Corrosion characteristics of single-phase Mg–3Zn alloy thin film for biodegradable electronics
2023-12-27JiWooGuJaeYoungBaeGuangzheLiHaeWonHwangaSoHyeonLeeSungGeunChoiJuYoungKimMyoungRyulOkYuChanKimSeungKyunKang
Ji-Woo Gu ,Jae-Young Bae ,Guangzhe Li ,Hae Won Hwanga, ,So-Hyeon Lee ,Sung-Geun Choi ,Ju-Young Kim,Myoung-Ryul Ok,e,Yu-Chan Kim,e,*,Seung-Kyun Kang,**
a Department of Materials Science and Engineering, Seoul National University (SNU), Seoul 08826, Republic of Korea
b Research Institute of Advanced Materials (RIAM), Seoul National University, Seoul 08826, Republic of Korea
cSoft Foundry Nano Systems Institute (NSI), Seoul National University, Seoul 08826, Republic of Korea
d Biomaterials Research Center, Biomedical Research Division, Korea Institute of Science and Technology (KIST), Seoul 34141, Republic of Korea
e Division of Bio-Medical Science and Technology, KIST School, Korea University of Science and Technology, Seoul 02792, Republic of Korea
fDepartment of Materials Science and Engineering, Ulsan National Institute of Science and Technology (UNIST), Ulsan 44919, Republic of Korea
Abstract Biodegradable metals as electrodes,interconnectors,and device conductors are essential components in the emergence of transient electronics,either for passive implants or active electronic devices,especially in the fields of biomedical electronics.Magnesium and its alloys are strong candidates for biodegradable and implantable conducting materials because of their high conductivity and biocompatibility,in addition to their well-understood dissolution behavior.One critical drawback of Mg and its alloys is their considerably high dissolution rates originating from their low anodic potential,which disturbs the compatibility to biomedical applications.Herein,we introduce a single-phase thin film of a Mg–Zn binary alloy formed by sputtering,which enhances the corrosion resistance of the device electrode,and verify its applicability in biodegradable electronics.The formation of a homogeneous solid solution of single-phase Mg–3Zn was confirmed through X-ray diffraction and transmission electron microscopy.In addition,the dissolution behavior and chemistry was also investigated in various biological fluids by considering the effect of different ion species.Micro-tensile tests showed that the Mg–3Zn alloy electrode exhibited an enhanced yield strain and elongation in relation to a pure Mg electrode.Cell viability test revealed the high biocompatibility rate of the Mg–3Zn binary alloy thin film.Finally,the fabrication of a wireless heater demonstrated the integrability of biodegradable electrodes and highlighted the ability to prolong the lifecycle of thermotherapy-relevant electronics by enhancing the dissolution resistance of the Mg alloy.© 2023 Chongqing University.Publishing services provided by Elsevier B.V.on behalf of KeAi Communications Co.Ltd.
Keywords: Biodegradable alloy;Mg–3Zn binary alloy;Solid-solution thin film electrode;Biodegradable conductor;Transient electronics.
1.Introduction
Biodegradable electronics,or transient electronics,show unusual physical configurations under temporal conditions and are a novel class of emerging electronics in the fields of biomedical implantable therapeutics.Clinically relevant electronics include wireless stimulators for accelerating the regeneration of peripheral nerves,temporary intracranial pressure sensors for monitoring unexpected pressure increases due to traumatic injury,and drug delivery vehicles for acute and short-term chemotherapy [1–6].Biodegradable metals are key components in transient electronics;they are used as electrodes,interconnecting wires,conducting pads of a device,and even conductive pastes for adhesives [1–3,7].Thus,the functional lifetimes of implantable transient electronics tend to rely on the dissolution rates of these biodegradable metallic circuits [8–10].
To date,several biodegradable metals (e.g.,Mg,Zn,Fe,Mo,and W) have been investigated for use in transient electronics;however,Mg has received particular attention owing to its high conductivity and biocompatibility,in addition to its well-understood dissolution behavior [11].For example,Mg foil has been used to cuff the electrodes and inductor coils of biodegradable nerve stimulators [12];it has also been employed in the triggerable metal gates of a biodegradable drug delivery device [13].Furthermore,Mg has been used as a structural vehicle for the generation of trenches in biodegradable pressure sensors [14,15].However,despite these promising advances,Mg exhibits a relatively high dissolution rate in biofluids owing to its extremely low anodic potential.Therefore,biodegradable electronics consisting of Mg thin films as electrodes rapidly exhibit a loss of conductance,which leads to the premature failure of the implanted device.The limited mechanical elongation of the dislocation slip systems in hexagonal close-packed (HCP) Mg increases material brittleness,which can lead to the mechanical abruption of devices on soft substrates [16,17].
As a bulk metal alloying strategy,the doping of Mg-based alloys with Ca,Al,and Zn can impart desirable electrical and mechanical properties;however,alloy compositions and microstructures need to be carefully controlled [18].For example,the inclusion of a specific amount of Ca can induce the grain refinement effect,which improves mechanical strength and corrosion resistance [19].Moreover,Ca doping can promote tissue regeneration,thereby leading to beneficial health effects caused by the abundance of Ca in the human body[20].Zuo et al.reported the effects of Ca doping on the grain sizes of Mg–xCa alloy systems [19].They found that upon increasing the Ca content,the fraction of the secondary Mg2Ca phase primarily increased along the grain boundaries,inducing the grain refinement effect and increasing the mechanical strength of the alloy [20].Dopants such as Al and Zn have been widely employed to enhance the mechanical and electrochemical stabilities of Mg-based alloys through the formation of interstitial intermetallic phases.In addition,dopants can be used as biodegradable metals to fabricate Mg alloy systems that exhibit optimized device characteristics and are applicable in biomedical electronic devices.For example,Mg-based alloys with Zn dopants have been employed as key components in bio-implantable materials owing to their superior electrochemical and mechanical properties [21].In Mg–Zn binary alloys,solid-solution doping with Zn has a positive effect on the mechanical properties and corrosion resistance of the Mg matrix [22].In addition,Setiawan et al.reported that the corrosion resistance of Mg–Zn binary alloys improves at Zn content of 1–5 wt% [23].Furthermore,Zhang et al.described the positive effects of Zn on the mechanical strength of Mg–Zn alloys,while Nayak et al.studied the possibility of strengthening Mg-based alloys through the grain refinement effect induced upon Zn doping [24,25].
Among the various alloy systems reported to date,the Mg–3Zn alloy has exhibited superior biocompatibility and corrosion resistance [26–28].Although Mg–Zn binary alloys possess attractive properties in terms of biodegradability,their application in the development of clinical devices is challenging.For example,the use of pyrometallurgy to prepare Mg–Zn binary alloys leads to the formation of a secondary phase (i.e.,MgxZnyprecipitates) during the cooling process,which has a detrimental effect on the mechanical properties and corrosion behavior of the alloys [29].More specifically,the presence of phase-separated particles along grain boundaries accelerates non-uniform galvanic corrosion and promotes the random delamination of materials [30,31].The precipitated inclusions present in Mg–Zn–X cast alloy systems have been shown to rapidly degrade the properties of theα-phase matrix [32–34].To overcome these limitations,sputtering has been widely utilized in secure,facile approaches to the manufacturing of microscale electrodes,which do not exhibit non-uniform dissolution behavior owing to the formation of a secondary phase in a corrosive environment[35,36].Moreover,vapor-phase deposition can be executed at low temperatures to prevent any physical impacts on polymer substrates with low glass transition temperatures (Tg) [37].
Herein,we design and fabricate a solid-solution Mg–3Zn alloy with a homogeneous single phase in the form of a sputtered thin film microelectrode.The microstructure,electrochemical characteristics,and biodegradable behavior of the thin film are characterized and compared to those of a pure Mg thin film.Then,fabrication conditions and geometrical considerations are manipulated to tune the kinetics,mechanical properties,and electronic integrability of the alloy.The directly deposited Mg–3Zn alloy thin film microelectrodes exhibited enhanced corrosion resistance and dissolution kinetics in relation to pure Mg thin film microelectrodes.Highresolution transmission electron microscopy(HRTEM)and Xray diffraction (XRD) confirm a single-phase,solid-solution Mg–3Zn thin film.In addition,electrochemical analyses,including X-ray photoelectron spectroscopy (XPS),reveal the dissolution kinetics and corrosion mechanism of the Mg–3Zn thin film.The corrosion behavior of Mg–3Zn alloy thin films was explored in various corrosive media [deionized (DI) water,pH 6.4;phosphate-buffered saline (PBS),pH 7.4;and NaCl,pH 7.0].The mechanical properties of the Mg–3Zn alloy were measured using a micro-tensile test.Furthermore,the Mg–3Zn alloy thin film microelectrode with a serpentine geometry showed stretchability and durability with higher yield strain and elongation than a Mg-based microelectrode.The biocompatibility of the Mg–3Zn alloy was also assessed using anin vitrocell viability test.Finally,the applicability of the Mg–3Zn alloy in biomedical-related thermotherapy systems was demonstrated.
2.Materials and methods
2.1. Fabrication of biodegradable Mg–3Zn alloy thin films
Slices of as-cast Mg–3Zn ingots were used as targets(5.08 cm diameter,1.27 cm thickness) for the magnetron sputtering of biodegradable Mg–3Zn binary alloy thin films directly on Si wafers (KRTLAB,P type,10.16 cm diameter,525 μm thickness).The sputtering process (Custom-made magnetron 4 gun sputtering system,JVAC,Korea) was performed at a base pressure of 8.0 × 10-4Pa and a working pressure of 0.67 Pa under a constant Ar gas flow of 30 sccm at 25 °C.Bar-(3 mm width,30 mm length,500 nm thickness) and serpentine-type (2 mm width,130 mm length,500 nm thickness) microelectrodes were formed by sputtering with selective deposition using a shadow mask(polyimide(PI) tape,65 μm,Alphaflon,Korea).The Mg–3Zn specimens for electrochemical testing were also fabricated by sputtering,wherein the alloy edges were covered with PI tape to avoid the delamination of the thin film from the substrate during the immersion test.The as-deposited Mg–3Zn alloy traces were heated to 250,300,350,or 400 °C (10 °C·min-1) at the center of a horizontal tube furnace (Thermal Chemical Vapor Deposition system,ScenTech,Korea) for 1 h.
2.2. Characterization of Mg–3Zn thin films
The surface morphologies and cross-sectional profiles of the solid-solution Mg–3Zn thin films were examined through scanning electron microscopy (SEM;FEI,Quanta 250 FEG,USA).The crystallinities and crystalline structures of the single-phase Mg–3Zn alloy thin films were confirmed by XRD (Bruker,D8 Advance,USA) and HRTEM conducted using a field-emission transmission electron microscope (FETEM;JEOL Ltd,JEM-3000F,Japan).The byproducts of the corrosion process and atomic compositions were observed through energy-dispersive X-ray spectroscopy(EDS;Thermo Fisher Scientific EDAX system,USA) during SEM.The elemental compositions of the films were analyzed by XPS (PHI Versaprobe III Scanning XPS Microprobe,USA)conducted using a monochromatic X-ray source induced by 12 kV and 18 mA Al Kαradiation.After Ar+sputtering to a depth of 15–30 nm from the surface,the oxygen content was found to be 6–10 at%.The relative contents of the alloy elements were calculated using the deconvolution of the obtained XPS peaks.Grain size determination was manually conducted from the SEM images by dividing the number of intersections by the actual line length in the SEM image (grain size average=1/number of intersections/actual length of the line).
2.3. Electrochemical analyses
The open circuit potential(OCP)was measured on an electrochemical workstation (VersaSTAT3 Potentiostat,AMETEK Applied Research,USA) with a three-electrode system comprising the as-cleaned Mg–3Zn alloy as the working electrode(WE) (machine-sliced to 7.5 mm diameter and 1 mm thickness),a platinum wire as the counter electrode (CE),and 3 M Ag/AgCl as the reference electrode(RE).Prior to carrying out the measurement,the specimens were mechanically polished using SiC paper (2000 grit).The OCP (Eocp) was recorded until it stabilized in the electrolyte solution (350 mL PBS at 37 °C).Potentiodynamic polarization curves were obtained using the three-electrode system (saturated Ag/AgCl and a platinum bar were used as the RE and CE,respectively) with a μStat 4000 instrument (Metrohm,Switzerland).The corrosion potential (Ecorr) and corrosion current density (Icorr)were derived from the polarization curves by Tafel extrapolation in the potential range of-2.5 <Eocp<3.0 V and a scan rate of 5 mV·s-1with an exposed electrode area of 1 cm2.A delay of 300 s was set to ensure a steady state for the electrochemical measurement.Electrochemical impedance spectroscopy (EIS) was performed at Eocpusing an electrochemical test system (WBCS3000L,WonATech,Korea) with a scan frequency ranging from 1 MHz to 1 Hz and a perturbation amplitude of 100 mV.The specimens were cut into rectangular plates (15 mm × 30 mm) and immersed in the PBS solution (pH 7.4,37 °C) with a three-electrode system.
2.4.In situ micro-tensile tests
The edges of the samples were undercut via XeF2gas etching to eliminate the Si substrate.Dogbone-shaped tensile samples with a gage width of 4 μm and a length of 8 μm were patterned using a focused ion beam (FIB;FEI,Quanta 3D FEG,USA).The micro-tensile samples were transferred to a push-to-pull microelectromechanical system (MEMS) device using a micromanipulator (Oxford Instruments,OmniProbe,UK)and attached to the devices by the deposition of platinum on the grip sections of the tensile samples.The mechanical properties were measured using a picoindenter (Bruker,PI-87,USA) in a field-emission scanning electron microscope(FESEM;FEI,Quanta 200 FEG,USA) at a constant strain rate of 0.001 s-1.The micro-tensile test process was optically recorded,and the strain was calculated using the change in the gage length in the image over time.Machine compliance was calculated by the tensile testing of the push-to-pull MEMS device in the absence of a sample,and the true stress–strain curve was corrected by considering the machine compliance.
2.5. Stretchability and fatigue testing
Samples for mechanical property characterization were prepared by the direct deposition of an Mg–3Zn alloy thin film (500 nm thickness) on a polybutylene adipate terephthalate (PBAT) substrate (150 μm thickness) at a sputtering power of 50 W.PBAT was solvent-cast on UV-treated glass substrates and sequentially pre-cut by laser ablation(MD-U1000C,Keyence,Japan) to prevent electrode microcracking caused by the thermal expansion of PBAT.A commercial red light emitting diode (LED;3.2 mm × 1.6 mm,Rohm Semiconductor,Japan) was connected to the contact electrode pads between jigs.The serpentine-shaped electrode samples were uniaxially stretched in a custom-made uniaxial stretching jig.Fatigue testing of the Mg–3Zn alloy thin film microelectrodes was conducted using a cyclic fatigue tester(CKMF-12P,CKSI,Korea) at a speed of 1 Hz.The changes in the electrical resistances of the Mg–3Zn serpentine electrodes were recorded using a digital multimeter (NI USB-4065,National Instruments,USA) connected to each end of the electrode during fatigue testing.All mechanical tests were repeated with pure Mg samples as a comparison.
2.6. Experimental setup of Mg–3Zn wireless heating trace
A biodegradable Mg–3Zn wireless coil trace composed of a receiving coil and a heating trace was fabricated with direct deposition on a glass substrate using the stencil PI tape as a shadow mask.Patterning using a laser cutter (MD-U1000C,Keyence,Japan)was conducted to fabricate the entire wireless metal trace.To demonstrate wireless powering and heating,the Mg–3Zn wireless coil was placed in the center of a Petri dish and immersed in PBS.The external RF coil was connected to a function generator and a power amplifier in series to continuously supply wireless AC power to the receiving coil.The heater fabricated as a transient passive device dissipated the heat from the heating trace,which was captured by an infrared camera (A615,NOVITECH,Republic of Korea).
2.7.In vitro cell viability test
Mg–3Zn alloy dot patterns (10 μm width × 10 μm length × 500 nm thickness) were deposited on a glass substrate via a lift-off process with a negative resist(AZ nLOF2070,Germany) solution.A polydimethylsiloxane(PDMS) well (10:1,Dow Corning,USA) was prepared using a 5–8 mm-thick PDMS specimen and a biopsy punch (8 mm hole diameter).O2plasma was applied to the surface of the Mg–3Zn-patterned glass and the punched PDMS specimen to ensure strong attachment.The plasma-treated surfaces of the samples were affixed and baked in an oven at 70 °C for complete adhesion.L929 cells (NCTC clone of strain L) were maintained in Dulbecco’s Modified Eagle Medium(DMEM;Welgene,Korea),which contained 10% fetal bovine serum (FBS;VWR International,PA,USA) and 1% penicillin/streptomycin (Gibco,USA).Prior to experimental use,the L929 cells were stabilized for 48 h in a humidified cell incubator at 37 °C and 5% CO2atmosphere for up to~90%confluence.The prepared L929 cells were washed with 1×PBS (Welgene) twice and dissociated using 0.05% trypsin-EDTA (Gibco) for 3 min in an incubator.After neutralizing with DMEM in 5–10 times the volume of the trypsin-EDTA solution,the cell-containing medium was centrifuged at 125 ×gfor 5 min.In each well of the device,2000 cells were seeded.The cell medium (DMEM) was replaced daily.
2.8. Fluorescence cell imaging
Confocal laser scanning microscopy(LSM 700,Carl Zeiss,Germany) was conducted to obtain fluorescence images of the L929 cells.Previously stained L929 cells (DNA and filamentous actin (F-actin)) were imaged using two excitation/emission channels.The cells at each point in time were fixed with 4% paraformaldehyde (Bylabs,Korea) for 20 min.For the cell penetration experiments,0.25% Triton X-100 (Sigma,USA) in PBS was used along with 0.5%Tween 20 (Sigma,USA) and 2% FBS in 1× PBS.DNA was stained with a conjugated fluorescence marker,namely Hoechst 33,342 (1:1000,Invitrogen,USA),while F-actin was stained with Alexa Fluor 488 phalloidin (1:400,Invitrogen).The cells were incubated with the staining solutions at 4 °C,and PBS washing was performed at each step of the staining process.
3.Results and discussion
3.1. Formation of solid-solution Mg–3Zn alloy thin film
Fig.1 shows the crystallographic and electrochemical characteristics of the solid-solution Mg–3Zn alloy and pure Mg.The sputtering of a pre-homogenized alloy ingot with a composition of 97 wt% Mg–3 wt% Zn homogeneously formed a single-phase solid-solution thin film of Mg–3Zn without the generation of secondary phase precipitates,which had been previously observed in cast alloys.More specifically,as-cast Mg–3Zn ingots with a binary alloy composition (see Fig.S1 for details) have been reported to generally precipitate intermetallic particles(γ-phase MgxZny)along the grain boundary in theα-phase Mg matrix due to thermodynamic phase separation [35,36].However,the sputtered Mg–3Zn alloy created a homogeneous solid-solution thin film,which is attributed to two potential factors.First,the strong preferential growth orientation in (0002)HCPcauses growth concentration and energy stabilization of the sputtered metal atoms along the [0002]direction,thereby suppressing phase separation.Secondly,the ultrahigh cooling rate employed during the sputtering process prevents phase separation and induces the formation of a secondaryγ-phase in the Mg matrix.Indeed,the formation of a thin film of a high-entropy alloy (CrCoCuFeNi) also exhibited suppression mechanisms similar to those for precipitate formation during the sputtering process [38].
Fig.1a shows the relative weight concentrations of Mg(97.02 wt%) and Zn (2.98 wt%) in the Mg–3Zn alloy,as determined by XPS.In addition,the obtained XPS results clearly show the Zn LMM and Mg KLL Auger spectra for the surface of the alloy thin film,wherein the O 1s peak can be attributed to surface contamination.In addition,Fig.1b shows the XRD pattern of the thin film,which confirms its polycrystalline nature,as characterized by the presence of strong and narrow reflection lines without the observation of any peaks related to precipitate formation.The major reflection peaks were indexed to the crystal structure of the HCP Mg–3Zn binary alloy,wherein the peaks at 2θvalues of 34.6°,36.8°,48.1°,57.7°,63.5°,and 69.1° correlated with the (002),(101),(102),(110),(103),and (112) crystal planes of the Mg–3Zn binary alloy,respectively (JCPDS #04–001–2771).Slight differences in the major 2θreflections were observed between Mg (34.48°) and Mg–3Zn (34.50°).These were attributed to the lattice strain induced by the interstitial Zn atoms [39].Importantly,the surface morphology and crystal structure of the as-deposited Mg–3Zn alloy clearly indicated the absence of secondary precipitates.Furthermore,Fig.1c shows the SEM image of the grain microstructure at the surface of the Mg–3Zn alloy thin film,wherein an array of hexagonal fiber textures can be observed in the basal plane parallel to the substrate surface;columnar growth with voided boundaries perpendicular to the substrate surface is also apparent in the cross-sectional image (see the inset of Fig.1c)[40,41].The HRTEM images of the solid-solution Mg–3Zn alloy presented in Figs.1d andS2confirm the presence of a single phase corresponding to the HCP crystal structure ofα-Mg.
3.2. Electrochemical characteristics of Mg–3Zn alloy
The solid-solution Mg–3Zn alloy thin film exhibits higher corrosion resistance,likely because of the higher anodic potential of the Mg–3Zn alloy matrix than that of pure Mg.Fig.1e shows the OCP of the Mg (-1.85 V) and Mg–3Zn alloys(-1.63 V)determined at the saturation point after immersion in PBS for 387 and 1030 s,respectively.The schematic illustration in Fig.S3 shows a series of anodic potentials for pure Mg,Mg–3Zn,and Mg-containingγ-phase intermetallic compound (Mg67Zn32).As previously reported,separate phase,intermetallic particles accelerate the electrochemical reaction at the interface between the precipitates and surroundingα-Mg matrix via galvanic corrosion,which is driven by the large differences in the anodic potentials of various phases [30].The corrosion potentials in the anodic dissolution reaction of the Mg–3Zn alloy thin film were therefore determined through three-electrode electrochemical analysis.Fig.1f shows the anodic polarization curves and corresponding corrosion current density of pure Mg and Mg–3Zn alloy thin films respectively,and the experimental setup during immersion in PBS is shown in Fig.S4.Initially,the passivation behavior indicates that the dissolution current density slowly increases with increasing anodic potential.This likely originates from the protective effect of the surface oxide (OX) films formed immediately in the corrosive medium[42].When the potentials became more positive,i.e.,between-1.7 and-1.5 V,the resulting current density increased,suggesting that film breakdown occurred owing to the fracture and corrosion of the Mg matrix.The higher breakdown potential of Mg–3Zn (-1.52 V) than that of pure Mg (-1.57 V)indicates that the Mg–3Zn alloy exhibited a higher corrosion resistance [43].This was attributed to overall uniform dissolution modality presented by the homogeneous solid-solution phase of the Mg–3Zn thin film.Fig.1g shows the polarization curves of the pure Mg and Mg–3Zn alloy thin films in the PBS solution (pH 7.4,37 °C).The two curves indicate identical corrosion behavior.In the cathodic region,the reaction is controlled by the hydrogen evolution reaction.The anodic side shows the passivation tendency,implying the presence of hydroxide films on the surface of the Mg and Mg–3Zn alloy thin films.The corrosion potentials (Ecorr) of the Mg and Mg–3Zn alloy thin films are-1.48 and-1.3 V,respectively,when the corrosion current density (Icorr) is 9 × 10-4and 1.7 × 10-5A·cm-2,respectively.The anodic current density of the Mg–3Zn alloy is lower than that of Mg at the same potential,indicating the decreasing anodic dissolution rate in the corrosive medium.
The complex impedances involved in the corrosion process were subsequently investigated through EIS;the results are presented in Fig.1h.The equivalent circuit in this system (Fig.S5) is assumed to consist of a serial connection of three electrochemical components,namely,an electrolyte that allows charge transfer to occur,a metal OX layer on the electrode surface,and an electrical double layer (EDL)at the interface between the OX layer and electrolyte [43].The Nyquist diagrams for all samples consisted of two loops,namely,the high-and medium-frequency-range capacitive loops.The high-frequency capacitive loop can be attributed to the charge transfer reaction in the EDL formed at the interface between the OX layer and corrosive electrolyte,as described by the charge transfer impedance including resistance and capacitance (REDLand CEDL).In contrast,the mediumfrequency capacitive loop originates from mass transport in the solid phase,which includes ion diffusion in the hydroxide or OX films and can be described as mass transfer impedance(ROXand COX).The electrochemical system in the EDL consists of a parallel connection of REDLand CEDL,and the OX layer is composed of a parallel connection of ROXand COX.The Nyquist plots show that the real part of the Mg impedance value (Zre) is lower than that of the Mg–3Zn alloy,which indicates that the passivation layer at the surface of the Mg–3Zn film (composed of Mg(OH)2and Zn(OH)2)has a higher corrosion degradation tolerance than that of the pure Mg matrix (composed of a single Mg(OH)2layer) [44].The characteristic frequency of the Mg film is 79 mHz at 330Ω·cm-2(Zre),while that of the Mg–3Zn alloy is 126 mHz at 370Ω·cm-2(Zre).This indicates that the Mg–3Zn alloy thin film has a higher corrosion resistance than the Mg thin film.The frequency responses of the Mg and Mg–3Zn alloy microelectrodes are shown as Bode plots in Fig.S6.They represent the modulus (|Z|) differences of impedance in the low-frequency range,indicating that the Mg–3Zn alloy has a higher impedance resistance than pure Mg.Fig.1i shows the electrical resistivity profile for the Mg–3Zn thin film resistor formed on Si with a length of 10–50 mm.The electrical resistivity of the Mg–3Zn resistor trace was calculated using the transmission line measurements,as follows:
whereρis the resistivity,andwandtare the width (1.5 mm)and thickness (200 nm) of the line resistor,respectively.The electrical resistivity of the Mg–3Zn alloy thin film is 0.84 × 10-7Ω·m,which is comparable to those of conventional biodegradable as-cast Mg (0.6 × 10-7Ω·m),as-cast Mg–6Zn alloy (0.55 × 10-7Ω·m),and as-cast Mg–0.5Ca alloy films (0.48 × 10-7Ω·m) [45,46].
3.3. Biodegradable behavior of Mg–3Zn alloy thin film
Fig.2a shows a series of optical microscopy (OM) and SEM images presenting the dissolution progress of the patterned Mg–3Zn alloy thin film on a glass substrate during immersion in PBS (pH 7.4,37 °C).The dissolution behavior of the Mg–3Zn alloy thin film proceeds in the form of intragranular and pitting corrosion sequentially,which is similar to the case of Mg (Fig.S7).More specifically,the edge and shape of the grain surface gradually fade after immersion in PBS,and a metal hydroxide layer is formed as the passivation layer.Subsequently,the chloride ions in PBS activate pitting corrosion on the OX layer,which leads to the formation and propagation of microcracks within the film[47].The Mg–3Zn thin film then undergoes partial dissolution and breaks into pieces after 6 h,reaching full dissolution after 18 h in PBS.Of note,disintegrated grain fragments were clearly observed after 2 h of immersion.
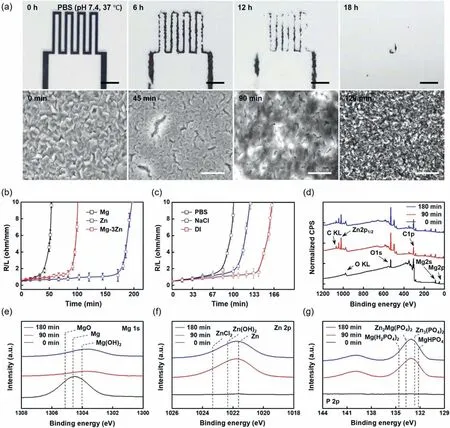
Fig.2.Degradation behavior and dissolution chemistry of the Mg–3Zn alloy thin film microelectrode.(a) Series of optical microscopy (OM;top) and SEM(bottom) images of the surface morphology of an Mg–3Zn microelectrode trace (2 mm width,130 mm length,500 nm thickness,) immersed in PBS at 37 °C.Top scale bar=5 mm,bottom scale bar=0.6 μm.(b) Dissolution rates of the Mg (black),Zn (blue),and Mg–3Zn (red) thin films (500 nm thickness,2 mm width,130 mm length) during immersion in PBS (pH 7.4,37 °C).(c) Variations in the dissolution resistance of the Mg–3Zn serpentine trace (30 mm length) immersed in PBS (pH 7.4,37 °C;red),0.01 M NaCl (pH 7.0,37 °C;blue),and deionized (DI) water (pH 6.4,37 °C;black).(d) Variations in the XP spectrum of the Mg–3Zn thin film with dissolution time in PBS (pH 7.4,37 °C) (black: 0 min,red: 90 min,blue: 180 min).(e)–(g) Atomic composition of Mg 1s,Zn 2p,and P 2p on a thin film surface after immersion in PBS (pH 7.4,37 °C) for 0 min (black),90 min (red),and 180 min (blue).
Subsequently,serpentine-shaped metal traces were introduced to track the changes in the electrical resistance of the Mg–3Zn microelectrodes (130 mm length,500 nm thickness)during the dissolution process (Fig.S8),wherein the changes in the dissolution resistance were normalized by the total length of the metal trace.Fig.2b shows the dissolution rates of Mg,Zn,and the Mg–3Zn alloys in PBS (pH 7.4,37 °C).The Zn metal traces showed the slowest change in the resistance transition curve owing to its higher anodic potential.In addition,the Mg–3Zn alloy shows a slower change than pure Mg,as expected from the OCP comparison presented in Fig.S3.The dissolution of Mg,Zn,and their OXs in PBS can be attributed to the following key reactions [48]:
Furthermore,the corrosion behavior of the biodegradable metal thin film was examined in various types of corrosive media (i.e.,DI water,pH 6.4;0.01 M NaCl,pH 7.0;and PBS,pH 7.4) at 37 °C,as presented in Fig.2c.More specifically,the lowest transitions in resistance were observed for the corrosion and film degradation behaviors of Mg–3Zn in DI water compared with those obtained in NaCl and PBS.This can be attributed to the fact that the H+and OH-ions present in DI water participate only in the hydrolysis reaction to form the metal hydroxide and generate a passivation layer on the metal surface;this layer prevents further degradation via corrosion [44].In NaCl,the chloride ions serve as corrosion accelerators by generating metal chlorides,which have a relatively higher solubility in NaCl [47].As a result,the resistance change in NaCl was relatively faster than that in DI water.Furthermore,the most pronounced dissolution rate and resistance changes were observed in PBS after 90 min owing to the presence of K+,Cl-,PO42-,and Na+ions.In PBS,the Mg and Zn atoms formed metal hydroxides with the water molecules.The phosphate ions also bind to the metal ions to generate metal phosphate species that form a passivation layer on the thin film surface [48].Because the concentration of chloride ions in PBS is 10 times higher than that in NaCl,pitting corrosion induces the delamination of the Mg–3Zn thin film,causing a sharp transition in the dissolution resistance.Therefore,these results indicate that the different dissolution rates observed in the various corrosion media are dependent on the ion species present in the corrosive solution.
To study the dissolution chemistry of the solid-solution Mg–3Zn alloy under biomimetic conditions,the corrosion byproducts were characterized by XPS.Fig.2d–g show the XP spectra obtained for the constituent elements on the outermost surface of the Mg–3Zn alloy thin film after immersion in PBS for 0,90,and 180 min at 37 °C.As mentioned above,the Mg and Zn metal atoms in the Mg–3Zn alloy form hydrated corrosion products [Mg(OH)2and Zn(OH)2,respectively]immediately after the reaction with ions and water molecules during immersion.These species act as a non-conductive passivation layer that prevents further degradation during the dissolution process,thereby leading to a slight resistance variation following the initial degradation period.Although Zn was not detected at a time of 0 min owing to this depletion behavior,high concentrations of pure Zn and Zn-containing compounds were detected in the corrosion specimen after the dissolution of the top native OX layer.In addition,various metal and alkaline phosphates[i.e.,Mg(H2PO4)2,Zn3(PO4)2,Zn2Mg(PO4)2,Ca(H2PO4)2,and MgHPO4]were detected as corrosion products after immersing the Mg–3Zn alloy in PBS(pH 7.4,37°C)for 90 min(Fig.S9) [49].These types of phosphates also act as a conversion coating layer to protect against the corrosion reaction.Further immersion (up to 100 min) resulted in a sharp increase in resistance owing to the complete dissolution of the thin film due to pitting corrosion by chloride ions.In this biodegradable alloy system,the corrosion behavior of the Mg–3Zn thin film involves intragranular corrosion accompanied by pitting corrosion [50].The signals of the elements of the dissolved Mg–3Zn alloy thin film at different immersion times were deconvoluted into specific binding energies(Fig.S10).
3.4. Tunability of dissolution kinetics of Mg–3Zn alloy thin film by variation of deposition conditions
Fig.3 shows the influence of the processing factors on the dissolution kinetics of the biodegradable Mg–3Zn alloy thin film electrode (2 mm width,130 mm length,500 nm thickness).Fig.3a shows that the dissolution rates of the Mg–3Zn microelectrodes deposited under various power conditions (50–100 W) to represent different kinetics according to the increase in sputtering power,as characterized by changes in the dissolution resistance during immersion in PBS.The microstructures of these thin films were also examined;the results are shown in Fig.3b.The grain sizes tended to increase at a higher applied DC power (95.3,119.59,and 148.56 nm in the 50,75,and 100 W samples,respectively),because the enhanced kinetic energy by the accelerated metal ions drives stronger collisions between the gaseous metal ions and substrate [51,52].The dissolution rate of the Mg–3Zn microelectrode decreased with larger grain sizes,indicating a decrease in the reactive surface area during the intragranular corrosion process.As shown in the XRD patterns of the Mg–3Zn alloy thin films deposited at different sputtering powers (Fig.3c),all samples exhibited the crystal planes of the Mg–3Zn alloy,thereby indicating that the corrosion behavior is mainly influenced by the different grain sizes and surface areas resulting from the variation in sputtering power.The dissolution kinetics when changing the surface OX layer via a heat treatment were also characterized.Fig.3d shows the resistance variations of the Mg–3Zn thin film resistors subjected to different heat treatment conditions (i.e.,250–400 °C for 1 h) upon their subsequent immersion in PBS at 37 °C.The slowest resistance change was exhibited by the Mg–3Zn thin film that had undergone thermal treatment at 400 °C.Upon examination of the oxidized and partially melted surface morphologies (Fig.3e) and subsequent EDS mapping,the oxygen content at the top of the surface of the Mg–3Zn microelectrode increased upon increasing the processing temperature.This infiltrative OX species likely acted as a passivation layer,suppressing further dissolution [53].Furthermore,the XRD patterns of the thermally treated thin film samples presented in Fig.3f confirm crystal planes different than those of the pristine,untreated film.As previously reported,when the temperature increases above the recrystallization temperature of Mg (195 °C),the nucleation and grain growth processes in the microstructure of the Mg–3Zn alloy thin film cause the rearrangement of the crystal orientation and grain textures[52].Therefore,the differences in the dissolution rate among the thermally treated Mg–3Zn films are expected to originate from surface oxidation and the reorientation of the grain microstructure.
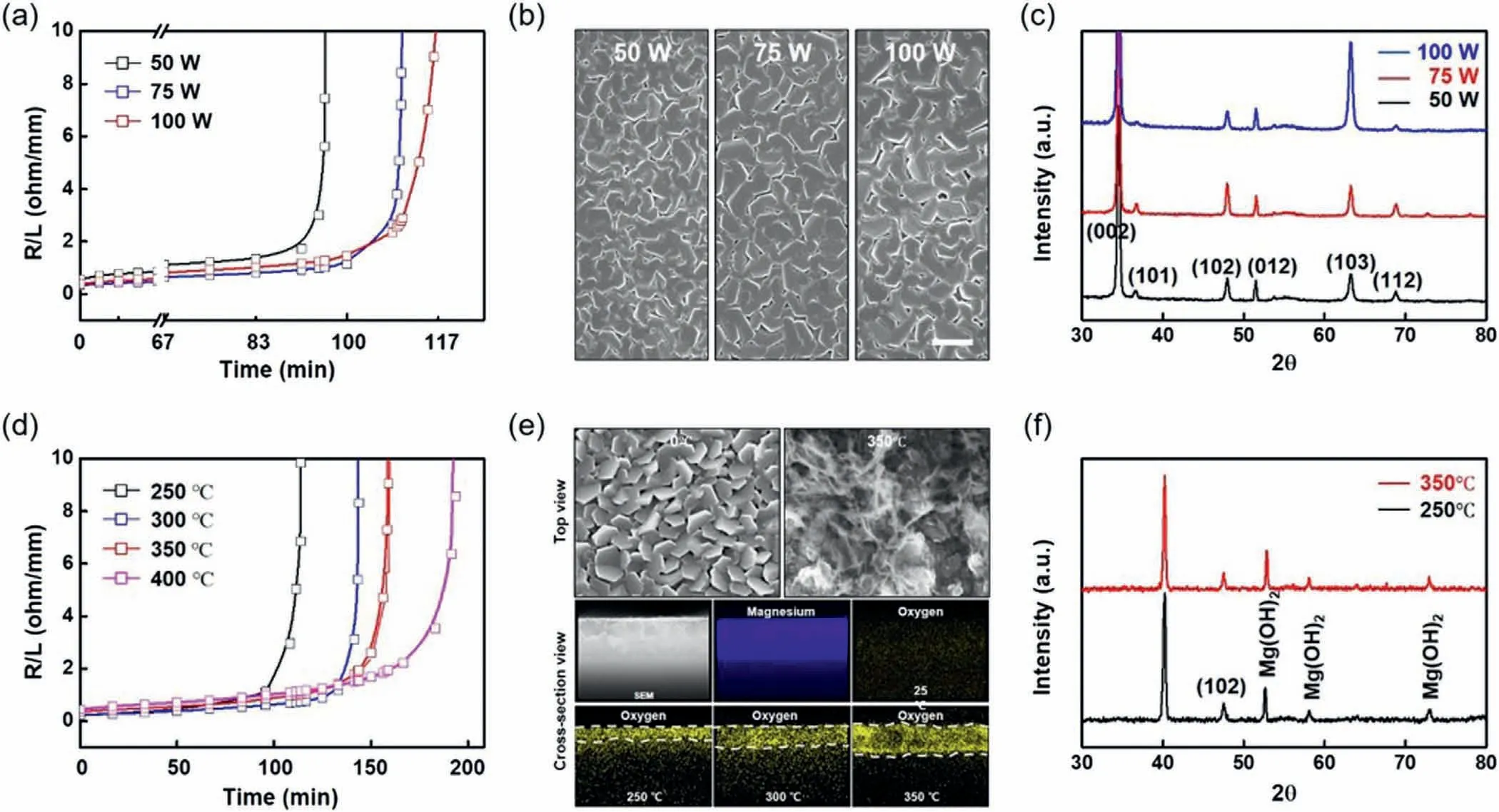
Fig.3.Tunability of the dissolution rate of the biodegradable Mg–3Zn alloy thin film.(a) Dissolution rates at different deposition powers for the determination of the electrical resistance of the Mg–3Zn trace (500 nm thickness) (black: 50 W,blue: 75 W,red: 100 W).(b) Grain structures and microstructures of the as-deposited Mg–3Zn alloy thin film electrodes fabricated under various deposition power conditions (50,75,and 100 W) on a Si wafer.Scale bar=250 nm.(c) XRD patterns of the Mg–3Zn thin films prepared at different deposition powers.(d) Dissolution rates of the Mg–3Zn thin films prepared under various post-heat treatment conditions (black: 250 °C,blue: 300 °C,red: 350 °C,and magenta: 400 °C) at 100 W for 1 h.(e) SEM and EDS mapping images of the Mg–3Zn alloy thin film after post-heat treatment at each temperature (250,300,and 350 °C),revealing the formation and diffusion of the oxide layer into the thin film.(f) XRD patterns of the Mg–3Zn alloy thin films treated at 250 °C (black) and 350 °C (red).
3.5. Mechanical properties of Mg–3Zn alloy thin film
Fig.4 shows the mechanical properties of the pure Mg and Mg–3Zn alloy thin films determined by uniaxial tensile testing.More specifically,Fig.4a shows the true stress–strain curves of the two specimens fabricated into micro-tensile samples (4 μm width,8 μm length,500 nm thickness) by means of FIB milling;linear elasticity and negligible plasticity are evident in both samples.In addition,the Young’s modulus,yield strength,and fracture strain of the Mg–3Zn alloy thin film were determined to be 22.31 GPa,666.33 MPa,and 3.30%,respectively,while those of the pure Mg thin film were determined to be 23.72 GPa,389.69 MPa,and 1.86%,respectively.The Mg–3Zn alloy thin film exhibits a larger fracture strain owing to the local non-uniformity and lattice strain into the crystal structure caused by added Zn dopants[54].In the bulk phase of the Mg–Zn binary alloy system,the yield strength and fracture strain typically increase upon increasing the Zn content,despite the similar Young’s modulus(Fig.S11).Even in microscale thin films,the improvement in yield strength and fracture strain can be attributed to the localized lattice strain caused by the presence of Zn dopants in the Mg crystal structure,which leads to the suppression of the dislocation slip and twinning in the HCP structure of the Mg–3Zn alloy [17].The mechanical behavior of the Mg–3Zn alloy was also analyzed by processing the digital image correlation on a free-standing Mg–3Zn thin film attached to the edge of a push-to-pull MEMS device forin situmicrotensile tests(Fig.4b).The dogbone-shaped micro-tensile samples (4 μm width,8 μm length) transferred onto a MEMS device were under uniaxial tensile force during the tensile testing.The edge of the micro-tensile samples fractured at a strain of 0.03%,while that of the pure Mg sample fractured at a strain under 0.02%.

Fig.4.Characterization of the mechanical properties of the Mg–3Zn thin film.(a) True stress–strain curves of the as-deposited pure Mg (black) and Mg–3Zn(red) thin films (500 nm thickness).(b) SEM images of the micro-tensile specimen (500 nm thickness,4 μm width,8 μm length) (left: before loading,right:after loading) for digital image correlation analysis.Scale bar=2 μm.(c) Resistance variations of the pure Mg (black) and Mg–3Zn (red) serpentine trace electrodes during uniaxial stretching up to 100% strain.(d) Stretching tests for the serpentine microelectrodes (top: Mg–3Zn,bottom: Mg) connected to a commercial light emitting diode (LED) during uniaxial stretching up to 100%.(e) Fatigue behavior of the Mg (black) and Mg–3Zn (red) serpentine-shaped electrodes during 25% cyclic stretching;the results are illustrated in terms of the relative changes in resistance.(f) OM image of the Mg–3Zn serpentine electrode loaded in the cyclic stretching jig (top),and SEM images of the surface of the Mg–3Zn trace before (bottom left) and after (bottom right) 10,000 cyclic tests carried out using 25% strain.Scale bar=500 μm.
Using a serpentine or wavy design to structurally modify metal electrodes has been shown to impart improved mechanical properties,including mechanical flexibility and stretchability,to the device-scale interconnectors beyond the yield strain of the materials.To induce this effect in a biodegradable Mg–3Zn alloy thin film,the electrical resistance during the uniaxial stretching of a single serpentine-shaped Mg–3Zn alloy microelectrode was varied (Fig.4c) in a hand-made jig.For this purpose,an Mg–3Zn alloy electrode (500 nm thickness) was deposited onto PBAT and patterned in a serpentine structure by laser cutting.The change in resistance of the Mg–3Zn serpentine electrodes was found to remain constant up to a stretching of 38% tensile strain,exhibiting a nearly six-fold increase at a stretching degree of 84%.In contrast,the change in resistance for the Mg electrode remained stable only up to a stretching degree of 28%,and electrical disconnection initiated upon stretching to 60%.The stability of the Mg–3Zn/PBAT serpentine electrode was further verified using a LED,as presented in Fig.4d.A commercial red LED connected with the Mg–3Zn alloy microelectrode is stable and remains connected at up to 90% strain,while that with the pure Mg microelectrode dims at 75% strain.This shows that the enhanced mechanical properties of Mg–3Zn more stably maintain electrical conductivity under a stretching condition than those of Mg.
Furthermore,the Mg–3Zn alloy thin film exhibited higher stability in the cyclic fatigue test over a mild strain range(0–25%) than the pure Mg thin film,as presented in Fig.4e,wherein the cyclic stretching behavior of the Mg–3Zn/PBAT and Mg/PBAT serpentine electrodes (10,000 cycles at 25%strain) is shown.More specifically,the serpentine-structured Mg–3Zn/PBAT electrode showed a relatively small change in electrical resistance after 10,000 cycles,while electrical resistance of the Mg/PBAT electrode increased by~2.8 times under the same conditions.In addition,Fig.4f shows the SEM images of the surface morphologies of the serpentinestructured Mg–3Zn/PBAT electrode before and after cyclic stretching (10,000 cycles at 25% strain).These results indicate the potential of structural design for enhancing the electronic stretchability and mechanical durability of rigid metal electrodes.Enhancement of the mechanical properties of the Mg–3Zn alloy thin film in relation to those of pure Mg can contribute to the durability of the microelectrode,which can be integrated into implantable electronics.
3.6. Demonstration of electronic integrability
Biodegradable metals are crucial for the achievement of transient electronics,wherein their degradation and decomposition periods must be carefully controlled under corrosive conditions in the operating environment.In addition,biodegradable metal electrodes as electrical interconnections in dissolvable device platforms can be used to impart improved structural compatibility to transient electronic devices containing elastic materials [55,56].In this context,the enhanced corrosion resistance and mechanical property of the Mg–3Zn alloy thin film could lead to a reliable and extended functional lifetime in relation to that of the Mg thin film when employed in biodegradable electronic devices.Moreover,the Mg and Mg–3Zn alloy thin film microelectrodes were fabricated with a custom-designed wireless trace coil to further investigate this.Fig.5 shows an example of the improved lifetime of a biodegradable wireless heating coil,achieved using an Mg–3Zn alloy microelectrode trace for hyperthermic sterilization.Fig.5a shows a schematic of the experimental setup for the wireless heater,which consisted of a receiver coil and a resistive heating trace.This heating system generated heat by scavenging AC power from the external transmission coil(Fig.5b).In addition,Fig.5c shows the increased temperature of the wireless coil trace upon increasing the applied transmission power from 0 to 50 Vppunder air at a distance of 2 mm from the transmission coil.As shown in Fig.5d,the wireless power transmission system based on electromagnetic induction coupling using the Mg–3Zn alloy thin film trace exhibited a longer operational time in the PBS solution (pH 7.4,37 °C) than a system based on pure Mg,with residual heat remaining after 2.4 h in the former;in contrast,the pure Mg system failed to operate after 1 h.This result shows that the biodegradable Mg–3Zn alloy can be applied to thermotherapy electrodes in the field of biomedical electronics.
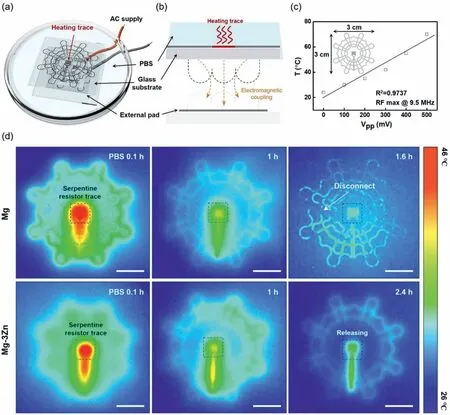
Fig.5.Application of the Mg–3Zn thin film in a wireless,implantable,and biodegradable thermotherapy device.(a) Schematic of the experimental wireless powering system based on an Mg–3Zn thin film resistor (1.5 μm thickness).A transmission coil directly connected to an external power supply applies an AC voltage to the serpentine trace under the PBS reservoir.(b) Schematic of the cross-sectional view of the wireless power transmission system integrated with a Mg–3Zn trace heater.(c) Saturated temperature changes of the wireless heater measured at the center as a function of the externally applied AC power at an 8 mm distance.(d) Degradation of the heating performance of the wireless heaters prepared from Mg (top row) and Mg–3Zn (bottom row) by dissolution in PBS (pH 7.4,37 °C).Scale bar=10 mm.
3.7. Biocompatibility
Finally,the biocompatibility of the Mg–3Zn alloy thin film was evaluated to confirm its potential for use as a biodegradable conductive material in implantable electronic devices.For this purpose,cell viability experiments were performed using Mg–3Zn alloy dot patterns and PDMS wells as the fluid reservoirs (Fig.6a).The dotted Mg–3Zn thin films were immersed in wells containing DMEM,and cell attachment was observed at four-time points over a period of 72 h.As shown in Fig.6b,the Mg–3Zn dot patterns became fully dissolved after immersion and spread adequately into the PDMS well space.Fig.6c shows the cell actins cultured in the PDMS well,indicating they are firmly attached to the glass substrate and ready to proliferate.The number of cells present after 24,36,48,and 72 h was determined and compared with those present in the control sample (i.e.,bare glass;Fig.S12).The Mg–3Zn alloy thin film resulted in a cell viability of>92%(Fig.6d),thereby confirming excellent biocompatibility.
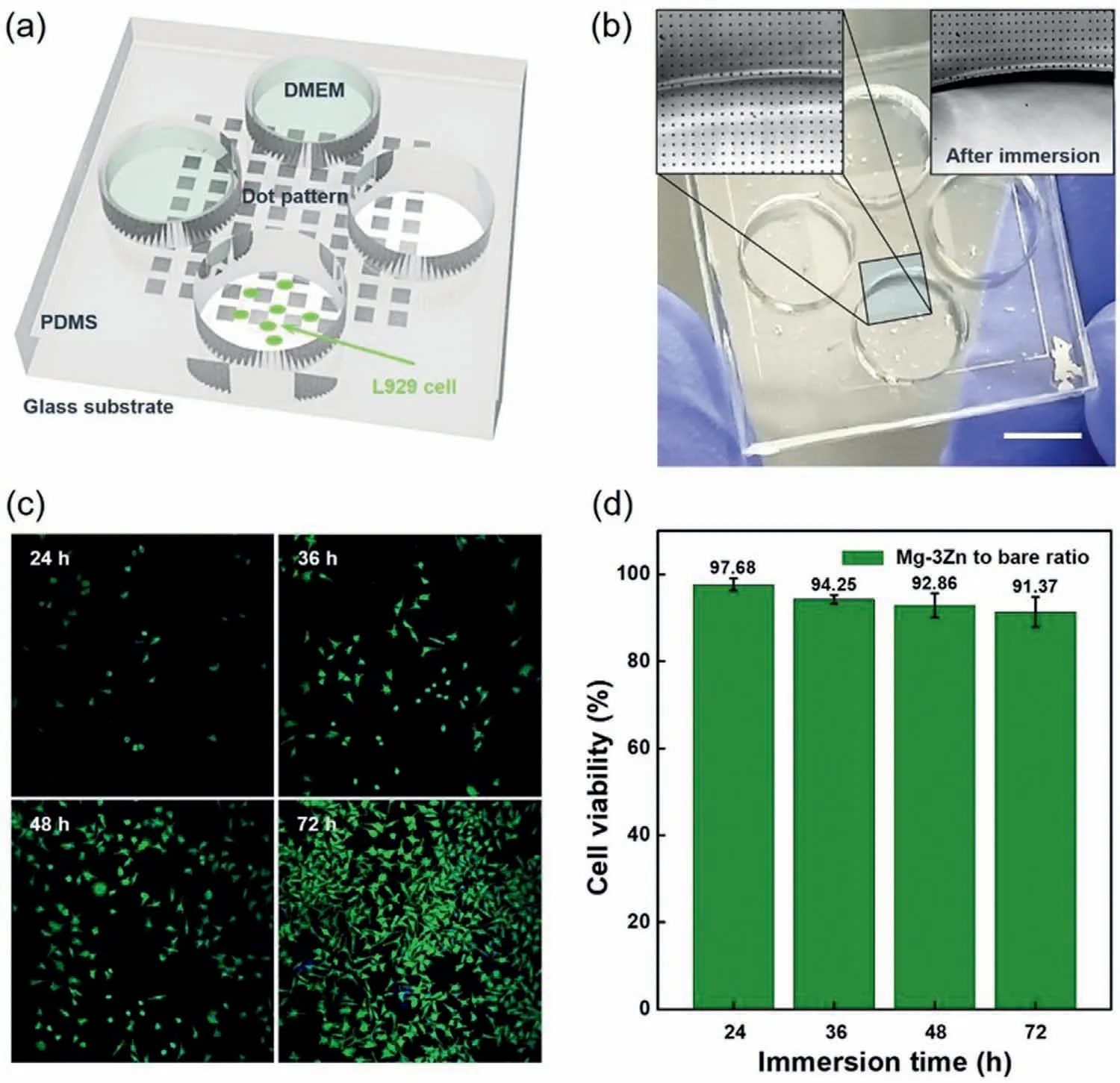
Fig.6.Demonstration of the cytotoxicity of the Mg–3Zn alloy thin film.(a) Schematic representation of the test platform for an in vitro cell viability test.L929 cells (green dots) were cultured on a UV-sterilized glass substrate,which was covered with an Mg–3Zn dot pattern array (10 μm width,10 μm length,500 nm thickness).(b) Image of the cell viability test sample (scale bar=10 mm).OM image (inset) showing the surface of the glass substrate covered with the Mg–3Zn dot pattern dissolved by the cell culture medium (Dulbecco’s Modified Eagle’s Medium,DMEM) after immersion.(c) Fluorescence image of the test cells surviving in the DMEM containing fully dissolved Mg–3Zn for 72 h.(d) L929 cell viability for 24,36,48,and 72 h.Cell viability was calculated from the number of cells attached to bare slide glass (control,Fig.S12) and the number of cells attached to the Mg–3Zn alloy dots.
4.Conclusions
A homogeneous solid-solution Mg–3Zn alloy thin film was successfully fabricated without any precipitated secondary phases by sputtering from a bulk ingot target and its applicability as a biodegradable microelectrode was verified.The resulting Mg–3Zn alloy thin film exhibited an enhanced corrosion resistance in relation to a pure Mg thin film in a biomimetic environment.Moreover,the dissolution kinetics of the alloy film could be controlled by tuning processing factors such as sputtering power and heat treatment temperature.A deposited Mg–3Zn microelectrode was mechanically characterized using a micro-tensile test;the results showed extended yield strain in relation to that of a Mg microelectrode.Furthermore,upon adopting a serpentine geometry,the Mg–3Zn alloy thin film possessed enhanced mechanical properties,including improved stretchability and fatigue resistivity,in relation to the pure Mg film.Because of its improved corrosion resistivity,elongation properties,and conductivity,the Mg–3Zn alloy can be applied in transient electronics.To confirm its applicability,a wireless heater was prepared using the Mg–3Zn alloy;this device possessed an extended functional lifetime in relation to a pure-Mg-based device.Moreover,the excellent biocompatibility of the solid-solution Mg–3Zn alloy thin film promotes its application in biomedical systems.Overall,this study opens new opportunities for the application of Mg alloys in biodegradable electronics by providing a means to tune their biodegradable kinetics and mechanical behavior.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was supported by the Renewable Energy Technology Development(Develop technology to enhance reliability and durability for parts of hydrogen storage tank system)(2022303004020B) grant funded by the Korea Energy Technology Evaluation Planning(KETEP)and the Ministry of Science and ICT (Development Project for Emerging Research Instruments Technology),(Project Number:(2022)ERIC)06_1 and Commercialization Promotion Agency for R&D Outcomes (COMPA).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.jma.2023.06.016.
杂志排行
Journal of Magnesium and Alloys的其它文章
- Corrosion behavior of composite coatings containing hydroxyapatite particles on Mg alloys by plasma electrolytic oxidation: A review
- Rational design,synthesis and prospect of biodegradable magnesium alloy vascular stents
- Antibacterial mechanism with consequent cytotoxicity of different reinforcements in biodegradable magnesium and zinc alloys: A review
- Preparation,interfacial regulation and strengthening of Mg/Al bimetal fabricated by compound casting: A review
- Pitting corrosion behavior and corrosion protection performance of cold sprayed double layered noble barrier coating on magnesium-based alloy in chloride containing solutions
- Designing strategy for corrosion-resistant Mg alloys based on film-free and film-covered models
