Pre-autism:What a paediatrician should know about early diagnosis of autism
2023-12-25MohammedAlBeltagi
Mohammed Al-Beltagi
Mohammed Al-Beltagi,Department of Pediatric,Faculty of Medicine,Tanta University,Tanta 31511,Algahrbia,Egypt
Mohammed Al-Beltagi,Department of Pediatric,University Medical Center,King Abdulla Medical City,Arabian Gulf University,Dr.Sulaiman Al Habib Medical Group,Manama 26671,Manama,Bahrain
Abstract Autism,also known as an autism spectrum disorder,is a complex neurodevelopmental disorder usually diagnosed in the first three years of a child's life.A range of symptoms characterizes it and can be diagnosed at any age,including adolescence and adulthood.However,early diagnosis is crucial for effective management,prognosis,and care.Unfortunately,there are no established fetal,prenatal,or newborn screening programs for autism,making early detection difficult.This review aims to shed light on the early detection of autism prenatally,natally,and early in life,during a stage we call as “pre-autism” when typical symptoms are not yet apparent.Some fetal,neonatal,and infant biomarkers may predict an increased risk of autism in the coming baby.By developing a biomarker array,we can create an objective diagnostic tool to diagnose and rank the severity of autism for each patient.These biomarkers could be genetic,immunological,hormonal,metabolic,amino acids,acute phase reactants,neonatal brainstem function biophysical activity,behavioral profile,body measurements,or radiological markers.However,every biomarker has its accuracy and limitations.Several factors can make early detection of autism a real challenge.To improve early detection,we need to overcome various challenges,such as raising community awareness of early signs of autism,improving access to diagnostic tools,reducing the stigma attached to the diagnosis of autism,and addressing various culturally sensitive concepts related to the disorder.
Key Words: Autism;Pre-autism;Biomarkers;Autism spectrum disorder;Biophysical profile;Neurodevelopment;Antenatal;Neonatal
INTRODUCTION
Autismis a word derived from the Greek word "autos," which means self,described in 1943 for the first time by Leo Kanner from Johns Hopkins University[1].This word reflects the different perspectives by which the child sees the world.Autism,or autism spectrum disorder(ASD),is a complex neurodevelopmental disorder affecting specific brain developmental areas,impacting communication abilities,social interaction,and child behavior.It is typically diagnosed during the first three years of life with a spectrum of symptoms ranging from mild to severe and varies from child to child[2].There is a wide variation of autism prevalence from one country to another and from one ethnicity to another,with an average prevalence of 0.1%.However,the prevalence could reach 1/64 in the United Kingdom and 1/36 in the United States.These differences could be related to the availability of diagnostic tools[3].In other countries,e.g.,the Kingdom of Bahrain,the prevalence could be much lower with underestimation(0.1%),probably due to missed diagnosis of some cases and the absence of official recording due to the fear of stigma[1].In addition,there are significant gender differences as boys are affected 4-5 times more than girls.Ethnicity also impacts the prevalence as it is more common in non-Hispanic white populations than in Hispanic and African American/black people,with vast differences in Asian/Pacific residents[4].
The exact pathogenesis of autism is still not precisely obvious.It is multifactorial mainly due to the interaction between genetic,environmental,advanced parental age,biological,psychological,and immunological factors[5].About 80%-90% of the autism risk is heritable,which explains why autism runs in families[6].With the enormous advances in genetic studies,we recently recognized various genetic mutations in the affected children,which can eventually provoke brain changes and inflammation during the early developmental phases[7].Many genetic disorders are implicated in autism pathogenesis,from simple genetic mutation to complex numeric variations with large deletions,inversions,duplications,or chromosomal translocations[8].These genetic mutations,such as Fragile X syndrome,may be inherited,de novo mutation,or due to accumulative changes over generations.These genetic factors raise the recurrence rate to between 2% and 8% in siblings of children with autism.Children with autism may have other chromosomal or genetic co-morbidities,such as Down syndrome,Fragile X syndrome,Duchenne muscular dystrophy,tuberous sclerosis complex,and neurofibromatosis type I[9].However,the increased risk of recurrence does not take root only from the genetic factor but also from sharing the same environmental and epigenetic factors[10].Various ecological factors could induce epigenetic changes through deoxyribonucleic acid(DNA)methylation,histone modifications,or microRNAs;all these can affect gene expression without modifying the primary underlying DNA sequence.These environmental factors include dietary modification,exposure to stressful conditions,environmental pollution,and toxin exposure.The increased awareness of autism,in addition to the interaction between genes and environment,are important causes for the rising autism prevalence[11].
Autism is classically diagnosed according to the basic criteria summarized in the Diagnostic and Statistical Manual of Mental Disorders(DSM-5)[12].ASD is characterized by the impairment of at least two out of three main domains that describe the fundamental features and symptoms of autism:impaired social interaction and/or communication and restricted,repetitive,or stereotyped behavior,interests,and activities with the onset of these changes occurs during early development[13].Impaired social interaction is manifested by difficulties in making friends,playing with others,or sharing their interests.Children with autism may struggle to make eye contact and understand social rules and their perspectives.Impaired social communication includes verbal and nonverbal communication difficulties,such as using and understanding facial expressions,gestures,body language,or voice tone.In addition,they have difficulty initiating and maintaining dialogues,understanding jokes or sarcasm,and using appropriate social clues[14].Restricted and repetitive behaviors observed in children with autism appear as repetitive or stereotyped actions,such as hand flapping and persistent routines with a strong interest or obsession with a limited spectrum of topics or activities.They may also have abnormal sensory processing mechanisms with atypical responses to sensory stimuli.The child becomes hypersensitive to specific sensory stimuli,such as lights,sounds,tastes,or textures,or hyposensitive to other stimuli,such as pain or temperature changes[15].It is crucial to note that the symptoms of these domains vary in severity and presentation from one child to another,with different strengths and challenges.Some individuals may also have other comorbidities or challenges.Diagnosis of autism is complex and not an easy task.It requires a thorough evaluation by a competent healthcare professional and teamwork,such as a paediatrician,child psychiatrist,developmental and behavioral paediatrician,and clinical childhood psychologist[16].
Diagnosis of autism is usually achieved around the age of two to four years,depending on many factors,including the severity of autism,availability of healthcare access,parents' education level,and cultural and community factors[17].Diagnosis of autism can be made at any age and could even be done for the first time during adolescence or adulthood.However,early diagnosis significantly impacts the child's management,prognosis,and welfare[18].Early diagnosis allows early intervention,which helps children develop proper communication and social skills and enhances patient functioning and independence later in life.It also allows parents and caregivers to better understand the child's strengths and challenges.In addition,early diagnosis helps to improve the child and family's quality of life with a significant reduction in the long-term cost[19].As the cost of autism may reach up to US$3.2 million lifetime per capita,and early intervention may improve the prognosis and decrease the cost,we need to detect autism early.Directed actions could delay the autism progression,especially for language and cognitive abilities,and may help autism regression before it becomes irreversible.No well-established early fetal or newborn biomarker screening programs exist for autism.Therefore,this review will try to shed some light on the early detection of autism prenatally and in early life.
METHODS
To establish an evidence-based view of this aim,we accomplished a systematic literature review by searching the available electronic databases,including PubMed,PubMed Central,Cochrane Library,Embase,Web of Science,Cumulative Index to Nursing and Allied Health Literature,Scopus,Library and Information Science Abstracts,and the National Library of Medicine catalog up until May 8,2023,using the keywords:Autism,Pre-autism,Biomarkers,Autism spectrum Disorder,Biophysical Profile,Neurodevelopment,Antenatal,Neonatal.We included a total of full-text articles(175 articles),including research articles(102 articles),metanalysis(9 articles),systematic reviews(4 articles),reviews(58 articles),and consensus guidelines(2 articles).We included articles that were written in English and concerned with the effects of early diagnosis of autism.Figure 1 shows the study flow chart.Reference lists were checked,and citation searches were performed on the included studies.We also reviewed the articles that are available as abstracts only.We excluded articles with a commercial background.
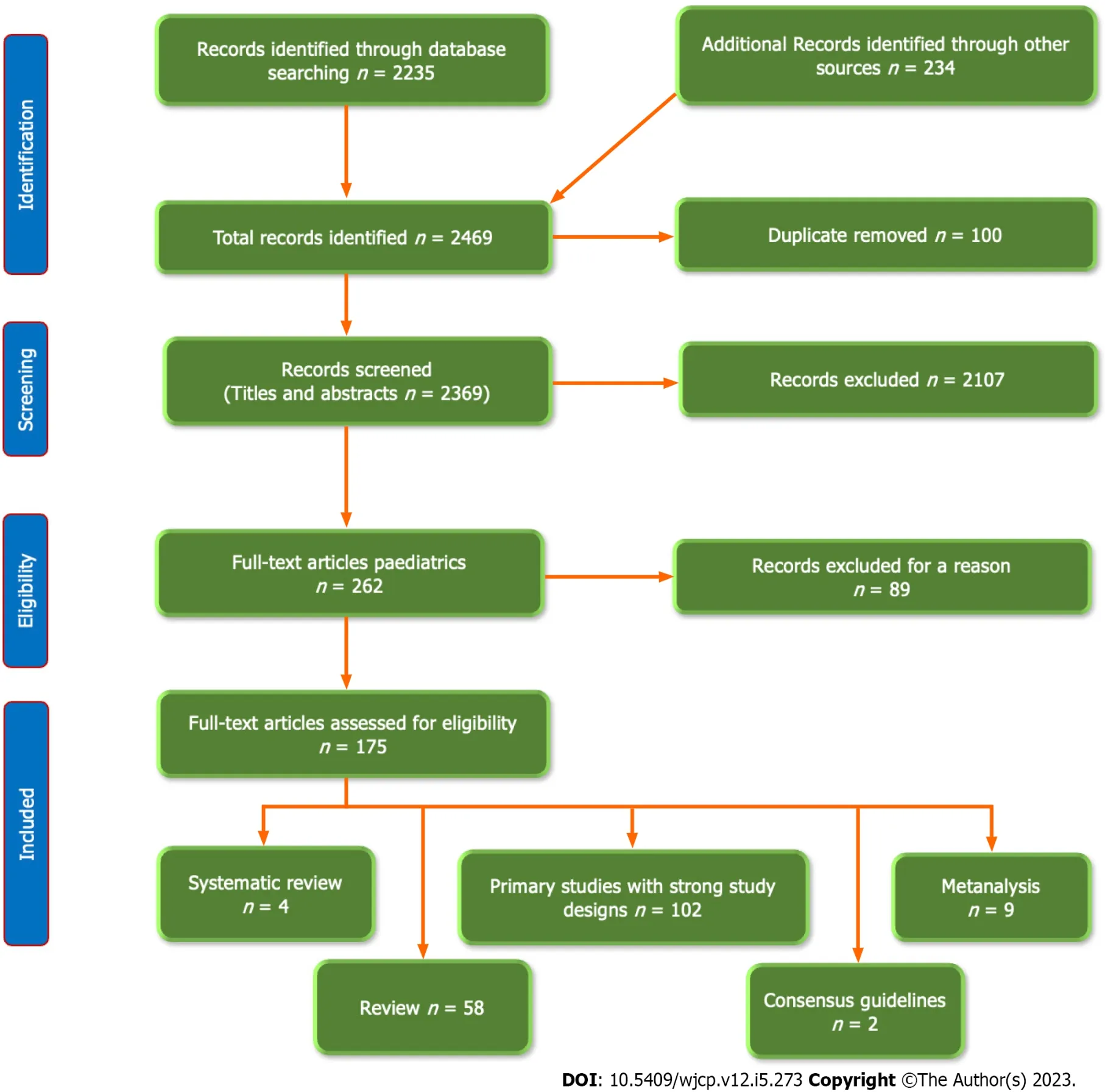
Figure 1 The flow chart of the included studies.
DISCUSSION
Pathophysiology of autism
Autism results from genetic and environmental developmental factors disrupting normal brain development and affecting many or all brain functions by activating pathological pathways.Despite the extensive efforts over the last few decades to elaborate the exact mechanism of autism,we are still in the first steps of a long way to be achieved,and its precise mechanism is still poorly understood.However,two critical domains are being tried to explain what could lead to the manifestations of autism.The first domain is the changes in the brain structure and pathophysiology usually observed in individuals with autism.The second domain is the neuropsychological and neurobiological links between the changes in the brain structure and the development of behavioral changes characteristic of autism[20,21].Different theories tried to explain the occurrence of autism.One of them is related to the presence of excess neurons with significant changes in synaptic density and plasticity,which causes local overconnectivity in critical brain regions,which explains the high brain weight and volume and the large head circumference observed in children with autism,together with the enhanced expansion of the grey matter of cortical surface area in children with autism resulting in reduced maturation of the cortical white matter.However,these pathological changes' molecular and cellular mechanisms are not fully addressed,nor is it known whether the brain’s overgrown causes the characteristic autism signs[22].
In addition,specific brain regions are affected in children with autism,such as the frontoparietal cortex,frontotemporal lobe,amygdala,basal ganglia,hippocampus,and anterior cingulate cortex[23].For example,defects in social attention and social language processing are associated with abnormalities in Broca's area(inferior frontal gyrus),Wernicke's area(located in the posterior part of the upper temporal convolution of the left-brain hemisphere and concerned with speech comprehension),and superior temporal sulcus[24].Impaired social behaviors are associated with abnormal functioning of the frontal lobe,parietal cortex,superior temporal cortex,and enlargement of the laterobasal amygdala[25].Meanwhile,abnormalities in size,structure,and activity of the orbitofrontal cortex(which is involved in decision-making,social behavior,and emotion regulation)and caudate nucleus(which is involved in motor control,reward processing,and learning)are associated with increased risk of autism[26].Accelerated cortical thinning was also observed in patients with autism aged 3-39 years after accelerated expansion in early childhood[27].
Neuronal migration is an essential process in fetal brain development,during which neurons migrate from their original place to their final destination.Disturbance of neuronal migration during early pregnancy alters neural connectivity and induces abnormal neural circuits,contributing to abnormal behavioral and impaired cognitive functions.Irregular neuronal migration with abnormal positioning and organization of neurons in the brain are implicated in developing a range of neurological conditions,including autism.Prenatal exposure to specific hazardous environmental hazards,such as environmental toxins,drugs(e.g.,thalidomide or valproic acid),and maternal infections,inflammation,or immune dysfunction,can induce gene mutation(e.g.,gene CNTNAP2 which regulates neuronal migration and axon guidance or gene RELN,which regulates neuronal migration in the cerebral cortex,Lis1,DCX,and TUBA1A genes)and altering their expression,interrupting the normal neuronal migration,and raising the risk of autism[28,29].Imaging studies showed that patients with autism have abnormal brain white matter organization,which could be related to neuronal migration disturbances during early development[30].
In addition to the structural and ultrastructural changes observed in patients with autism,there is growing evidence of altered brain connectivity and function due to imbalances in brain excitatory and inhibitory signaling.The excitatory neurons release the neurotransmitter glutamate,which promotes neuronal firing.In contrast,inhibitory neurons release the neurotransmitter Gamma-aminobutyric acid(GABA),suppressing neuronal activity[31].An overall rise in neuronal excitation and reduced neuronal inhibition led to the social and communication deficits commonly observed in autism.This imbalance mostly results from decreased GABAergic inhibitory neurons’ number or function,e.g.,reduced parvalbumin-positive interneurons,that naturally assist in controlling the excitatory neurons’ activity.This imbalance could result from several factors,including genetic mutations,harmful environmental factors,and disturbed neural circuit development during early childhood[32].This imbalance was proved by genetic analysis of the expression of the functional genes responsible for the inhibitory function and post-mortem and animal studies that demonstrated the reduction of the inhibitory neurons[33].Treating this imbalance using medications that boost GABAergic signaling,such as certain antiepileptics,benzodiazepines,and GABAB receptor agonist STX209,has been proven effective in treating some autism symptoms,with variable degrees of success[34].
We can admit from the previously described pathophysiological mechanisms that autism results from genetic and environmental interaction with some predominance of the genetic factor.However,a growing list of exposures for mother and baby may sway the odds and affect the onset time of symptoms.There are three different pathways or trajectories for the development of autism,according to the timing of the insult that triggers the changes observed with autism.The first pathway is caused by in-utero insult/injury;obstetric complications at birth activate the second pathway,while the third pathway is caused by environmental triggers of autism affecting infants 0-3 years of age[35].In addition,we can classify autism according to the presence or absence and the number of minor physical defects into essential and complex autism.In essential autism,there is no evidence of an early embryological abnormality with no or very few minor physical defects.Conversely,in complex autism,the affected children have six or more minor physical defects.They are more likely to have genetic disorders,brain anatomical abnormalities,seizures,and a low intelligence quotient.They may also have peculiar facial features with a broader upper face,a shorter middle face comprising cheeks and a nose,wider eyes,a bigger mouth,and a philtrum.These facial features are better recognized using a 3-D camera with the help of artificial intelligence[36,37].
Pre-autism("Preclinical")
We are using the term “pre-autism” for the first time to describe the early prodromal period that precedes the development of the full-blown and formal clinical picture of classic autism when the symptoms and signs of autism or ASD are not yet clearly evident.During the pre-autism phase,some risk factors,with few and/or non-classic signs,appear during the early developmental stage of the child’s life that could indicate the increased potential risk of autism later in life.These early signs are often subtle,not yet fully developed,or may not be easily recognizable.The pre-autism phase typically happens during the neonatal period,infancy,or early childhood.It could be challenging to distinguish these early signs from typical developmental variations.It is central to admit that not all kids who display these early signs or risk factors will develop autism,and some children who develop autism may not show all these early signs.Therefore,a comprehensive evaluation by a trained professional is necessary to determine if a child has autism.
Presently,no newborn biomarker screening programs exist to detect autism.We should think about pre-autism in the presence of a positive family history of a patient with autism and or other antenatal or post-natal risk factors.The more risk factors,the higher the risk of autism[38,39].These risk factors are summarized in Table 1.

Table 1 Factors that increase the risk for autism
Preconception risk
Having a child with autism increases the risk of having another child with autism by 20-fold,especially in full-siblings compared to half-siblings and in the maternal half-siblings than paternal half-siblings.This supports the genetic and uterine environment effects in the pathogenesis of autism[40].The risk further increases with a shorter time between the birth of a previous child with autism and the birth of the next sibling.The risk of autism recurrence reached 14.4% with an interbirth interval equal to or less than 18 mo,compared with 6.8% for an interval equal to or more than four years[41].
The birth order may also affect the risk of having autism.The first-born child might have a higher risk of having autism than later-born children.The increased risk in the firstborn child may be related to a higher exposure rate to pregnancy and delivery complications such as infections,stress,and inflammation.In contrast,the lower risk of the later-born children may be related to the social support and help provided by the first-born child to the later-born children[42].On the other hand,Alvaresetal[43] showed that intelligence scores and adaptive functioning decrease with increasing birth order.The later-born children are more prone to have an intellectual disability.However,they showed that first-born children without siblings have decreased cognitive functioning compared to those with siblings.This controversy may be related to other confounding factors,such as differences in the ethnicity of the studied group.
The age of the parents at the time of conception can predict the chance of having autism.Both the father and mother’s age at the time of conception is independently associated with an increased risk of autism in the offspring.The risk will be higher if both parents are old[44].Increased risk of autism with increasing paternal age may be due to increased risk of de novo mutations in their sperm or increased risk of having some features of autism phenotype that cause them to marry late[45].Conversely,mothers over 35 have a 1.5 higher risk of having a child with autism than mothers between 25-29 years.Younger mother age gives more protection against autism.Higher maternal age is associated with a higher risk of chromosomal abnormalities and more exposure to environmental risk factors that affect DNA methylation in germ cells,with an increased risk of developmental consequences on the offspring[46].The effect of higher maternal age is more evident in the male offspring,while higher paternal age is more evident in the female offspring[47].
Some data show that pre-conceptional maternal obesity might raise the risk of autism in the offspring.Wangetal[48] showed a linear dose-response relationship between pre-pregnancy maternal BMI and the risk of autism,with a relative risk of 1.16 for each 5 kg/m2increase in Mother BMI.Joetal[49] showed that children born to mothers with severe obesity before pregnancy had a high risk for adverse developmental outcomes,including autism.Hinkleetal[50] also observed an increased incidence of learning and behavior disorders by kindergarten among children born to mothers with severe pre-pregnancy obesity.Pre-conceptional maternal obesity may alter the intrauterine milieu,increasing inflammation,t affecting the function of insulin and other metabolic hormones,and affecting the developing fetal brain[51].
Antenatal Markers for the risks of autism
As mentioned before,no screening test can tell us that the baby will develop autism,as autism's aetiology and pathogenesis are not fully understood.However,some fetal and infant biomarkers may expect an increased risk of autism in the coming baby.These biomarkers could allow us to develop a biomarker array that,when properly developed and analyzed,could give us an objective diagnostic tool to diagnose and rank the severity of autism for each patient.These biomarkers could be genetic,immunological,hormonal,metabolic,amino acids,acute phase reactants,body measurements,or radiological markers[52](Table 2).
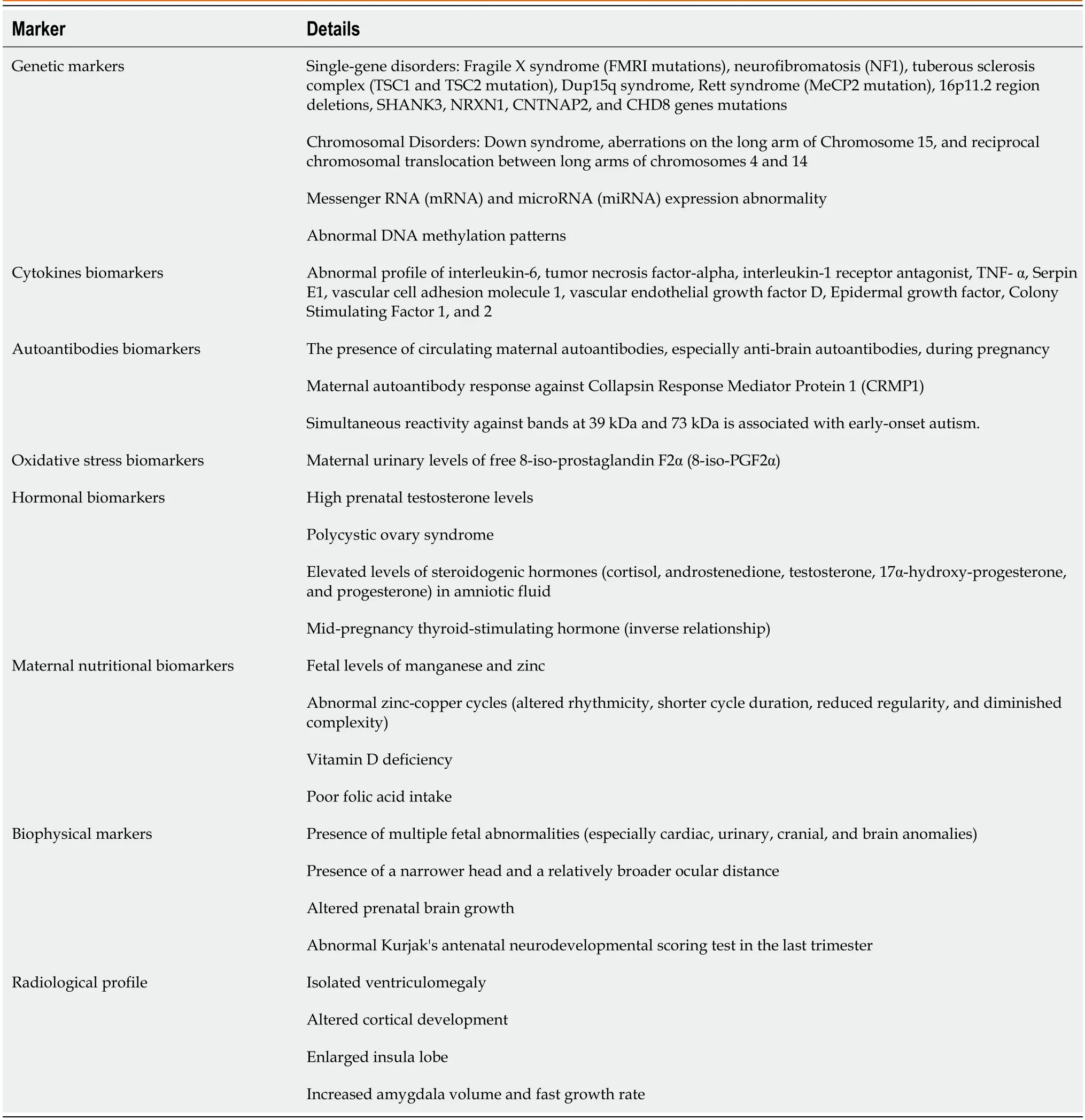
Table 2 Antenatal markers for the risks of autism
Genetic profile
As our understanding of the genetic and environmental factors that contribute to the development of autism is improving,it may be feasible to develop more accurate techniques to predict the risk of autism based on examining genetic profiling.The presence of specific genetic markers in amniotic fluids or cord blood may indicate an increased risk of developing autism.Single-gene disorders such as Fragile X syndrome(FMRI mutations),neurofibromatosis(NF1),tuberous sclerosis complex(TSC1 and TSC2 mutation),Dup15q syndrome,Rett syndrome(MeCP2 mutation),and deletions in the 16p11.2 region are present in about 3%-5% of patients with autism[53].Other chromosomal and genetic disorders,such as Down syndrome(40% of them have autism or autism-like symptoms),Duchenne muscular dystrophy,and tuberous sclerosis complex,are associated with an increased risk of autism[9].Large-scale genome-wide association studies have also identified numerous common genetic variants that are associated with an increased risk of autism.Among these mutations,mutations involving the SHANK3,NRXN1,CNTNAP2,and CHD8 genes increase the risk of autism[54,55].16p11.2 is a chromosomal region linked to autism and other neuropsychiatric conditions.The 16p11.2 genetic mutation with either deletion or duplication is associated with an increased risk of motor speech disorder,motor coordination difficulties,language disorder,and various psychiatric conditions,including autism.Detection of deletions or duplication in 16p11.2 in the cord blood might expect that the child may develop autism and other neuropsychiatric conditions[56].However,the resulting severity varies significantly,and not all the patients with the mutation or deletion have the same clinical picture.Not all patients with these mutations will develop autism[57].
Another important genetic variation that could increase the risk of autism is the presence of single nucleotide polymorphisms(SNPs).The SHANK2 gene is an important gene for glutamate neurotransmission and synaptogenesis.A synergistic interaction between SNPs and the SHANK2 gene increases the risk for autism[58].Other SNPs that affect the expression of genes that increase the risk of autism are MTHFR(Methylenetetrahydrofolate reductase has a significant role in folate metabolism.),RFC(genes encoding the reduced folate carrier),MTR(methionine synthase responsible for the regeneration of methionine from homocysteine and involved in the folate/homocysteine pathway),OXTR gene(a gene correlated with aggression,social dysfunction,and irritability),and CD38 gene(a gene linked to low CD38 expression and a lack of emotions)[59].
Not only structural gene changes can be used as a marker for risk autism,but also markers of gene expression,including messenger RNA(mRNA)and microRNA(miRNA)expression.mRNA is a single-stranded RNA molecule.It undergoes post-transcriptional modifications to carry the genetic information from nuclear DNA from the nucleus to ribosomes in the cell cytoplasm to be used as a template for protein synthesis[60].On the other hand,microRNA is a small,non-coding RNA molecule transcribed from DNA.It undergoes several processing steps before its incorporation into the RNA-induced silencing protein complex to guide this complex protein to its target mRNA through base pairing.Once base-paired with the targeted mRNA,it inhibits it by degradation or inhibiting its translation.A single miRNA can combine with several hundred targeted sites in the 3′ untranslated regions of mRNAs,thus controlling the expression of many genes.There are about 5104 miRNA variants have been identified till now[61].Studies have suggested the role of miRNAs in the development of autism.Wongetal[62] identified variants in five brain-expressed miRNAs,which target 326 genes,and 3′ UTR miRNA target regions of 152 genes potentially involved in neurodevelopmental disorders,including autism.miRNA could post-transcriptionally regulate autism-related risk genes and affect various molecular pathways related to autism and associated disorders.In addition,Noroozietal[63] identified five modules linked with neurexins and neuroligins,cell adhesion molecules,glutamatergic synapse,L1CAM interactions,MECP2 and circadian clock pathways,NOTCH,and neurotransmitter release cycle.Functional analysis of these genes helps to determine critical pathways related to neurological disorders and the pathogenesis of autism.
Methylation is a process of adding a methyl group to a DNA molecule to affect gene expression.Changes in the methylation patterns of certain genes could influence the development of autism.Patients with autism are in their DNA methylation patterns in specific genes from typically developing children.DNA methylation Epimutations can be developed throughout life.Reprogramming of the global DNA methylation is a dynamic process that occurs during the embryonic and the early postnatal period that parallels the peak time of synaptogenesis[64].Abnormal methylation of specific genes could result in an increased risk of autism.García-Ortizetal[65] detected higher Neural Cell Adhesion Molecule and Nerve Growth Factor methylation levels in children with autism.Testing abnormal methylation patterns in biological specimens obtained from the parents could help to predict the possibility that the child may be born with autism.Global DNA hypomethylation was observed in mothers of children with autism.In addition,specific methylation patterns were observed in sperms obtained from fathers of children with autism[66,67].Despite being a promising antenatal marker of autism,methylation has a complex nature and interaction with other environmental factors such as vitamin intake and prenatal tobacco exposure[68].
Cytokines profile
Cytokines are specific proteins that have an essential function in the immune system and could affect brain development and function.Specific cytokines,such as interleukin-6 and tumor necrosis factor-alpha,could play a role in brain development as they may initiate neuroinflammation,contributing to autism development.Therefore,the irregular profile of these cytokines,especially interleukin-6 in the fetus,might be related to the increased risk of autism during childhood[69].Another study by Abdallahetal[70] showed increased levels of TNF-alpha in the amniotic fluid in mothers of children with autism than in mothers of children with typical development.In addition,Cheetal[71] showed that the maternal mid-gestational and neonatal cord blood immune markers are strongly associated with increased risk of autism in the offspring,including but not limited to interleukin-1 receptor antagonist,TNF-alpha,Serpin E1,vascular cell adhesion molecule 1,vascular endothelial growth factor D,Epidermal growth factor,Colony Stimulating Factor 1,and 2.Maternal mid-gestational findings were outstanding,with remarkably great effect sizes in girls.However,Bryngeetal[72] found no strong evidence of the association of maternal immune markers during early pregnancy with autism.Therefore,it is critical to endorse that the relationship between abnormal intrauterine cytokine profiles and the risk of autism is not fully understood and needs more research.We should consider other genetic and environmental factors that can impact the development of autism.
In addition to the previous markers,Jietal[73] found in their prospective study that cord biomarkers of fetal acetaminophen exposure,such as 3-[N-acetyl-l-cystein-S-yl]-acetaminophen,acetaminophen glucuronide,or unchanged acetaminophen were associated with a significantly high risk of developing attention deficit hyperactivity disorder and autism during childhood in a dose-response fashion.Acetaminophen can cross the placental barrier and remain in the infant's blood circulation for a long time[74].However,no specific time window was confirmed during which the developing brain is most sensitive to acetaminophen exposure.At the same time,we could not ensure whether the effects are due to the direct acetaminophen exposure or the cause of its use,e.g.,maternal fever during pregnancy,which increases the risk of autism.Even the observed dose-response effects can be related to the severity of the cause of its use and not to acetaminophen itself.However,we need more animal studies to confirm acetaminophen's direct brain toxic effects.
Maternal plasma or fetal blood anti-fetal brain autoantibodies profile
Many studies correlated the presence of circulating maternal autoantibodies,especially anti-brain autoantibodies during pregnancy,to the occurrence of neonatal neuronal dysfunction.These autoantibodies can cross the placental blood barrier and access the developing fetal brain and inducing the development of autism[75-77].Daltonetal[77] performed a very interesting experiment.They injected 0.5-1 mL of sera obtained from mothers of children with autism and mothers of typically developed children into pregnant mice daily.They found that the offspring of mice injected with sera from mothers of children with autism developed altered motor coordination and exploration and had cerebellar changes in magnetic resonance spectroscopy in the mouse offspring,compared with offspring of mice injected with sera from mothers of typically developed children.Fox-Edmistonetal[78] recognized maternal autoantibodies against seven neurodevelopmental proteins associated with developing maternal autoantibody-related(MAR)autism in the offspring.Ramirez-Celisetal[79] found that maternal autoantibody response against Collapsin Response Mediator Protein 1 is associated with a significant increase in the chance that the child has autism,indicating the potential use of these autoantibodies as potential biomarkers of autism risk in up to 18% of autism cases termed as MAR autism.
The mechanism and the triggers for developing these autoantibodies are not clear.These autoantibodies against the fetal brain can be identified in the mother’s blood and can be used as a marker for autism risk[80].Croenetal[81] showed that maternal mid-gestation autoantibody against human fetal brain protein(especially reactivity to a band at 39 kDa)is associated with the risk of developing autism.In addition,simultaneous reactivity against bands at 39 kDa and 73 kDa is associated with early-onset autism.However,identifying these anti-fetal brain autoantibodies has two critical implications:diagnostic and potential therapeutic benefits.Preventing the development of these maternal autoantibodies can prevent the irreversible damage that could happen to the developing fetal brain.More searches are needed to elucidate the trigger for these autoantibodies,prevent their generation,and develop different therapeutic modalities to remove these antibodies,such asinvivoautoantibody competition orexvivoautoantibody removal.
Oxidative stress biomarkers
Oxidative stress may have a role in the pathogenesis of autism.Oxidative stress means excessive production of reactive oxygen species beyond the detoxification ability of cells,leading to damage of the cellular components such as lipids(peroxidation),proteins(e.g.,post-translational changes},DNA,and toxic build-up by these reactive radicles,causing cellular dysfunction and damage[82].Many studies have figured out the relationship between intrauterine oxidative stress markers and the increased risk of developing autism.For example,Rommeletal[83] found that maternal urinary levels of free 8-iso-prostaglandin F2α(8-iso-PGF2α)and its metabolites(markers of oxidative stress)were significantly increased in mothers of children who developed autism than in mothers of typically developing children.Maternal immune activation(MIA)during pregnancy is another example of the effects of oxidative stress and one of the common environmental risk factors for developing autism.MIA induces an unusual immune reaction in the pregnant woman,causing further inflammation and increasing oxidative stress in the placenta,crossing it to affect the fetal brain,causing activation and accumulation of microglia,dysregulating neurodevelopmental and autism-associated genes,inducing neurodevelopmental impairments in the developing fetal brain,and afterward triggering behavioral problems in the offspring[84-86].
In addition,oxidative stress is one of the possible mechanisms by which gestational diabetes and some antenatal infections increase the risk of autism.Lantéetal[87] experimentally prevented the harmful neurodevelopmental damage induced by bacterial endotoxin lipopolysaccharide with strong oxidant stress effects by using the antioxidant N-acetylcysteine,confirming the effect of oxidative stress on the pathogenesis of autism.However,Careyetal[88] found weak or no association between oxidative stress markers(glutathione,glutathione disulfide,8-oxo-deoxyguanine,and nitrotyrosine)in late pregnancy and the risk of autism in the offspring.This discrepancy between their results and previous studies can be explained by the differences in the markers used and the time points they investigated the mothers(late trimester).We should also consider that association is not equal to causation.Therefore,we need more research to confirm the role of oxidative stress,choose the proper marker,and also the proper timing for the test to give more reliable results.
Hormonal profile
Antenatal exposure to steroid hormones can directly impact fetal gene transcription and expression during vulnerable stages of embryonic development.High prenatal testosterone levels can predict an elevated prevalence of behavioral and cognitive traits that are usually associated with autism in both typically developing males and females from early to late childhood,even if they did not develop frank autism with long-lasting effects on brain structures and organization and emotional reward processing in late childhood[89,90].Therefore,female offspring(but not male)of mothers with hyperandrogenism,such as in polycystic ovary syndrome,have an increased prevalence of autism features than girls born to mothers without hyperandrogenism[91].The effect is higher when polycystic ovary is combined with maternal obesity due to more hyperandrogenism[92].Baron-Cohenetal[93] showed that.Elevated levels of steroidogenic hormones(cortisol,androstenedione,testosterone,17α-hydroxy-progesterone,and progesterone)in amniotic fluid samples are associated with an increased risk of autism due to their effects on early fetal brain development.The difference in sex steroid hormone exposure can explain why autism affects males more than females and can be used as an early biological risk factor marker.Steroid hormones have a substantial impact on the biological sexual differentiation of autism.Perinatal testicular androgen surge is responsible for brain sexual differentiation as prenatal sex hormones affect microglial activation during early neurodevelopment and consequently affect many neurodevelopmental disorders[94].An exciting study by Yauetal[95] showed an inverse association between autism and mid-pregnancy thyroidstimulating hormone levels in maternal serum samples,indicating the critical role of thyroid hormones for in-utero normal brain development.Therefore,the in-utero hormonal environment could increase or alleviate the risk of autism,explaining the male dominance and female protection of autism[96].
Maternal nutritional profile
Metals are essential to neurodevelopment.Essential elements deficiency during specific critical developmental windows could increase autism risk and severity,indicating a state of systemic elemental dysregulation in autism.Arora found that fetal levels of manganese and zinc were reduced in infants who developed autism than their monozygotic or dizygotic twin discordant for autism[97].In addition,Curtinetal[98] studied zinc-copper cycles in a Sweden nationwide study of twins using unique tooth-matrix biomarkers that directly measure fetal elemental uptake.They found an altered fetal and postnatal zinc-copper rhythmicity in autism with shorter cycle duration,reduced regularity,and diminished complexity than in those who did not develop autism.This altered pattern of zinc-copper cycles had a sensitivity between 85% and 100% and a specificity between 90% and 100% for the diagnosis of autism.Some studies showed that maternal vitamin D deficiency in the first or second trimester is associated with an increased risk of autism[99,100].However,a more recent study by Madley-Dowd found no significant association or causation of maternal vitamin D deficiency during pregnancy with an increased risk of autism in the offspring[101].These contradictory results of studies may need more research to confirm or rule out the effects of vitamin D deficiency on the increased risk of autism.Folic acid is an essential nutrient for cell division and rapid tissue growth during fetal development,affecting all the tissues,including the brain[102].In the same way as vitamin D,gestational folic acid has a complex relationship with autism development.There are some contradictory results about the effects of folic acid deficiency during pregnancy and the risk of the development of autism.It is suggested that poor folic acid intake during pregnancy may raise the risk of neurodevelopmental disorders,including autism[103].Steenweg-de Graaffetal[104] found no association of maternal folic acid concentration at 13 wk of gestation with the risk of autism in the offspring.However,they found that maternal antenatal supplementation with folic acid is associated with fewer child autistic traits.On the other hand,Egorovaetal[105] found weak evidence that high maternal serum folate during early pregnancy may increase the risk of autism in offspring.This contradiction of the result may be explained by the differences between the reduced(folinic acid or methyl-tetrahydrofolate)and the synthetic oxidized forms of folic acid.The oxidized form inhibits folate metabolism,while reduced forms do not[106].Because of the conflicting results of different studies,we need to do more research at different gestational periods and with reduced forms of folic acid supplementation.
Biophysical profile
Fetal ultrasound is an outstanding tool for studying abnormal fetal development.It is commonly used to follow up on fetal growth and detect early fetal anomalies throughout pregnancy.Multiple fetal abnormalities(especially cardiac,urinary,cranial,and brain anomalies)are significantly more prevalent in patients with autism,especially in severe cases[107].Fetuses with autism have a narrower head and a relatively broader ocular distance than typically developed fetuses.The more anomalies present,the more severe the autism is.The association between fetal anomalies and autism may be due to underlying genetic and/or environmental factors that cause autism and congenital disabilities,or the birth presence of abnormalities may predispose a child to develop autism[108].The presence of these multi-anomalies may also indicate the abnormal multiorgan embryonic development in patients with autism and suggest the importance of using fetal ultrasonography as a biomarker for autism.Regevetal[109] observed that female patients with autism have significantly more ultrasonography-detected fetal anomalies and a higher prevalence of multiple abnormalities than male patients with autism.
During antenatal life,the brain goes through complex and coordinated differentiation and growth processes,which are critical for typical brain structure and function development.Abnormalities in these processes can cause brain structure and function changes and increase the risk of neurodevelopmental disorders[110].Altered brain growth is often observed in individuals with autism,specifically during the first year of life.Several studies have recognized numerous abnormalities in different brain regions and structures in individuals with autism,particularly the cortex,amygdala,and cerebellum[22].Blankenetal[111] also reported this abnormal brain growth,who prospectively examined the altered fetal brain growth trajectories by examining prenatal head growth in babies who further developed autism.They compared them with typically developed children and related this finding to the severity of autism symptoms.They studied 3,820 children from two population groups:The Netherlands and Australia;60 children developed autism.They used latent growth curve models to examine the relationship between fetal head circumference measured by fetal ultrasound at three different time points(at first,second,and third trimesters)and autistic features measured in postnatal life using the Social Responsiveness Scale or the Autism-Spectrum Quotient.They found a weak association between lower initial prenatal head circumference and increasing autistic traits in the Dutch cohort but not in the Australian cohort,nor when the two cohorts were analyzed together.These mixed findings suggest that we need more research on this point.Altered prenatal brain growth seems to be a significant risk factor for autism.Although the exact mechanisms underlying this association are still not fully realized,studies suggest that neural circuit development and connectivity changes may have a role[112].
Fetal ultrasound can provide useful information about fetal motor activity,heart rate variability,and other behavioral markers.Many studies have suggested that there may be some differences in fetal neurodevelopmental behavior between children who later have typical development and those who develop autism[113].Fetal ultrasound is also used to assess fetal neurodevelopmental behavior,which could predict the neuronal outcome of the baby.Hataetal[114] used antenatal four-dimensional ultrasound to assess fetal neurodevelopment using Kurjak's antenatal neurodevelopmental scoring test between 28 and 38 wk of gestation.A low score between 0 and 5 is abnormal and denotes a strong possibility of postnatal developmental disabilities,including autism.Conversely,a score between 6 and 9 is borderline,and a score between 10 and 16 is considered normal.They suggested that the KANET assessment may be a helpful diagnostic tool to predict postnatal developmental disabilities.However,many confounding factors could affect the fetus’s behavior,such as maternal stress or medication use and other environmental factors that could affect the fetus and consequently affect the scoring.These factors make it challenging to determine whether fetal behavior differences accurately indicate the increased risk of autism.
Radiological profile
Antenatal magnetic resonance imaging magnetic resonance imaging(MRI)brain is a diagnostic method that can be used to assess the fetal brain during pregnancy and might be applied as a potential tool to identify the risk of autism in infants.Recently,there has been a growing interest in the relationship between fetal MRI brain and the risk of having autism in childhood[115].However,studies brought mixed results.Kyriakopoulouetal.investigated the presence of autism in twenty-four children [20 males/4 females] with isolated ventriculomegaly and altered cortical development by fetal MRI brain,compared with ten controls.Children with ventriculomegaly were more liable to have difficulties in sustained attention,working memory,and sensation-seeking behaviors and were more likely to have autistic features[116].In addition,a group of scientists from Harvard University and Boston Children's Hospital,United States,examined MRI scans of thirty-nine fetuses with an average gestational age of 25 wk.They noted two enlarged brain areas in fetuses who developed autism in infancy and childhood.Babies who developed autism had an enlarged insula lobe(seen as early as 25 wk).Insula is linked to sensory stimuli processing,socialization,and decision-making processes.It appeared more prominent than the typically developed fetuses.They also showed increased amygdala volume.The amygdala is also responsible,among other brain areas,for emotional processing and interpretation of the facial expressions of others[117].This observed hypertrophism was previously discovered a few years ago in school-age children with autism[118].
On the other hand,a study from the University of North Carolina,United States,showed that the amygdala was normal in babies who developed autism up to 6 mo of age,then began to enlarge between 6 and 12 mo before the appearance of the clinical manifestations of autism,and continue to grow to 12-24 mo,the age at which the first clinical manifestations of autism including interpersonal difficulties usually appear.There is a positive correlation between the Amygdala growth rate and the severity of autism.The faster the growth rate,the worse the autism manifestations of the condition[119].We should consider the safety of fetal MRI as it may induce maternal stress,high acoustic noise,and induction of preterm labor.However,the benefits of fetal MRI mostly outweigh the risks,mainly when experienced radiographer technicians perform the procedure.Table 2 summarises the various antenatal markers of autism.
Neonatal and early infancy markers for the risks of autism
Early identification of autism allows the timely launching of targeted therapies,which can help optimize different cognitive functions impaired in autism.Different studies tried to identify autism early in newborns and early infancy,depending on different physical,behavioral,biochemical,and imaging markers(Table 3).

Table 3 Neonatal and early infancy markers for the risks of autism
Neonatal physical and behavioural profile of autism
Even though clinicians are identifying autism earlier than before,some children still have a delayed diagnosis till the age of six years.Differences in screening tools and diagnostic abilities in various clinical settings can affect the timing of autism diagnosis.A neonatal physical and behavioral profile of autism refers to the physical and behavioral characteristics identified in infants who later develop autism.However,we should emphasize that there is no one standard set of features that could predict the development of autism.Some patterns have emerged and are associated with an increased risk of autism[120].
Many studies focused on studying the motor and behavioral response changes that could be observed during the first year of life and their relation to later diagnosis of autism.Physically,neonates with future diagnoses of autism may have large or abnormal head sizes at birth and throughout early childhood,which may reflect early brain overgrowth in children with autism[121].However,in their meta-analysis,Crucittietal[122] showed age and sex differences in the head circumference of neonates and infants who developed autism.They found that girls with autism aged 12-17 mo had smaller head sizes,while boys were more likely to have significant head size differences at birth and between 60 and 100 mo,being small between 6 and 11 mo and large between 12 and 17 mo.They also found that average head size was not atypical finding in autism.Moreover,girls were more likely to have significant head size differences between 36 and 59 mo and were less likely at birth.
They may also have hypertelorism(Increased intra-ocular distance),anteriorly rotated ears,long nose back,abnormal mouth shape,and facial asymmetries[123].Gorczycaetal[124] found a statistically non-significant connection between the degree of dysmorphia and the presence of some physical disorders in first-degree relatives.In addition,some studies reported that infants who develop autism later in childhood tend to have longer birth body lengths than typically developed infants[125].This finding may be related to genetic or environmental effects on fetal growth and development.
Some studies have reported that infants who later develop autism may show subtle changes in the neonatal period before the onset of more overt symptoms.Therefore,it could be challenging to be recognized.They may have abnormalities in motor development during the first year of life.For example,by the age of one month,they may have arm tone deficits and asymmetric visual tracking.They may also have hypotonia,hyperreflexia,poor movement quality,head lag,delayed or missing major motor development,and delayed milestones,such as sitting or crawling,or prefer using one hand over the other[126,127].Children with autism have lower heart rate variability,as shown by Loryetal[128],who found significantly lower tonic heart rate variability in children with autism than in children with typical development.Reducing noradrenergic activity could enhance and improve different aspects of network processing and thus improve cognitive abilities,such as verbal problem-solving,in children with autism[129].Moreover,the presence of reduced heart rate variability could predict the response to propranolol therapy in improving cognitive functions in children with autism[130].In addition,Nyströmetal[131] found that the presence of enhanced pupillary light reflex during infancy is associated with a greater risk of developing autism in toddlerhood,which may indicate the important role of sensory atypicality in the pathogenesis of autism.They also are more liable for sleep disturbances and gastrointestinal symptoms,such as constipation,diarrhea,or gastroesophageal reflux,in the neonatal period than typically developing infants[132,133].
As abnormal motor behaviors are a very characteristic feature of autism,movement analysis during early infancy may be a valuable tool for the early diagnosis of autism.However,autism-associated movement disorders vary from child to child,with disturbances in some or all of the development milestones,including lying,righting,sitting,crawling,and walking[134].Disturbances of movement could be identified obviously at the age of 4-6 mo and occasionally even at birth,using the Eshkol-Wachman Movement Analysis System using still-frame videodisc analysis.Movement disturbances represent an intrinsic part of autism that could present at birth.It can be used to diagnose the presence of autism even during the first few months of life[135].
Infants later diagnosed with autism may demonstrate early deficits in social behavior,specifically in joint attention,eye contact,orienting to names,facial expressions,social smiles,attention,and tolerance of social touch.They may exhibit atypical sensory processing,with hypersensitivity or hyposensitivity to touch,sounds,or visual stimuli,representing manifestations of atypical sensory processing.They dislike being touched or cuddled,constantly lie in the bassinet,and cry when held up.Infants who later develop autism may also show differences in eye gaze compared to typically developing infants.They may avoid eye contact or have difficulty following a person's gaze when directing the infant's attention to something[136-138].They also do not look at the face and try to avoid eye contact even during nursing,which may appear too early by the age of two weeks.They may have reduced visual attention to social stimulation and impaired orienting to novel stimuli at the age of two months[139].They may also show differences in social responsiveness,such as reduced interest in social interaction or decreased responsiveness to social cues.They also lie on one of the extremes of having very low or very high needs,e.g.,needing to be held constantly or avoiding touching.They also may lack proper response to sounds after rolling out hearing problems[140,141].These early signs could be hard to detect and are not always apparent in all infants who will develop autism.Still,they may be crucial and helpful for early autism identification and intervention.
Prematurity is a significant risk factor for autism.Wongetal[142] showed that premature babies below 30 wk of gestation had more significant social-communication problems and autistic behavior in early childhood than seen in fullterm babies as assessed by the Quantitative Checklist for Autism in Toddlers(Q-CHAT).Less attention maturation observed in preterm babies can negatively impact how long they can stay actively interested in social interaction.Eye gaze is an indisputable means of communication.Preterm babies are different from full term in many behavioral aspects.They typically avert their gaze more often and for longer periods in early social interactions than full-term infants due to less optimal attention maturation in preterm children[143].Therefore,preterm babies who demonstrated gaze aversion and endpoint nystagmus had better language scores on the Bayley-III.On the other hand,Pinedaetal[144] found that preterm babies with absent gaze aversion and absent endpoint nystagmus during the neonatal period showed positive screening for autism using Modified Checklist for Autism in Toddlers(M-CHAT)at the age of two years.
In neonates,a fascinating Japanese study by Tokunagaetal[145] prospectively demonstrated the relationship between behavioral features during the neonatal period and social behavior and sensory disorders at 18 mo to early detect and intervene in children with autism.They studied apparently healthy 105 full-term neonates for the behavioral features using the Neonatal Behavioral Assessment Scale(NBAS)between 2 and 7 d after birth.Then,they were re-assessed at 18 mo of age using the Japanese version of M-CHAT(M-CHAT-JV)and the Infant/Toddler Sensory Profile(ITSP).They found that 15.2% of the infants were M-CHAT-JV-positive,with significant differences between the M-CHAT-JV-positive and M-CHAT-JV-negative groups in two of the NBAS clusters:motor and orientation.They also found a significant negative correlation between the NBAS orientation cluster and the "low registration" and "auditory processing" sections in the ITSP.In addition,they found a negative correlation between the NBAS motor cluster and the "sensation avoiding" and "tactile processing" sections in ITSP.Logistic regression analysis also showed a significant association between the NBAS orientation cluster and ITSP low registration with the M-CHAT-JV at 18 mo.Their results suggested a significant relationship between the NBAS orientation cluster in full-term neonates and the social behavior and sensory features at 18 mo[145].These findings imply the need to develop therapeutic methods that can be applied from the first few months of life to manage autism.
Neonatal immunological profile of autism
As previously mentioned,prenatal and/or postnatal exposure to anti-brain antibodies plays an important pathoplastic role in autism as they enhance autism severity by damaging cognitive processes and adaptive functioning,boosting motor stereotypies,altering the sleep/wake cycle,and delaying or halting neurodevelopment,primarily verbal and nonverbal language.Some mothers of children with autism(about 7%)had antibodies to 39kDa and 73kDa proteins during gestation.Detection of antibodies against both 39kDa and 73kDa proteins during mid-gestation predicts early and severe autism in the offspring[81].Anti-brain antibodies can be used as biomarkers predicting autism severity and clinical features and possibly providing new avenues for preventive and therapeutic strategies,including predicting the response to treatment.A systematic review and meta-analysis by Rossignoletal[146] showed abnormalities in the total IgG and IgG 4 subclass concentrations related to social impairments and aberrant behavior.They also studied the immunomodulatory effects of intravenous immunoglobulins in children with autism.Using intravenous immunoglobulins showed clinical improvements in irritability,hyperactivity,cognition,attention,communication,social interaction,eye contact,speech,response to commands,echolalia,drowsiness,and decreased activity.In some cases,the early use of intravenous immunoglobulins led to the complete resolution of autism symptoms.The presence of anti-dopamine D2L receptors and anti-tubulin autoantibodies and the ratio of the anti-dopamine D2L to D1 receptor antibodies can predict the treatment response to intravenous immunoglobulin.These antibodies can serve as a marker to predict patients who may respond better to intravenous immunoglobulin therapy[147].
Another interesting finding is the high prevalence of folate receptor-alpha autoantibodies in children with autism(71%)compared to children with typical development or children with developmental disabilities but not with their siblings[148].These antibodies induce cerebral folate deficiency and consequently induce autism symptoms.Consequently,the presence of folate receptor-alpha autoantibodies in infancy may help to predict the risk of autism as well as the response to leucovorin(folinic acid)treatment[149].Another crucial immunological marker of autism in infants and children is immunoglobulin A(Ig A)in the stool.Ig A is the main immunoglobulin secreted by the gastrointestinal immune cells.Zhouetal[150] found higher stool Ig A levels in children with autism than in typically developed children,suggesting the presence of gut immune abnormalities in patients with autism and can explain the gene-environmental interaction in the pathogenesis of autism.It also suggests the possible use of stool Ig A as a possible marker of autism.
Neonatal and infancy inflammatory profile of autism
As previously mentioned,the immune activation and signaling pathways can impact the child’s neurodevelopment and may contribute to the pathogenesis of autism.However,this effect varies considerably with the genetic background and many perinatal environmental factors[151].High neonatal C-reactive protein levels are consistently associated with an increased risk of autism compared to the controls.On the other hand,decreased levels of α-2-macroglobulin,ferritin,and serum amyloid P are associated with increased autism risk in the matched sibling comparison.The changes observed in these acute-phase reactant proteins are indications of maternal immune activation,which in turn increases the risk of autism[152].In addition,a meta-analysis by Masietal[153] showed that elevated levels of IL-1β,IL-6,IL-8,interferongamma,eotaxin,and monocyte chemotactic protein-1,and lower levels of transforming growth factor-β1 in children who have autism than healthy control.Another study by Krakowiaketal[154] showed that high neonatal levels of IL-1β are associated with an increased risk of mild to moderate autism.Furthermore,high neonatal levels of IL-4 are independently associated with an increased risk of severe autism.However,there is no single cytokine abnormality can be used as a marker of increased risk of autism.We need more extensive prospective studies to investigate the abnormal cytokine profiles that could be strongly associated with autism at different stages of development.
Neonatal and infancy biochemical and metabolic profile of autism
Brain-derived neurotrophic factor(BDNF)is essential for neuronal survival and growth.It serves as a neurotransmitter modulator and contributes to neuronal plasticity,which is critical for learning and memory[155].A Meta-analysis by Liuetal[156] found that the blood levels of BDNF were lower in neonates who later developed autism than in children with typical neurodevelopment.However,contradictory results with previous studies warrant more studies to facilitate a more robust conclusion[157].There is substantial evidence for the possible association between neonatal hyperbilirubinemia and the later risk of developing autism.The risk increases in the presence of prematurity,severe jaundice,insufficient milk intake,phototherapy,and dark-skinned babies due to a heightened risk of missed diagnosis,delayed care access,and poorer settings[158,159].
Neonatal and infancy hormonal profile
Besides the antenatal effects of sex and steroid hormones on brain development and the risk of autism,other hormones could also play a role.Vasopressin,a social neuropeptide arginine,is found to be significantly lower in the cerebrospinal fluid(CSF)of children with autism than in healthy controls.Oztanetal[160] studied CSF samples from 913 febrile infants between 0-3 mo as a part of their medical care.They stored the collected samples at-70°C.Eleven children out of 913 developed manifestations of autism.They compared the vasopressin concentration in the CSF samples obtained from these eleven children with 22 children with typical neurodevelopment(Ratio 1:2).They found a significant reduction of vasopressin concentration in the neonatal CSF samples from children who developed autism compared to those with typical development,with the highest accuracy when patients who had comorbid attention-deficit/hyperactivity disorder with autism were removed from the analysis.These findings suggest the beneficial use of CSF vasopressin levels as an early marker for autism in neonates with a high risk of developing autism and in behaviourally symptomatic infants.In addition,Zhangetal[161] found lower plasma levels of vasopressin in mothers of children with autism than in mothers of typically developed children.They also found that children with autism with higher vasopressin levels are less likely to have repetitive behavior.They also found that children with higher plasma oxytocin levels have less verbal communication impairment[161].Moreover,Pichuginaetal[162] found lower salivary oxytocin levels in children with autism and intellectual disabilities than in children with Intellectual disabilities without autism.They also found a direct negative correlation between salivary oxytocin levels and the severity of autism.Another study by Gottliebetal[163] showed that the severity of autism is inversely proportional to the normalized number of oxytocin receptors,indicating the significant role of the oxytocin receptor number in the severity of autism.It also suggests the potential use of vasopressin and promising therapeutic tool to improve social cognition in children with autism[164].
Neonatal brainstem function
Only the lower parts of the nervous system(the brain stem and spinal cord)are very well developed by birth.In contrast,the higher regions(the limbic system and cerebral cortex)are still relatively primitive.Therefore,the lower brain mainly controls a newborn’s behavior.Therefore,kicking,grasping,rooting,crying,sleeping,and feeding are mainly functions of the brain stem and spinal cord[165].The brainstem has a revolutionary role in developing humans’ social development.Therefore,the integrity of brainstem sensory information transmission during the final weeks of gestation and the early neonatal period may support the development of social engagement[166].
Cohenetal[167] evaluated the contribution of initially abnormal neonatal auditory brainstem responses(ABRs)and 4-month arousal-modulated attention visual preference to later autism behaviors in neonatal intensive care unit graduates.They compared NICU graduates with normal ABRs(n= 28)to those with initially abnormal ABRs(n= 46)that later resolved.At four months post-term age,visual preference(measured after feeding)for a random check pattern flashing at 1,3,or 8 Hz and gestational age served as additional predictors.Outcome measures were Pervasive Developmental Disorder Behavior Inventory(PDDBI)scores at 3.4 years and developmental quotients(DQ)obtained around the same age with the Griffiths Mental Development Scales(GMDS).They found that the preferences for higher stimulation rates at four months were highly correlated with PDDBI scores and the GMDS Hearing and Speech DQ,but only in those with initially abnormal ABRs.The effects were most potent for a PDDBI social competence measure most associated with a diagnosis of autism.For those with abnormal ABRs,increases in preference for higher stimulation rates as infants were linked to nonlinear increases in the severity of autism at three years and an autism diagnosis.Therefore,abnormal ABRs were associated with later reports of repetitive and ritualistic behaviors irrespective of a 4-mo preference for stimulation.The common occurrence of initially abnormal neonatal ABRs and preference for more stimulation at four months,both indices of early brainstem dysfunction,may be a marker for the development of autism in this cohort[167].
Challenges to early detection of autism
Several reasons make early detection of autism a real challenge.Lack of parental,family,caregivers,or even healthcare providers' awareness about the disorder is one of the main challenges that enface early detection of autism.Despite rising awareness of autism,many people are not familiar with the symptoms and signs of autism,resulting in delayed diagnosis and treatment.Another critical challenge is the variability in symptoms[136].Autism is a complex and common neurodevelopmental disorder that affects children differently,with various symptoms and severity levels.Symptoms may also vary depending on the child's age,gender,and cultural background.This observation is particularly evident in young infants and children under 24 mo of age who may not yet display many characteristic symptoms[168].In addition,the lack of specific biological markers or diagnostic tests for a definitive diagnosis of autism is another real challenge(Table 4).
Furthermore,the diagnostic criteria for autism are changing over time,and experts continuously debate about what establishes a diagnosis of autism.Diagnosis is typically based on behavioral observations and evaluations,and the screening tools are not always accurate.These diagnostic tools are very subjective and vary between clinicians.All these together can result in false positives or negatives,leading to overdiagnosis,underdiagnosis,misdiagnosis,or delayed diagnosis[169].Moreover,autism frequently occurs with many neurological,psychological,and physical co-morbidities and overlaps with many other conditions,such as attention deficit hyperactivity disorder,intellectual disability,and language disorders,making it hard to differentiate between them[170].
Differences in cultural and linguistic backgrounds harden the abilities of healthcare providers to recognize early signs of autism,remarkably in minority or immigrant populations with significant cultural and linguistic barriers.Healthcare providers may not be familiar with cultural customs,norms,and practices that can impact the presentation of symptoms[171].Even with the increased awareness about autism,many families still suffer from the stigma and fear surrounding autism,making it difficult for parents and caregivers to ask for help for their children.This stigma may prevent early diagnosis and intervention,significantly impacting children's development,well-being,and long-term outcomes of children with autism[172].In addition to all these obstacles,limited funding may prevent the early detection and treatment of children with autism.Early detection and intervention for autism require resources and funding,which may be limited in some areas or for some families[173].
In addition,screening tools,such as the Modified Checklist for Autism in Toddlers(M-CHAT),which are used to identify children who may be at risk for autism,are not always readily available,and many pediatricians and healthcare providers may not be familiar with them,particularly in rural or low-income areas.These limited financial and expert resources can make it challenging to obtain a timely diagnosis of autism,resulting in delayed treatment and poorer outcomes for children with autism[174].Handling these challenges requires raising awareness,improving access to diagnostic tools,dealing with culturally sensitive concepts,and defining straightforward approaches to screening and diagnosis.Early recognition and management are critical for improving outcomes for children with autism.It is crucial to continue addressing these challenges to enhance the quality of life of children with autism and their families[175].
Limitations of the study
As autism is a multi-dimensional disorder,various techniques and approaches have been used to develop different biomarkers to diagnose autism.However,only some biomarkers underwent validation studies.In addition,many other potential diagnostic biomarkers underwent just preliminary investigation and still need further optimization.Moreover,many of the included studies were based on questionnaires depending on the parents or the caregiver’s memories and subjective judgment,therefore carrying the risk of memory effects bias such as rosy retrospection,egocentric bias,and cross-race effect,and consequently affecting the accuracy of the results.In addition to the memory bias,many studies used typically developing non-sibling children as controls and did not include typically developed siblings or children with developmental delays without autism.These two types of controls are clinically ideal and relevant as a comparison population as they share many risk factors with the children who developed autism and consequently improve the biomarkers’ sensitivity,specificity,and predictive values.One more limitation of the suggested biomarkers is the stage of measurement.Many of the suggested biomarkers were measured after the child was diagnosed with autism,therefore missing the early detection phase and may not be accurate or sensitive enough if used early.
CONCLUSION
Autism is a complex neurodevelopmental disorder,typically diagnosed during the first three years.Of life with a spectrum of symptoms ranging from mild to severe and varies from one child to another.Pre-autism refers to the early stage of autism and the signs and symptoms that appear during the developmental stages of a child that may indicate a potential risk for developing autism later in life.Early detection and intervention are crucial for improving outcomes for individuals with autism.Various physical,biochemical,hormonal,and imaging biomarkers are developed to assist in prenatal and early-life diagnosis of autism.However,every biomarker has its accuracy and limitations.To improve early detection of autism,we should try to overcome the various challenges that enface the diagnosis,such as improving the community awareness of early signs of autism,easing the access to diagnostic tools,trying to remove the stigma attached to the diagnosis of autism,and dealing with the different culturally sensitive concepts related to it.In addition,the scientific community must work hard to replicate studies with more different targeted populations,perform more randomized controlled studies with larger numbers of participants,and define straightforward and accessible gold standard screening and diagnostic approaches to detect autism early among newly developing young infants and children.We also should consider that autism is a heterogeneous spectrum with different presentations,symptomatology,and severity.Therefore,we may need to develop a group of biomarkers and diagnostic tools that could fit the various conditions we may face,aiming to provide optimized and individualized types of treatment.Therefore,we can say that we have just started our first step in screening autism.However,the journey is too long to achieve the aim.
ACKNOWLEDGEMENTS
We thank the anonymous referees and editors for their valuable suggestions.
FOOTNOTES
Author contributions:Al-Biltagi M collected the data,wrote,and revised the manuscript;The guidelines of the PRISMA 2009 were adopted while writing the manuscript.
Conflict-of-interest statement:Authors declare no conflict of interests for this article.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial(CC BY-NC 4.0)license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See:https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Egypt
ORCID number:Mohammed Al-Beltagi 0000-0002-7761-9536.
Corresponding Author's Membership in Professional Societies:Faculty of Medicine,Tanta University,Egypt;University Medical Center,King Abdulla Medical City.Arabian Gulf University.
S-Editor:Liu JH
L-Editor:A
P-Editor:Zhang XD
杂志排行
World Journal of Clinical Pediatrics的其它文章
- Situs inversus totalis in an asymptomatic adolescent-importance of patient education:A case report
- Comments by opponents on the British Medical Association’s guidance on non-therapeutic male circumcision of children seem one-sided and may undermine public health
- Prediabetes in children and adolescents:An updated review
- Renal calcification in children with renal tubular acidosis:What a paediatrician should know
- Brain metabolic profile assessed by magnetic resonance spectroscopy in children with Down syndrome:Relation to intelligence quotient
- Clinical factors predicting rotavirus diarrhea in children:A crosssectional study from two hospitals
