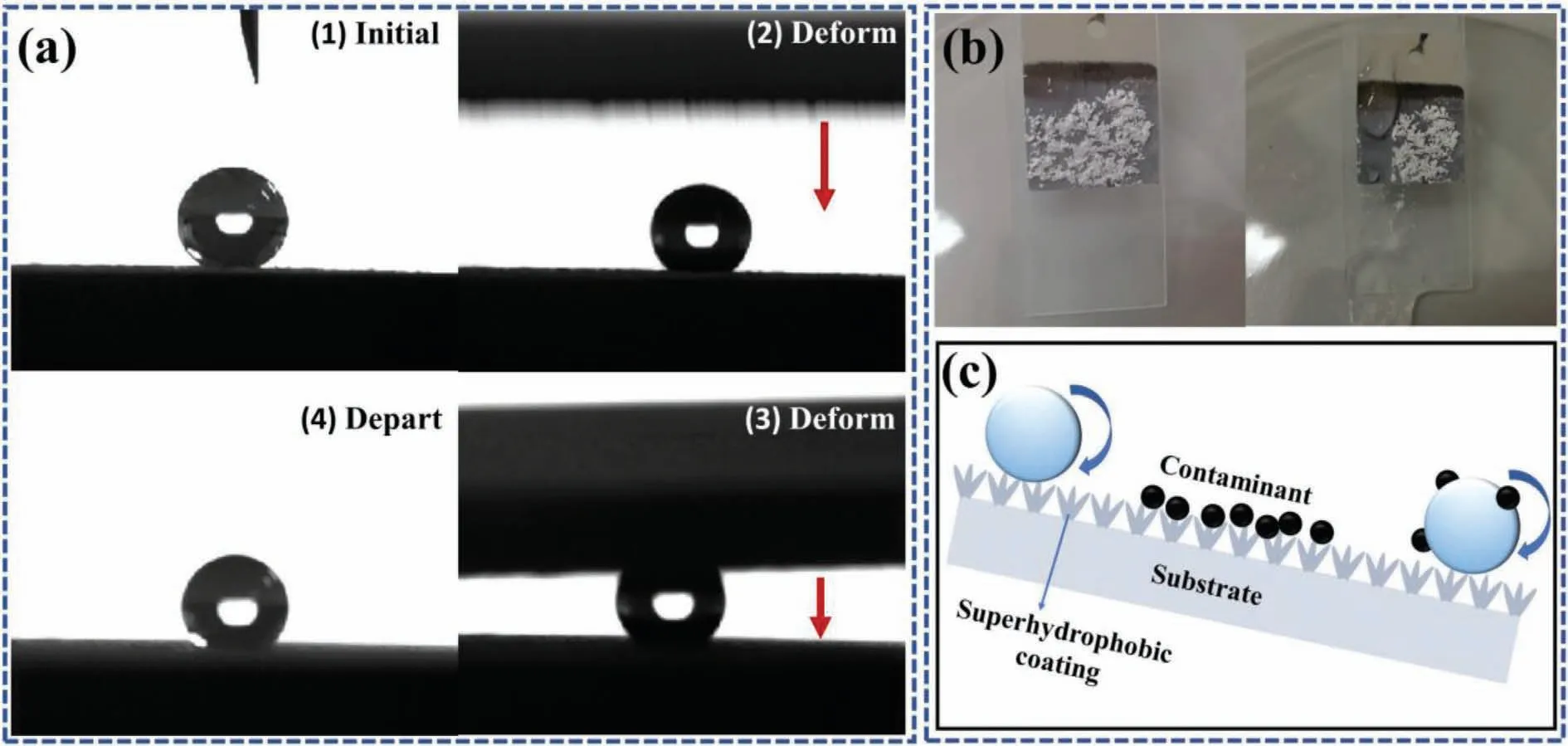
Fig.7.(a) Deformation process of droplets on ZnO@ZIF-8 superhydrophobic surface,(b) self-cleaning test of ZnO@ZIF-8 superhydrophobic coating,(c)schematic illustration of self-cleaning process.
Among them,fSLis the fraction of the coating and air in contact with liquid,θfis the apparent contact angle,andθois the intrinsic contact angle of the flat surface.In this work,θf=154.81°,θo=79.08°,sofSL=0.080,indicating that the contact area between the coating and the droplet was about 8.0%.The low adhesion of superhydrophobic coating was demonstrated by the self-cleaning test.As shown in Fig.7b,chalk dust as the pollution source,water droplets could easily take away the pollution source on the surface,indicating that the superhydrophobic coating has good self-cleaning and prevents liquid penetration.The self-cleaning schematic illustration more intuitively describes the self-cleaning process of the superhydrophobic coating (Fig.7c).When the inclination angle of the coating was greater than the sliding angle of the droplets,the contaminants on the surface could be easily removed.
3.3. Anti-corrosion property
The corrosion resistance evaluation of superhydrophobic coating was of great significance to its practical application.To explore the corrosion resistance and mechanism of superhydrophobic coatings,the corrosion characteristics of AZ91D,MAO coating and ZnO@ZIF-8 superhydrophobic coating in 3.5 wt.% NaCl solution were studied through simple potentiodynamic polarization curves (Fig.8).Generally,the potentiodynamic polarization curves could explain the corrosion reaction of the cathode and anode of the coatings,a more positive corrosion potential (Ecorr) and lower corrosion current density (Icorr) reflect that the coating has better corrosion protection performance [40].The inhibition rate (η) could be calculated by the following Eq.(2) [41]:
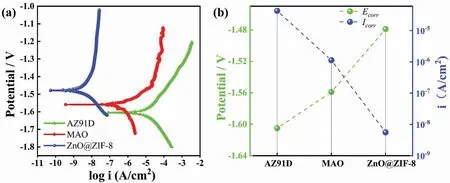
Fig.8.(a) Potentiodynamic polarization curves of AZ91D,MAO coating,ZnO@ZIF-8 superhydrophobic coating in 3.5 wt.% NaCl solution and (b) the corresponding Ecorr and Icorr.
Among them,IoandIcorrrepresents the current density of the AZ91D and the superhydrophobic coating,respectively.And theηof ZnO@ZIF-8 superhydrophobic coating was 99.96% Fig.8a demonstrate that the potentiodynamic polarization curves of the AZ91D and different coatings,and theEcorrandIcorrwere shown in Fig.8b.Compared with AZ91D,the superhydrophobic coating ofEcorrhas positive shift,which was due to the superhydrophobic coating changing the chemical composition of the substrate surface.In addition,Icorrhas dropped sharply,theIcorrof AZ91D was 10-5A/cm2,and theIcorrof superhydrophobic coating was lower,about 10-9A/cm2.The cathodic polarization of Tafel curve was related to the hydrogen evolution reaction on the coating surfaces,and the anodic polarization represents the dissolution of the substrates or the corrosion of the coatings [42].The current density of superhydrophobic coating in the cathode region was lower than that of the AZ91D,indicating that the coating could effectively inhibit the hydrogen evolution reaction of the AZ91D.The curve of the anode region was low and gentle,and weak anode polarization corresponds to lower current density and weaker corrosion tendency.It shows that the superhydrophobic coating has good corrosion resistance.The reason was that the presence of an air layer in the superhydrophobic coating,which effectively reduces the contact area between the NaCl solution and the coating,and prevents the contact between the corrosive medium and the substrate.
In addition to the potentiodynamic polarization curves,the potentiostatic polarization curve test was conducted at open circuit potential for 2 h in 3.5 wt.% NaCl solution,the results are shown in Fig.9.The current density of all curves increased in the initial stage,and stabilized with the extension of time,indicating a passive film was formed.After the current densities were stable,the superhydrophobic coating exhibits lower current density.Compared with the AZ91D,reduced by 4 orders of magnitude.These results demonstrate that the superhydrophobic coating has a good protective effect on the Mg alloy matrix.
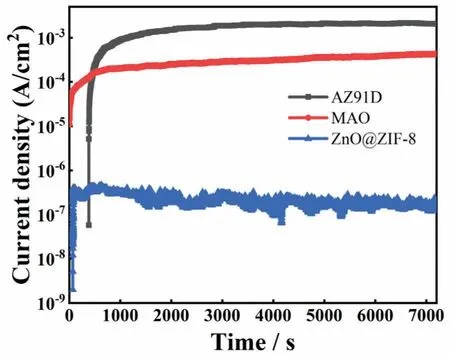
Fig.9.Potentiostatic polarization curve of different samples at open circuit potential in 3.5 wt.% NaCl solution.
EIS is an important method to evaluate the corrosion resistance of superhydrophobic coatings,its capability of “in situ”and non-destructively probing relaxation phenomena over a wide range of frequencies [43].In order to better understand the corrosion mechanism of the coatings,the coatings were analyzed in detail via EIS.Fig.10 shows the EIS of AZ91D and different coatings in 3.5 wt.% NaCl solution.From Fig.10a,AZ91D has a capacitive arc at the high frequency region and an inductive arc at the low frequency region.The inductive arc is related to the corrosion products formed on the surface of AZ91D.However,after MAO treatment,Nyquist plots present one capacitive arc,and the diameter of the capacitive loop was larger than AZ91D.Generally,the larger the diameter of the capacitive loops means that the coatings have good corrosion resistance.The superhydrophobic coating present two capacitive arcs.The capacitive arc at the high frequency region was attributed to the charge transfer process caused by the double layer capacitance,and the capacitive loop at the intermediate frequency region was caused by the coating resistance [44].Compared with other coatings,the superhydrophobic coating shows a larger capacitance loop,indicating that the barrier performance of the superhydrophobic coating is significant Fig.10b shows the charge transfer resistance of different samples,and the increasing trend was obvious,which further shows the excellent corrosion resistance of the superhydrophobic coating.In addition,the impedance modulus-frequency Bode plots shows the same result (Fig.10c).The MAO coating provides limited protection to the substrate,while the superhydrophobic coating has the largest |Z|0.01Hzvalue.The |Z|0.01Hzof ZnO@ZIF-8 coating (106Ω·cm2) was 3 orders of magnitude higher than the AZ91D (103Ω·cm2),which indicated the excellent protection ability for the substrate.Meanwhile,the phase angle of the superhydrophobic coating was higher than the other specimens,and a higher phase angle could maintain a wider frequency range (as shown in Fig.10d),which indicates that the superhydrophobic coating has better uniform density and means that it provides a good protective effect to the substrate.
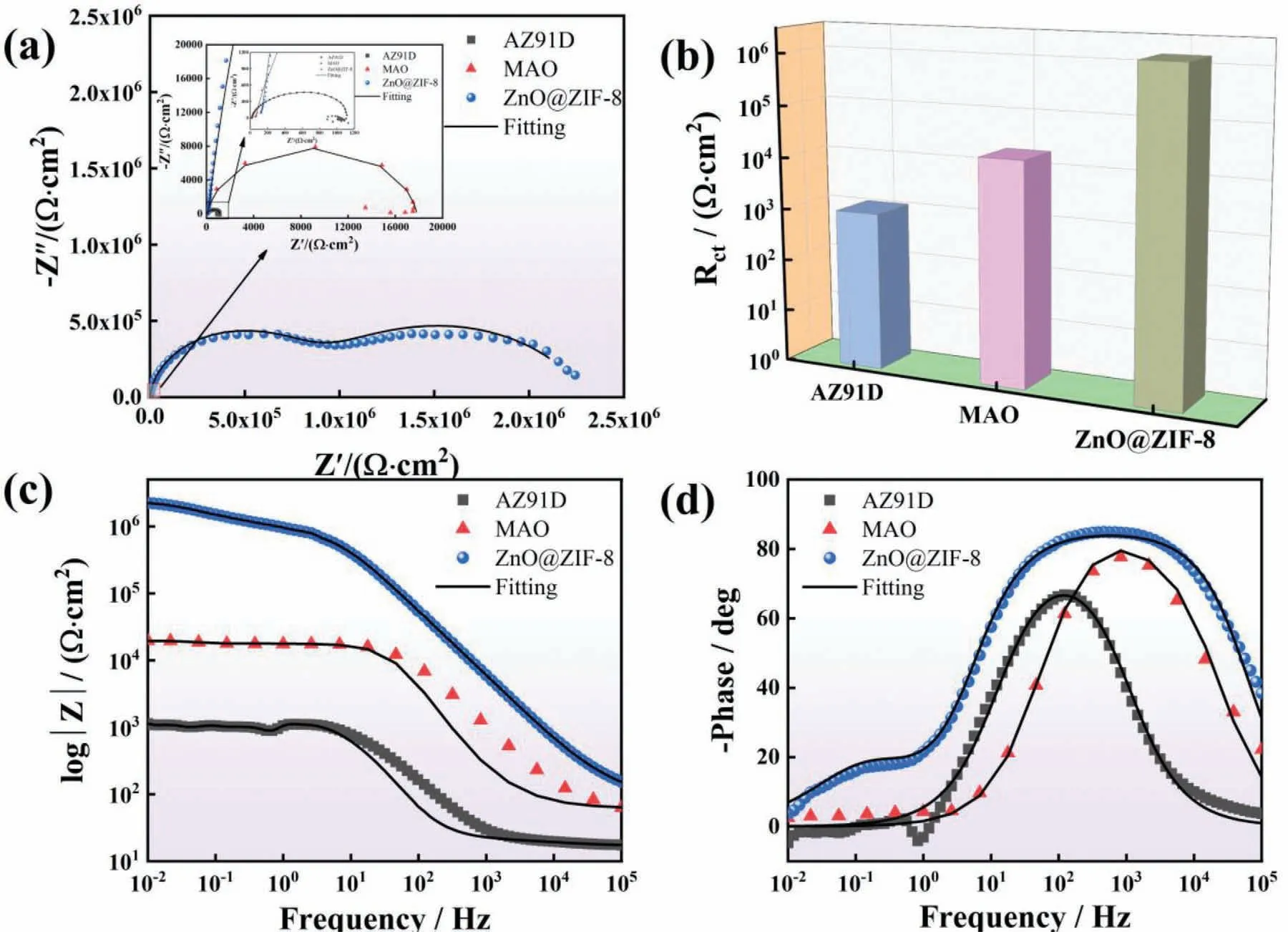
Fig.10.(a) Nyquist plots,(b) charge transfer resistance,(c) impedance modulus-frequency Bode plots,(d) phase-frequency Bode plots of different samples in 3.5 wt.% NaCl solution.
The equivalent circuits were fitted to illustrate the corrosion process of the coatings,and the contribution of the corresponding components were systematically studied.Among them,Rsis the electrolyte resistance,which was closely related to the distance between the electrolyte solution and the electrode;RcandQcare the resistance and capacitance of the coatings,respectively;RctandQdlrepresents the charge transfer resistance and the electric double layer capacitance,respectively;andLandRLrepresent the inductance and resistance,respectively.In order to fitted a more accurate equivalent circuit,the capacitive is replaced by aQ(constant phase element,CPE) [45].The equivalent circuit models as shown in Fig.11.The MAO coating on the substrate belongs to metallurgical bonding,the interface was not obvious,and combined with its electrochemical impedance spectroscopy,so the equivalent circuit in Fig.11b was used for fitting.In combination with Fig.10,the fitting curves accurately describes the experimental data,which shows that the model of Fig.11 was reasonable.The relevant parameters fitted from the equivalent circuits are summarized in Table 1.Among them,Rctwas an important indicator of the corrosion resistance of the coatings,and a high value indicates excellent corrosion resistance[46].It can be found that theRctof AZ91D,MAO coating and ZnO@ZIF-8 superhydrophobic coating were 1.044×103Ω·cm2,1.776×104Ω·cm2,1.414×106Ω·cm2,respectively,and theRctof superhydrophobic coating was higher than AZ91D for 3 orders of magnitude.The results indicating that the superhydrophobic coating has excellent corrosion resistance,which was consistent with the polarization curve results.Generally,the n reflects the roughness of the coatings.The larger the n,the smoother the coatings,and vice versa [47].The n value of the superhydrophobic coating was smallest,indicating that it has the largest roughness,which is caused by the flower-like structure of ZnO@ZIF-8 array.For superhydrophobic coating,the rough structure was more conducive to trapping air in the gaps,thereby forming an air layer on the surface,reducing the contact area between the corrosive solution and the coatings,and achieving the effect of isolating the corrosive medium from direct contact with the substrate.
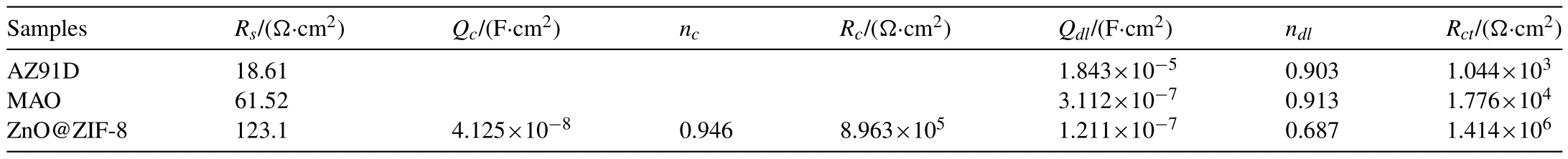
Table 1Fitting parameters obtained from equivalent circuits.
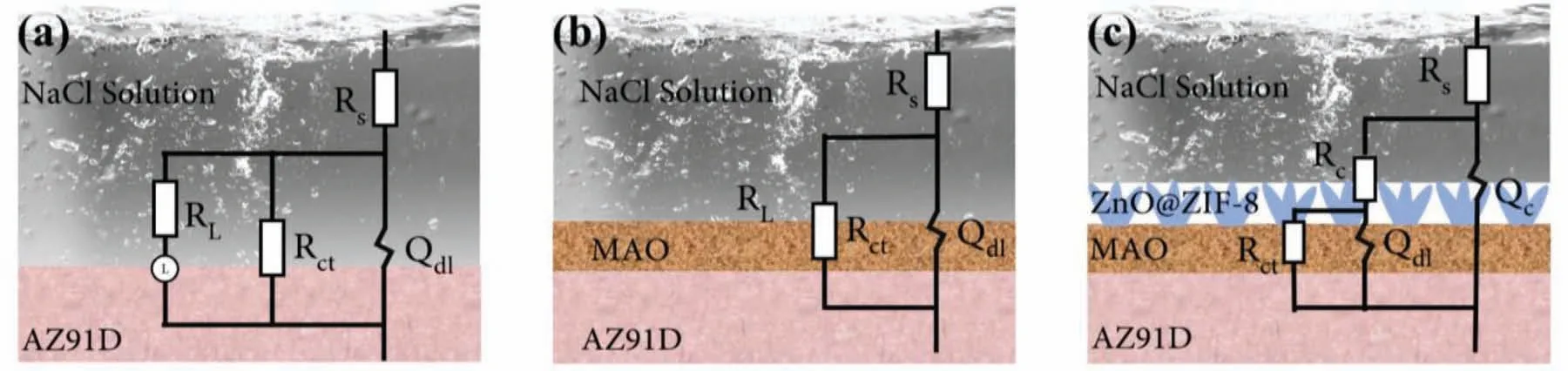
Fig.11.Equivalent circuits for (a) AZ91D,(b) MAO coating,(c) ZnO@ZIF-8 superhydrophobic coating in 3.5 wt.% NaCl solution.
In order to further evaluate the corrosion resistance of the coatings,salt spray test was used to characterize the long-term stability.During the test process,the coatings were placed in salt spray cabinet with continuous spraying (5 wt.% NaCl solution,35°C) Fig.12.shown the salt spray test photos of the coating at different times and SEM images.According to Fig.12a and b,the corrosion of the coatings was closely related to the topography and structure.After 48 h of exposure,the MAO coating appeared corrosion,and the corrosion area increased with the prolonged exposure time.This was mainly due to the fact that the micropores on the surface of the MAO coating provided opportunities for the corrosive medium to contact the substrate.However,the ZnO@ZIF-8 superhydrophobic coating showed slight pitting corrosion after 192 h of exposure.At the same time,the morphology of the coating after exposure for 216 h (Fig.12c and d) were observed.The morphology of the MAO coating was severely damaged,and a large number of cracks appeared,which could not provide good corrosion resistance to the substrate.The morphology and structure of the ZnO@ZIF-8 superhydrophobic coating are slightly damaged,and the original morphology was still maintained.This is because the air layer on the surface of the superhydrophobic coating hinders the penetration of the corrosive medium and avoids the contact between the corrosive medium and the substrate.The results shown that ZnO@ZIF-8 superhydrophobic coating has good corrosion resistance and long-term stability.

Fig.12.Photos of salt spray tests and SEM images of exposure time of 216 h for (a,c) MAO and (b,d) ZnO@ZIF-8.
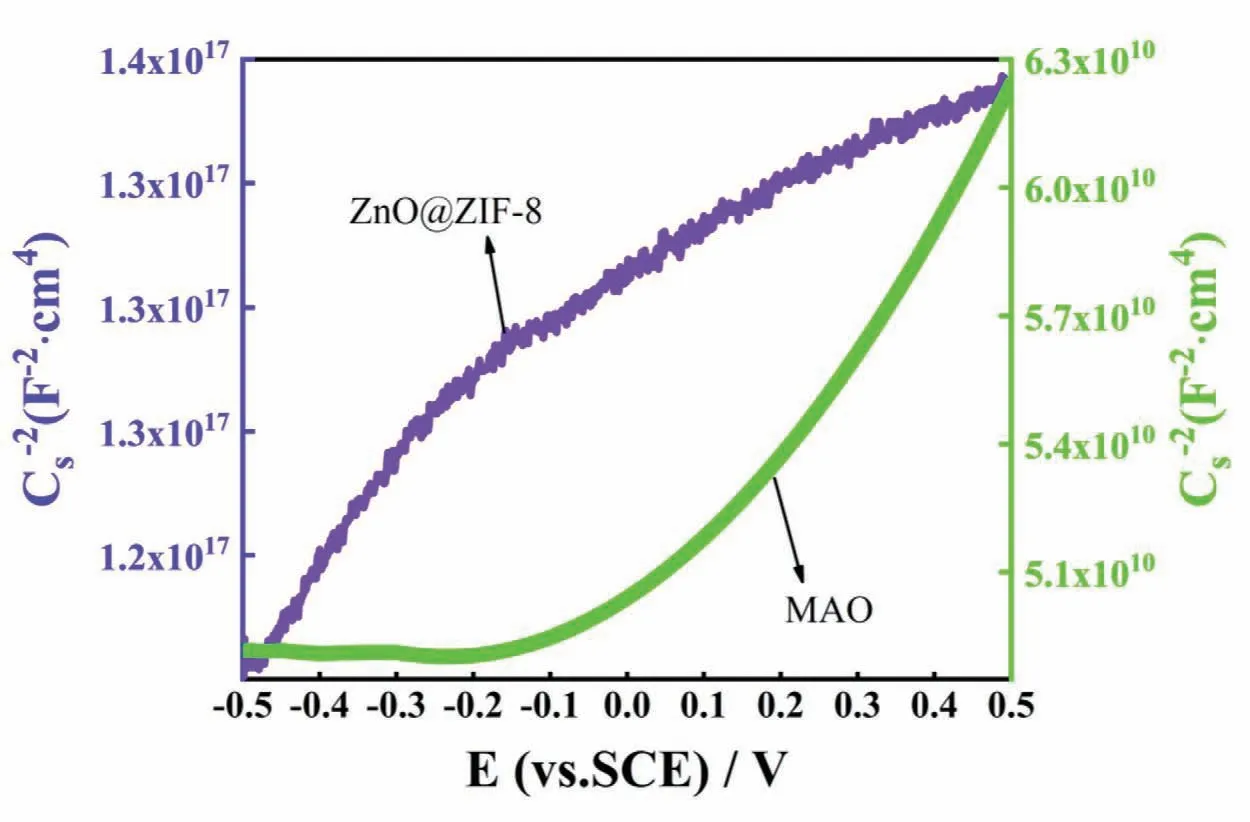
Fig.13.Mott-Schottky of ZnO@ZIF-8 superhydrophobic coating in 3.5 wt.% NaCl solution.
3.4. Electronic properties of superhydrophobic coating
Generally,the passivation films formed on the metal surface exhibits semiconductor properties.When the films were in contact with the solution,the semiconductor films and the solution were charged with opposite charges,and the excess charges in film were distributed in the space charge layer.The relationship between the space charge layer capacitance(Cs) and the applied potential (E) can be analyzed by the Mott-Schottky in solid-state physics [48].For n-type semiconductors,Cs~Ecan be written as [49]:
Whereε0is the spatial permittivity(8.85×10-14F/m),εis the dielectric constant of the film at room temperature,Ndis the donor concentration (cm-3),Efbis the flat band potential (V),kis the Boltzmann constant,Tis absolute temperature (K),eis the electron charge(1.602×10-19C),ThekT/eat normal temperature is about 25 mV,which can be ignored.In the Mott-Schottky,Ndcan be obtained from the slope of linearCs-2~E,andEfbcan be obtained from the intercept.
Figure 13 shows the Mott-Schottky of the MAO coating and ZnO@ZIF-8 superhydrophobic coating.It can be found that the slope of the linearCs-2~Eappears positive within the potential region from-0.5 V to 0.5 V,indicating that the passivation film has n-type semiconductor properties.For n-type semiconductors,the parameterNdwas the positive defects (such as cation interstitials or anion vacancies),which reflects the uniformity of the passivation film,and is used to evaluate the anti-corrosion performance of the passivation film [50].TheNdof ZnO@ZIF-8 superhydrophobic coating was 1015cm-3,and for the MAO coating was 1021cm-3.The results shown that ZnO@ZIF-8 superhydrophobic coating had a lowerNdthan that of MAO coating.Usually,Clexists in the solution in the form of Cl-·H2O at the solidliquid interface,and by reacting with O vacancies to generate more metal ion vacancies.These excess metal ion vacancies will pass through the passivation film and combine with the matrix metal atoms.A large number of metal ion vacancies accumulate and combine with the matrix atoms to cause the separation of the film from the matrix,which leads to corrosion [51].However,the ZnO@ZIF-8 superhydrophobic coating can reduce the contact area between the solution and the substrate,reduce the adsorption of Cl-at the interface,and effectively slow down the generation of metal ion vacancies,thereby avoiding the corrosion of the metal substrate.
3.5. Anti-corrosion mechanism
Compared with other engineering materials,Mg alloys with lower electrode potential result in high activity and corrosion easily occurs.Therefore,the anticorrosive coating deposited on Mg alloys can effectively avoid the failure of Mg alloy substrates and reduce economic losses caused by corrosion,which is of great significance to the practical application of Mg alloys.It was considered that corrosion pits will easily occur in the synthetic solution for a long-time,due to the non-homogeneous electrochemical property and high chemical activeness of Mg alloys in aqueous solutions [52].As an in-situ grown ceramic coating,the MAO coating is attached to the surface of Mg alloy by metallurgical bonding,which can provide the substrate with good corrosion resistance.However,the MAO coating is distributed with many micropores.When the MAO coating is without post-processing,the surface micro-porous will become the transmission channel of the corrosive medium.The corrosive ions will preferentially penetrate the micropores and contact the substrate to corrode,which eventually lead to physical barrier is damage(Fig.14a).Therefore,a single MAO coating fails to provide long-term corrosion protection for the substrate.On the contrary,a superhydrophobic coating constructed on the MAO coating will provide synergistic protection for the substrate.The micropores of MAO coating can provide interlocking points for the ZnO@ZIF-8 superhydrophobic coating,which is conducive to the strong adhesion of the superhydrophobic coating to the substrate surface.Secondly,ZnO@ZIF-8 superhydrophobic coating can rehabilitate the porous defects,reduce the transmission channel of corrosive ions,and inhibit the contact of corrosive medium with the substrate.Simultaneously,the flower-liked ZnO@ZIF-8 array can trap air in the gap to form an air layer,effectively reducing the contact area between the corrosive liquid and the surface,and further enhancing corrosion protection (Fig.14b).It is noteworthy that the combination of semiconductor materials and MOFs has shown exciting results,and the superhydrophobic state can be achieved without the requirement of fluorination or silane treatment,which is rarely reported in previous studies.The experimental results shown that the ZnO@ZIF-8 core-shell structure has good corrosion resistance,excellent self-cleaning property and superhydrophobicity (Fig.14c).It is proved that the ZnO@ZIF-8 core-shell structure is an effective corrosion inhibitor for metal substrates,and they play an important role in improving the corrosion resistance of metal substrates.
4.Conclusion
A self-templating method was used to successfully fabricate the ZnO@ZIF-8 nanorod with core-shell structure on the surface of AZ91D.The ZnO nanorods not only act as sacrificial template but also provide Zn2+for the formation of ZIF-8.Researches shown that the ligand concentration,synthesis time and temperature are extremely important for the growth of ZnO@ZIF-8 nanorods,because they have important impact on the balance between the dissolution rate of ZnO nanorods and the formation rate of ZIF-8.The as-prepared ZnO@ZIF-8 nanorods with micro-nano hierarchical structure had a contact angle of 154.81°.Ascribed to the hydrophobicity of ZIF-8 and the ZnO@ZIF-8 nanorod array captures a layer of air.ZnO@ZIF-8 superhydrophobic coating showed excellent chemical stability and long-term durability with exposure to harsh environments,as well as outstanding selfcleaning properties.Compared with the untreated AZ91D,the ZnO@ZIF-8 superhydrophobic coating also developed a significantly enhances the corrosion resistance.Icorrwas reduced by 4 orders of magnitude,andRctwas increased by 3 orders of magnitude.In addition,the superhydrophobic coating without surface modification is an environmentally friendly coating,which provides a new pathway for anti-corrosion coating systems.ZnO@ZIF-8 core-shell structure nanorods superhydrophobic coating has widespread application,and can be extended to the combination of a variety of semiconductor materials and metal organic frameworks,and is suitable for protecting different metal substrates.Therefore,this new-type of semiconductor@MOFs core-shell structure is expected to develop into a superhydrophobic and corrosion resistant coating.
Declaration of Competing Interest
None.
Acknowledgements
This work was financially supported by Guangxi Natural Science Foundation of China (No.2020GXNSFAA159011),National Natural Science Foundation of China(No.51664011).
杂志排行
Journal of Magnesium and Alloys的其它文章
- Corrosion behavior of composite coatings containing hydroxyapatite particles on Mg alloys by plasma electrolytic oxidation: A review
- Rational design,synthesis and prospect of biodegradable magnesium alloy vascular stents
- Antibacterial mechanism with consequent cytotoxicity of different reinforcements in biodegradable magnesium and zinc alloys: A review
- Preparation,interfacial regulation and strengthening of Mg/Al bimetal fabricated by compound casting: A review
- Pitting corrosion behavior and corrosion protection performance of cold sprayed double layered noble barrier coating on magnesium-based alloy in chloride containing solutions
- Designing strategy for corrosion-resistant Mg alloys based on film-free and film-covered models
