CTAB-assisted fabrication of hierarchical flower-like magnesium oxide adsorbent for enhanced removal performance towards phosphate
2023-12-27SaeedAhmed
Saeed Ahmed
aState Key Laboratory of Chemical Resource Engineering, College of Chemistry, Beijing University of Chemical Technology, Beijing 100029, China
b Department of Chemistry, The University of Lahore, Lahore 54590, Pakistan
c Department of Chemistry, University of Chakwal, Pakistan
Abstract In this work,a series of hierarchical flower-like magnesium oxide (MgO) adsorbents were successfully fabricated in a cetyltrimethylammonium bromide (CTAB) assisted solvothermal route using hexamethylenetetramine (HMTA) as a precipitating agent.Effects of CTAB feeding amount on the structure,morphology,pore structure,and corresponding adsorption behavior were investigated.The hierarchical gardenias flower-like MgO demonstrated a surface area of 336.54 m2·g-1 at a minimum ratio of the CTAB/Mg2+ was 0.02 in the reaction system.The hierarchical MgO phosphate removal capacity was 348.32 mg·g-1,which followed the pseudo-second-order and Freundlich isotherm model obtained from the large surface area and appropriate pore size.The value of n also suggests the feasible nature of phosphate adsorption under the examined conditions.Indeed,this CTAB assisted solvothermal method can provide a new understanding to tune the desired properties of a material by merely adjusting the reaction parameters of MgO.
Keywords: Adsorption;CTAB-assisted;Magnesium oxide;Phosphate removal;Solvothermal.
1.Introduction
The explosive growth of the world population and increase in food requirements led to more usage of phosphate fertilizer,and phosphate over-discharge into the environment is a threat to the ecosystem [1].Phosphate pollution has attracted increasing attention since the blue-green algae accident in Lake Taihu in 2007,resulting from excessive usage of phosphoruscontaining chemical fertilizer for crop yields.It is well known that phosphorous is necessary for plant growth,but its inappropriate use leads to serious health risks to the ecological system.Therefore,it is of great interest and urgency to develop a highly efficient technology to reduce phosphate pollution from the environment [2–6].
Several phosphate removal technologies and methods have proved efficient [7].Sorption is considered the most efficient due to its high efficiency and can achieve a stringent discharge standard for phosphate[2,8].Several adsorbent materials were highly selective and effective for phosphate remediation [6,9].Among the available sorption materials,magnesium oxide(MgO) is considered a low-cost and high-performance adsorbent towards phosphate removal from an aqueous solution[10–12].Besides,the factors like morphology,pore structure,and active sites also influence the adsorption capacity and corresponding adsorption rate of sorbents [13,14].Furthermore,three-dimensional MgO with a high surface area and fully exposed binding sites is one kind of desirable adsorbent to attain high adsorption capacity and a fast uptake rate of phosphate[15,16].
Among the reported structural properties of MgO,the hierarchical flower-like morphologies generally favor forming more active sites,high packaging density,and large transport passages for pollutants [17].Furthermore,three-dimensional nanostructures/microstructures MgO can effectively overcome the sorbent limitations [18–22].For instance,various methods have been developed to tune the morphology and pore structure of three-dimensional hierarchical flower-like MgO with improved removal of multiple pollutants [23–25].Also,the surfactant like cetyltrimethylammonium bromide (CTAB)is a structure-directing agent and pore-forming agent in the synthesis system that can influence the morphology and pore structure of the various metal oxides or hydroxides [26–28].Yet,it is still a big challenge to design and fabricate hierarchical MgO with a large surface area and high removal performance towards phosphate anions from an aqueous phase[26,29,30].The available methods of MgO synthesis are not suitable for forming various morphologies with tunable properties.Still,there is a need to explore new routes to tune the MgO morphologies with a high surface area and suitable pore size.
Here,we fabricated hierarchical MgO adsorbents with different morphologies in a CTAB-assisted solvothermal route by adjusting the feeding molar ratio between CTAB and Mg2+from 0 to 0.1,as shown in Fig.1 and investigated the removal performance towards phosphate anions from the aqueous solution.
2.Experimental section
2.1. Chemicals
Ammonium molybdate ((NH4)6Mo7O24),cetyltrimethylammonium bromide (C19H42BrN,abbreviated as CTAB),ethanol (C2H6O),hexamethylenetetramine (C6H12N4,abbreviated as HMTA),hydrochloric acid (HCl,38% v/v),magnesium nitrate (Mg(NO3)2·6H2O),sodium dihydrogen phosphate (NaH2PO4·2H2O),sodium hydroxide (NaOH),and sulphuric acid (H2SO4,98% w/v),and thiourea (CH4N2S) were of analytical grade and purchased from Beijing Chemical Reagents Co.The deionized water was used in all the experiments.
2.2. Synthesis of hierarchical magnesium oxide
A series of MgO samples were fabricated in a surfactantassisted solvothermal route using Mg(NO3)2·6H2O as a magnesium source,HMTA as a precipitating agent,and CTAB as a surfactant with different ratios between CTAB and Mg2+from 0.00 to 0.10.For MgO-0.10,for example,a mixed solution was prepared containing 12.82 g Mg(NO3)2·6H2O in 25 mL of water,0.88 g HMTA in 10 mL water,and 1.82 g CTAB in 10 mL water and 5 mL ethanol,and then was transferred into a Teflon lined autoclave and subsequently placed into the preheated oven at 140 °C for another 6 h After natural cooling down to room temperature,the obtained precursors were collected after washing twice with deionized water,one time with ethanol,and dried at 60 °C for 24 h Another five precursors were synthesized following a similar process with different feeding ratios between CTAB/Mg2+.Finally,six MgO samples were obtained after calcination of the corresponding precursors at 500 °C for 3 h at 1 °C/min.The corresponding MgO sample was marked as MgO-0.00 to MgO-0.10 according to the feeding ratio between CTAB and Mg2+.
2.3. Characterization
The crystalline structure of MgO samples were analyzed using an X-ray diffractometer (ULTIMA 3,Rigaku) with Cu Kαas a radiation source (λ=0.154 nm,40 kV,30 mA).The scanning electron microscope (SEM-5500) was used for the morphologies and nitrogen adsorption-desorption isotherm using Micrometrics (ASAP 2460).The surface area was calculated using the Brunauer-Emmett-Teller (BET) method and density functional theory (DFT) for pore size estimation.The various functional groups were analyzed using a furrier transform infrared (FT-IR,Nicolet 8700) spectrophotometer.The phosphate was measured using a UV–visible spectrophotometer using a wavelength of 840 nm(UV-2501 PC)based on the molybdenum blue method [31].The change in the chemical composition of MgO after phosphate uptake was measured using photoelectron spectroscopy (XPS,PHI 5600,USA).
2.4. Adsorption experiments for phosphate
Adsorption kinetic experiments were performed for MgO with a sorbent concentration at 0.1 g L-1and phosphate solution of 5 mg L-1into a conical beaker with pH0=5,and initial pH of phosphate solution was adjusted using dilute sodium hydroxide.The conical beakers were placed in a thermostat shaker at 30 °C with a shaking speed of 170 rpm.Approximately 1 mL of phosphate solution was taken at different time intervals and separated through a microfiltration membrane (Φ0.45 μm).The removal capacity with the contact time was evaluated and calculated based on Eq.(1)).The phosphate concentration was determined following the molybdenum blue method [31].
where,qt(mg g-1) is the removal quantity at contact time t,C0(mg L-1) is the initial phosphate concentration,Ct(mg L-1)is the phosphate concentration at time t,V(L)is the initial volume of phosphate solution,and m (g) is the mass of the used MgO sample.The obtained data were linearly fitted for the pseudo-first-order (Eq.(2)) and pseudo-second-order(Eq.(3)).Where qe(mgg-1) is the equilibrium adsorption capacity,k1is the pseudo-first-order constant,and k2is the pseudo-second-order rate constant.
For adsorption isotherm,the MgO of 0.1 g L-1was added to the phosphate solution with a different concentration from 25 mg L-1to 250 mg L-1at pH0=5.The solutions were placed in a thermostat shaker at 30 °C with a shaking speed of 170 rpm.Approximately 5 mL of phosphate solution was taken after 4 h and separated through a microfiltration membrane (Φ0.45 μm).
The phosphate concentration was estimated using a UVvisible spectrophotometer based on the molybdenum blue method using a wavelength of 840 nm.The equilibrium adsorption capacity was estimated using Eq.(4).The isothermal data were linearly fitted for the Langmuir (Eq.(5)) [9]and Freundlich (Eq.(6)) [32]isotherm models.
where,KL,and KF,are the Langmuir constant and Freundlich constant,respectively.
3.Results and discussion
3.1. Structure analysis of MgO samples
Fig.2 demonstrates the X-ray diffraction patterns for six MgO samples after calcination at 500 °C of the corresponding precursors,prepared in the surfactant-assisted solvothermal route with feeding ratios between CTAB and Mg2+from 0 to 0.10.For all of six samples,one observes five diffraction peaks located at ca.36.69°,42.56°,61.99°,74.27°,and 78.43°/2θfor (111),(200),(220),(311),and (222),respectively,and consistent with the standard pattern (JCPDS,card no.36–1451) for the cubic close-packed MgO.These results suggest that the CTAB-assisted solvothermal route is available to synthesize MgO precursors.
3.2. Morphology of MgO samples
Fig.3 shows the morphologies of six MgO samples formed in the presence of a different amount of surfactant developed using the CTAB-assisted solvothermal route.Each MgO sample exhibited different morphologies by the variation between CTAB and Mg2+from 0.0 to 0.1 in the reaction system.A globe thistle-like flower was formed without CTAB in the synthesis system (Fig.3A and B),while ranunculuslike flowers had the highest amount of CTAB (Fig.3K and L).Each feeding ratio resulted in a unique morphology of MgO (Fig.3).Briefly,Fig.3A-B shows a hierarchical globe thistle flower of MgO formed in the absence of CTAB under solvothermal conditions.Loosely packed nanosheets form the globe thistle MgO flower with ragged edges,further arranged to form a nanowhisker of variable width (Fig.3B).The hierarchical gardenias flower-like MgO-0.02 with a diameter of~2 μm,formed by thin sheets’ aggregation (Fig.3C).Each flower is formed by the high stacked density of ultrathin sheets with a large exposed surface (Fig.3D) [33].The flower-like morphology for MgO-0.04 was formed by the arrangement of thin sheets (Fig.3E),and each sheet was formed by the aggregation of nano units (Fig.3F) [34].The urchin-like MgO morphology was obtained for the MgO-0.06 and self-assembled to form nano pedals,which rearrange to give urchin-like spheres (Scheme I) [35,36].The nano pedals can be connected through the center to give a 3D urchin structure (Fig.3G).Each petal comprises various interconnected nanothrones (Fig.3H) [37]Fig.3I shows the cyclamen flower-like for the MgO-0.08 formed by the interlinked flakes.Each flake was formed by staking porous sheets (Fig.3J) [16]Fig.3K exhibits the ranunculus flowerlike morphology for MgO-0.10,formed by the aggregation of several sheets with a uniform size (Fig.3L).These different flower-like MgO morphologies built by a minimal variation of CTAB amount in the reaction system can play a key role in tuning the MgO morphologies with different pore structures,which could influence the adsorption behavior of MgO material towards pollutants [38].Furthermore,one can say,a slight variation in surfactant feeding amount in the reaction system can strongly affect the MgO formation and can provide a new pathway to tune the desired morphology of metal oxides.
3.3. Pore properties of MgO samples
Fig.4 illustrates the nitrogen adsorption/desorption isotherm and pore size distribution for the series of MgO samples.According to the IUPAC system,all the samples possess type II isotherm with H3 hysteresis loop with slit-like pores except MgO-0.04 with H4 without any slit-like pores(Fig.4A)[39],and also consistent with the observed from the morphology in Fig.3.The pore structure was calculated based on the DFT method.Each sample has exhibited different pore sizes (Fig.4B) and was further divided into three levels of pores: (1) 0.7–2 (2) 2–70 (3) 70–225 nm with the main difference in the second region for each sample.The MgO-0.06 exhibits the highest incremental pore volume,and MgO-0.04 has the lowest in the investigated series of samples,which can influence the adsorption capacity and adsorption rate [21].
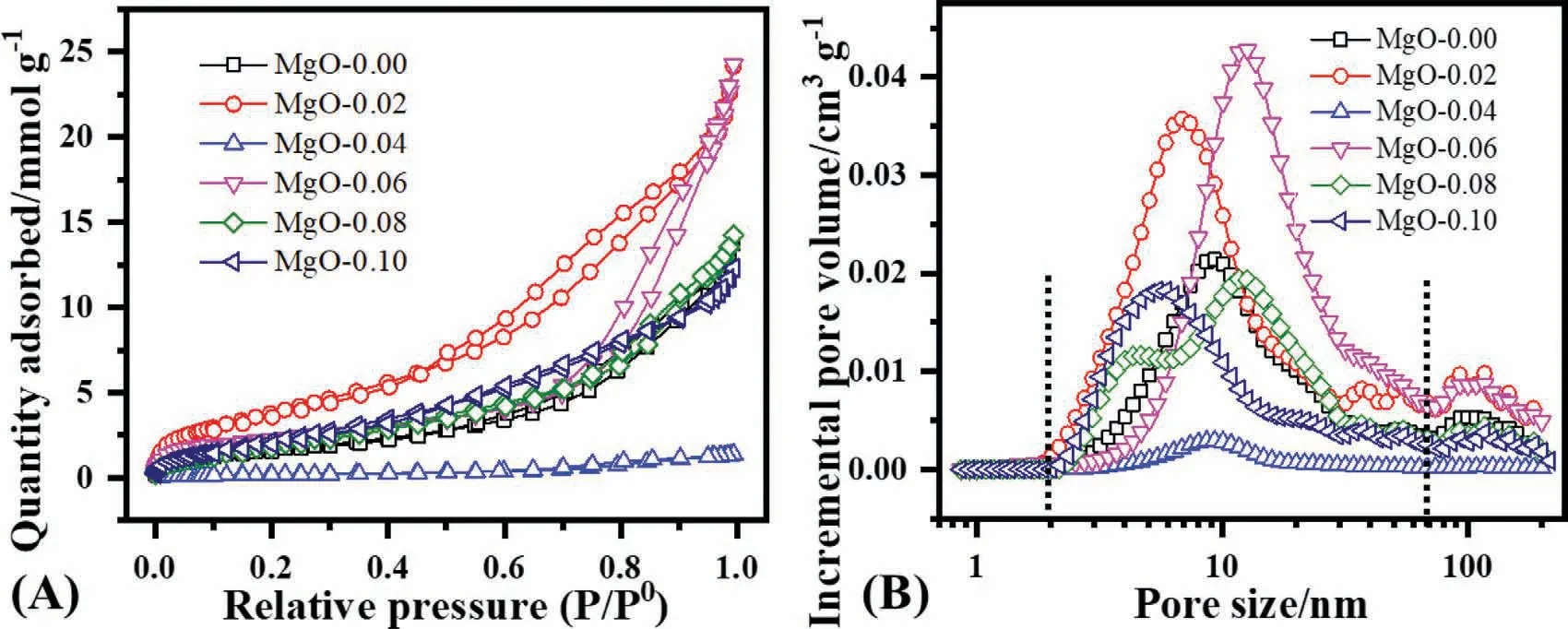
Fig.4.(A) Nitrogen adsorption-desorption isotherm,and (B) pore size distribution.
Table 1 summarizes the surface area,which varies from 15 m2g-1(MgO-0.04) to 336.54 m2g-1(MgO-0.02) for a series of MgO samples with the largest pore size of 18.07 nm(MgO-0.06) and pore volume of 0.843 cm3g-1.The surface area increased 1.41 times and pore volume 0.75 times with the small introduction of CTAB (0.001 mol) in the reaction system for MgO.These results suggest that the pore structure can also be tuned by a slight variation of the surfactant amount,which aids in the adsorption capacity and corresponding adsorption rate.The surface area of 336.54 m2g-1was the highest value developed by the CTAB method in the literature [40],suggesting that the CTAB assisted solvothermal route is available to develop an extended surface area MgO.
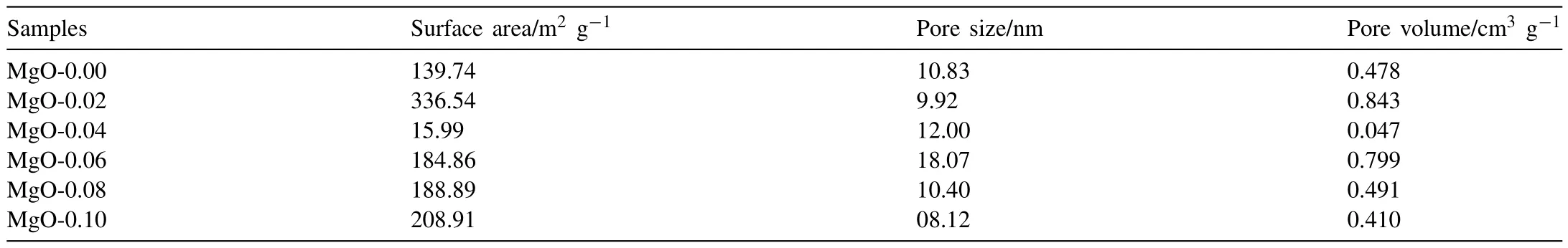
Table 1Surface area and pore parameters of various MgO samples.
3.4. Adsorption of phosphate by MgO
Fig.5 demonstrates the adsorption kinetics and adsorption isotherms for the phosphate for six MgO samples Fig.5.A shows the time-dependent phosphate uptake by MgO samples with an initial concentration of 50 mg L-1at pH0=5.The adsorption is very fast in the first two hours and attains an equilibrium within a short time of ca.3 h.MgO-0.02 establishes the highest equilibrium adsorption capacity of 266.43 mg g-1compared to other samples,probably related to the large surface area and appropriate pore size Fig.5B and C demonstrate the linear fitting results based on the pseudofirst-order,and pseudo-second-order kinetic equations,while Table 2 lists the corresponding fitting parameters for phosphate.The pseudo-second-order describes phosphate sorption kinetic by MgO much better,based on correlation coefficient values,comparable experimental and calculated removal capacities,and percentage standard deviation(S.D).The pseudosecond-order kinetic model indicates the mixed process of physiosorption and chemisorption during the phosphate uptake by MgO [41,42].
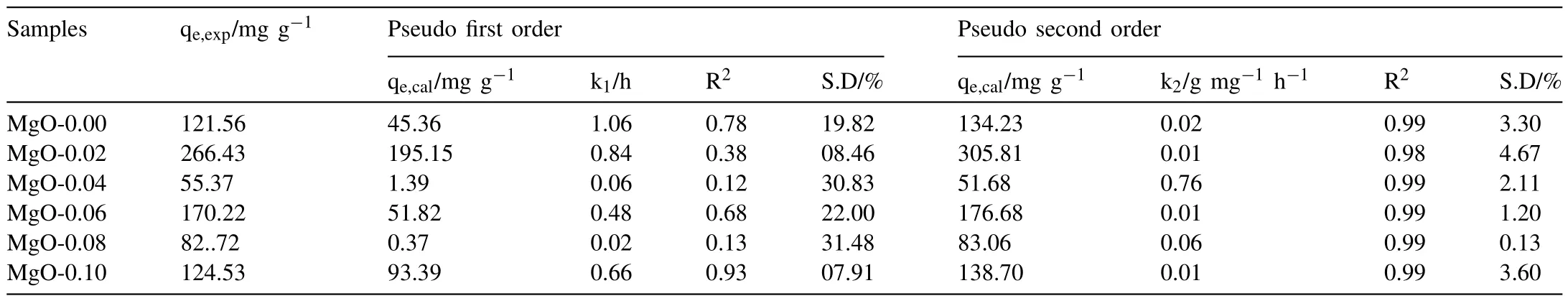
Table 2Adsorption kinetic order parameter of various MgO samples.
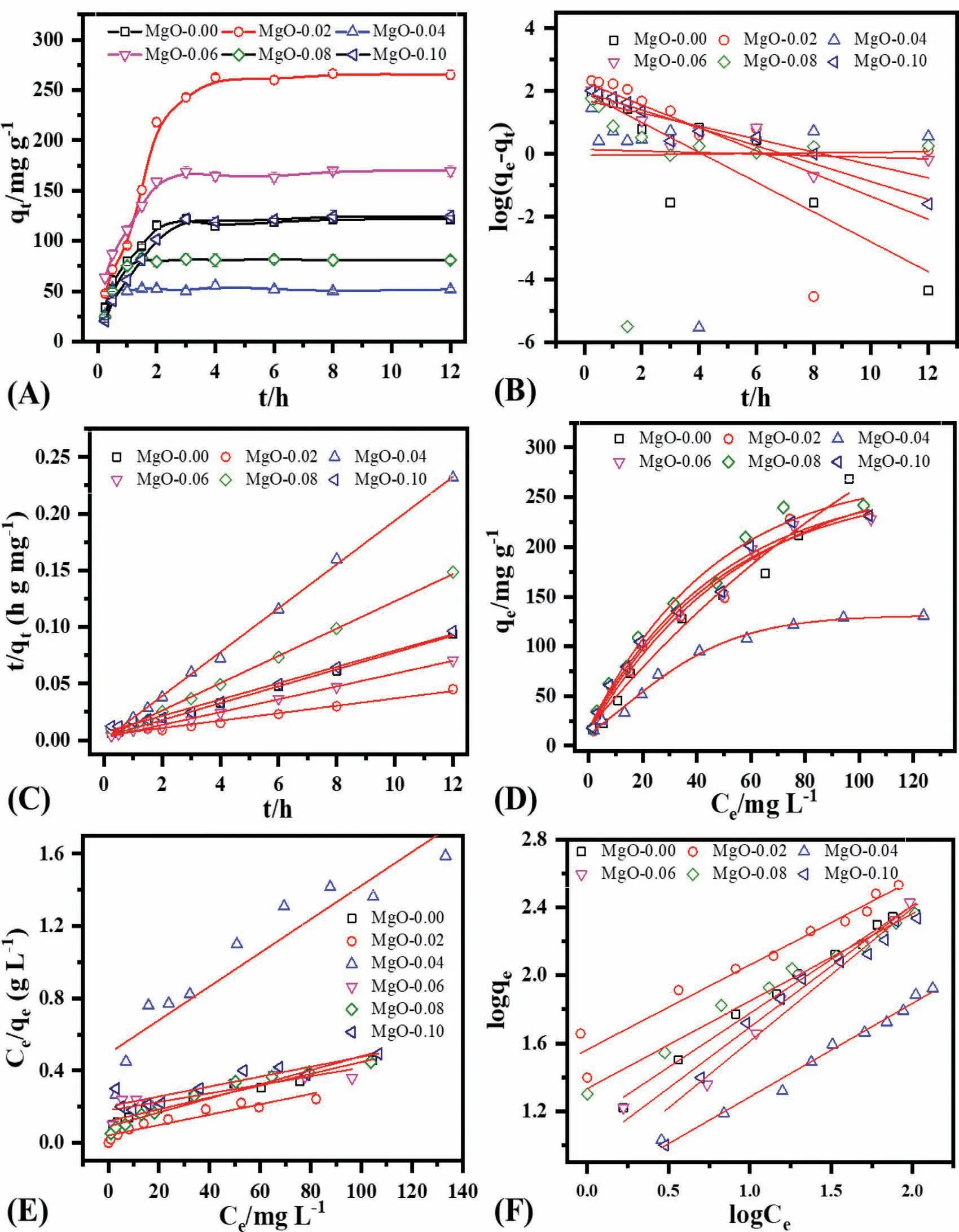
Fig.5.(A)Effect of time on removal capacity when phosphate=50 mg L-1,volume=100 ml,pH0=5,shaking speed=170 rpm,and temperature=30°C,(B) pseudo first order kinetic,(C) pseudo second order kinetic,(D) adsorption isotherm when phosphate=5–150 mg L-1,volume=25 ml,pH0=5,shaking speed=170 rpm and temperature=30 °C,(E) Langmuir isotherm model,(F) Freundlich isotherm model.
Adsorption isotherm experiments for six MgO samples were conducted to estimate the highest removal capacity by changing phosphate concentration between 5 and 150 mg L-1at the initial pH0of 5 (Fig.5D) Fig.5E and F demonstrate the linear fitting results following Langmuir and Freundlich isotherm models,while Table 3 presents the corresponding parameters.The Freundlich isotherm model explains the phosphate adsorption much better than the Langmuir isotherm model,based on the correlation coefficient values.Freundlich isotherm model suggests the heterogeneous coverage of MgO surface by phosphate under the investigated conditions.The value of n from the Freundlich isotherm model also suggests the favorable nature of phosphate uptake by different flower-like MgO [43–45].The highest removal capacity of 265.11 mg/g for the phosphate by the MgO-0.02 is related to the large surface area of 336.54 m2/g.The large surface area can lead to a high removal capacity,and large pore size can aid the fast uptake of phosphate Table 4.further demonstrates the comparison of removal capacities and surface area of various sorbents for phosphate removal.These results exhibit a higher removal capacity than many other materials owing to their high surface area and unique morphology.

Table 3Adsorption isotherm parameter of various MgO samples.
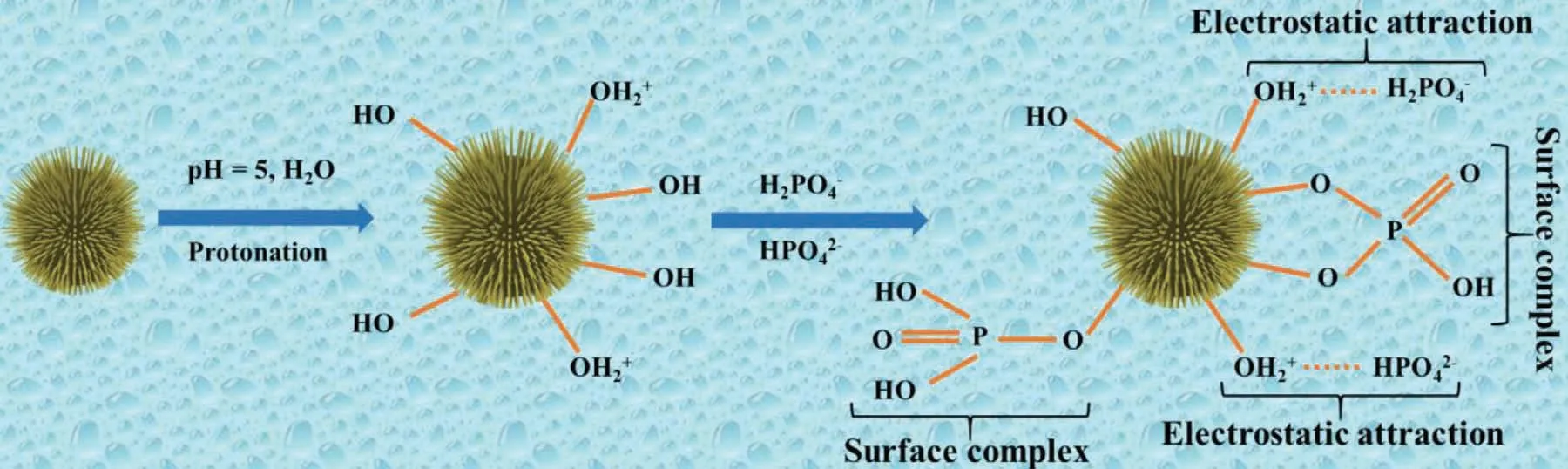
Scheme II.Schematic illustration of the phosphate sorption mechanism on the MgO surface at pH 5.
To understand the phosphate adsorption mechanism,the 50 mg MgO-0.06 was dispersed into a 500 mg L-1phosphate solution and then placed into a thermostat shaker for an equilibrium time of 4 h.After equilibrium,the MgO powder was collected by centrifugation and dried in an oven for the X-ray diffraction and FT-IR characterization to understand the phosphate removal’s mechanism Fig.6A compares the X-ray diffraction pattern of MgO-0.06 before and after the phosphate adsorption.In comparison,all the MgO peaks are converted into new diffraction peaks in the range of 12–80°/2θ,which belong to the Mg3(PO4)2·8H2O,(33–0877 and JCPDS 33–0319) Fig.6B demonstrates the FT-IR spectra of MgO-0.06 before and after the adsorption in which new peaks appeared in the range of 750–1200 cm-1belong to the phosphate captured by MgO [56–58]Fig.6C further indicates the presence of monohydrogen phosphate on the MgO surface and the phosphate,suggesting that the electrostatic interaction and complex formation are the main mechanisms of phosphate uptake.The MgO will convert into positively charged MgOH2+in acidic environment,which have more affinity to bind with the negatively charged phosphate species (Eq.(7))Scheme II further illustrates the possible binding mechanism in which phosphate can bind with the magnesium oxide by the electrostatic interaction and surface complexation.
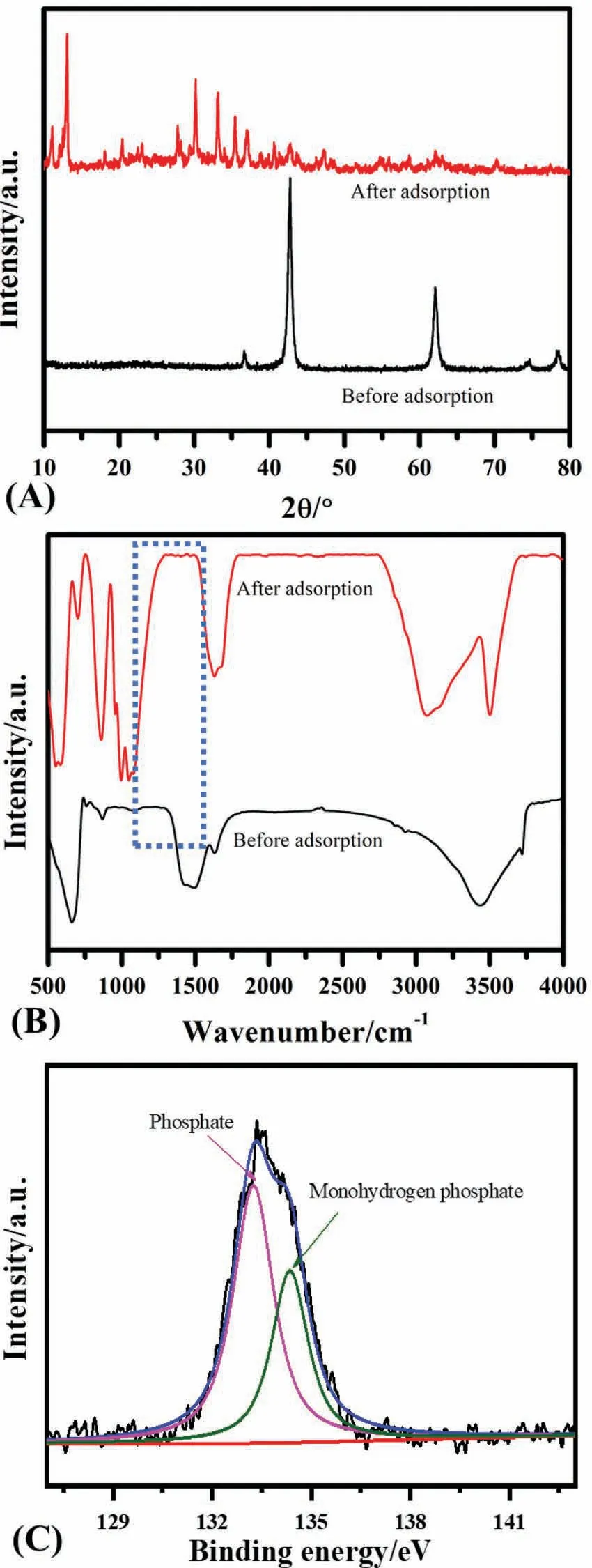
Fig.6.(A)X-ray diffraction pattern,and(B)FT-IR spectrum before and after adsorption of phosphate when phosphate=500 mg L-1,volume=50 ml,MgO-0.06=50 mg,time=4 h,pH0=5,and(C)XPS spectra for phosphate after sorption.
4.Conclusions
In summary,the solvothermal route successfully developed various flower-like morphologies by adjusting the feeding ratio between CTAB and Mg2+in the synthesis system.The XRD and SEM characterization confirmed the successful formation of different hierarchical flower-like morphologies resulting from changing the feed ratios of CTAB and Mg2+in the reaction system.The feeding amount of CTAB and Mg2+has changed the morphology,pore properties,and MgO adsorption behavior for phosphate.The hierarchical gardenias flower-like MgO possessed the highest surface area of 336.54 m2g-1and a total pore volume of 0.843 cm3g-1with the highest adsorption capacity of 348.32 mg g-1for the phosphate removal with a short equilibrium time of 4 h.This study provides a new insight for tuning the desired morphology and pore structure parameters to improve phosphate adsorption,ultimately reducing the eutrophication of water bodies.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Declarations
Not applicable.
Funding
Not applicable.
Availability of data and material
The raw data related to this work will be available on demand.
Code availability
Not applicable.
杂志排行
Journal of Magnesium and Alloys的其它文章
- Corrosion behavior of composite coatings containing hydroxyapatite particles on Mg alloys by plasma electrolytic oxidation: A review
- Rational design,synthesis and prospect of biodegradable magnesium alloy vascular stents
- Antibacterial mechanism with consequent cytotoxicity of different reinforcements in biodegradable magnesium and zinc alloys: A review
- Preparation,interfacial regulation and strengthening of Mg/Al bimetal fabricated by compound casting: A review
- Pitting corrosion behavior and corrosion protection performance of cold sprayed double layered noble barrier coating on magnesium-based alloy in chloride containing solutions
- Designing strategy for corrosion-resistant Mg alloys based on film-free and film-covered models
