Effectiveness of an amino acid beverage formulation in diarrheapredominant irritable bowel syndrome: A pragmatic real-world study
2023-12-21SamanthaNilesPhilBlazySamuelCheuvrontRobertKenefickSadasivanVidyasagarAdamSmithNeilFawkesWilliamDenman
Samantha E Niles,Phil Blazy,Samuel N Cheuvront,Robert W Kenefick,Sadasivan Vidyasagar,Adam B Smith,Neil Fawkes,William Denman
Abstract BACKGROUND Amino-acid based medical foods have shown promise in alleviating symptoms of drug induced gastrointestinal side effects;particularly,diarrhea-predominant symptoms.Irritable bowel syndrome (IBS) is a gastrointestinal disorder that affects up to 9% of people globally,with diarrhea predominant IBS (IBS-D) being the most prevalent subtype.Further trials are needed to explore potential added benefits when integrated into standard care for IBS-D.AIM To assess the effectiveness of an amino acid-based medical food as an adjunct to standard of care for adults with IBS-D.METHODS This is a pragmatic,real world,open label,single arm study comparing a 2-week baseline assessment to a 2-week intervention period.One hundred adults,aged 18 to 65 years,with IBS-D,according to Rome IV criteria,were enrolled after completing a 2-week baseline assessment period and received a 2-week supply of an amino acid based medical food which was consumed at home twice daily on top of their standard of care.The primary outcome was an assessment of tolerability after 2-weeks of consumption,while secondary outcomes included changes in stool consistency (Bristol Stool Form Scale),severity of abdominal pain &discomfort,symptoms of urgency,Global Improvement Survey (GIS),and the IBS severity scoring system (IBS-SSS).RESULTS The test product was well-tolerated as each participant successfully completed the full 14-day trial,and there were no instances of dropouts or discontinuation of the study product reported.Forty percent of participants achieved a 50% or more reduction in the number of days with type 6-7 bowel movements (IBS-D stool consistency responders).Fifty-three percent of participants achieved a clinically meaningful reduction of 30% in mean weekly pain scores,and 55% experienced the same for mean weekly discomfort scores (IBS-D pain and discomfort responders).Participants experienced a mean -109.4 (95% confidence interval: -130.1,-88.8) point reduction on the IBS-SSS and 52% experienced a minimally clinically important difference of >95 points.An IBS-SSS category shift from severe to moderate or mild occurred in 69% of participants. For functional symptoms,76% of participants reported symptom relief on the GIS.CONCLUSION The amino acid-based medical food was well-tolerated,when added to the standard of care,and demonstrated improvements in both overall IBS symptom severity and IBS-D symptoms within just 2 wk.
Key Words: Diarrhea-predominant irritable bowel syndrome;Amino acid beverage formulation;Pragmatic real-world study;Medical food;Bristol Stool Form Scale;Irritable Bowel Syndrome– Severity Scoring System
INTRODUCTION
Irritable bowel syndrome (IBS) is a chronic and debilitating gastrointestinal disorder with a population prevalence of 4%-5% in North America and 4%-9% globally[1,2].Healthcare professionals employ the Rome IV criteria for diagnosis and treatment decisions.As defined by Rome IV criteria,the predominant diagnosis for IBS is the presence of recurrent abdominal pain or discomfort in conjunction with changes in bowel habits[3].IBS is further categorized based on the primary stool pattern,with 40% of IBS patients experiencing changes in stool frequency and consistency equating to diarrhea,thus making diarrhea predominant IBS (IBS-D) the most prevalent subtype of the syndrome[4] and one of the most common causes of chronic diarrhea within the United States (est.5% general population)[5].International gastrointestinal societies characterize IBS-D for patients who experience >25% of bowel movements having Bristol[6] stool types of 6 or 7 (watery) and <25% of Bristol stool types of 1 or 2 (hard)[7].While the pathophysiology of diarrhea in IBSD is not precisely understood,the frequent passing of loose or liquid stool can be attributed to some imbalance in the normal function of the small and large intestine responsible for the secretion and absorption of various substrates,ions,and consequently water[5,8].
The management of patients with IBS is tailored to the individual and a holistic approach is recommended that can include dietary,lifestyle,medical and behavioral interventions[9].While diet modifications such as low fermentable oligosaccharides,disaccharides,monosaccharides,and polyols (FODMAP) diets are recommended to help alleviate global symptoms[10],strict food avoidance can become unsustainable for some patients and can also compound malabsorption nutrient deficits[11,12].More specifically,these dietary recommendations do not address water and electrolyte losses that result from diarrhea suffered by IBS-D patients[13,14].The correction and replacement of these nutrients often go overlooked,with the mainstay of treatment strategy focusing instead on treatments to reduce diarrhea[15].
Providing nutritional support for other conditions where patients suffer from recurrent watery diarrhea has been demonstrated to be a successful approach.For example,an amino acid based medical food is being used by patients undergoing cancer treatments who suffer from prolonged watery diarrhea associated with radiation and chemotherapy.The effects of chemotherapy on the small intestine include nutrient malabsorption,hypersecretion (diarrhea),and increased intestinal permeability[16,17].Select amino acid formulations have been shown to reduce secretions and improve nutrient absorption in animals[18].
The present study investigated a medical food which contains a select amino acid blend,in combination with ingredients designed specifically to support IBS-D nutritional deficits occurring with diarrhea[11,12].The objective was to assess the tolerability of the product when added to the standard of care in adults with IBS-D,whilst also measuring any additional benefits conferred to functional gastrointestinal symptoms in a pragmatic real-world setting.
MATERIALS AND METHODS
Study population
The study population consisted of adults between the ages of 18 and 65 years,residing across the United States,who had received a confirmed diagnosis of IBS-D from a qualified physician.Recruitment for this study was conducted through a decentralized approach,leveraging virtual platforms and online resources to reach and engage eligible participants nationwide.To confirm adherence to the Rome IV criteria,participants underwent a 2-week run-in period during which they utilized an electronic diary each day to record their predominant stool type on the Bristol Stool Form Scale (BSFS)[6]and the severity of abdominal pain/discomfort using numeric rating scales graded on an 11-point scale.Eligibility for the study was determined by specific criteria: participants had to experience diarrhea,defined as stool types 6 and 7 on the BSFS,occurring at least four times weekly and constituting the predominant (>25%) stool pattern.Additionally,they were required to report abdominal pain and/or discomfort occurring at least once a week and possess a Functional Bowel Disorder Severity Index (FBDSI) score exceeding 60[19].Throughout their participation in the trial,eligible participants were allowed to maintain their current therapeutic regimens,supplements,and dietary practices.
Exclusion criteria encompassed a diagnosis of IBS-C or unclassified IBS according to the Rome IV criteria,as well as diagnoses of other gastrointestinal disorders such as Crohn's disease,colitis,and celiac disease.Women who were pregnant or breastfeeding were ineligible for participation.Additionally,patients with known allergies to the components of the test product or those anticipating modifications to their regular medications that might impact bowel habits were excluded from the study.
Study design
The clinical study was conducted in a fully decentralized manner within the United States,adhering to the principles outlined in the International Council on Harmonization E6 Good Clinical Practice,and in compliance with local regulatory requirements.The study was approved and monitored by an independent institutional review board (Advarra Institutional Review Board,Columbia,MD).
This is a pragmatic real-world study,conducted in an open-label,single-arm design,comparing a 2-week baseline assessment to a subsequent 2-week intervention period.The study was administered through the Laina Clinical Research Platform.The study population was recruitedviavirtual channels,including online advertisements,and in partnership with the Walmart Health Research Institute,which facilitated the validation of participants' health records and physician diagnoses after obtaining informed consent.Eligible participants underwent a 2-week run-in period to assess baseline parameters,thereby informing their inclusion in the study.Data were collected electronically through a mobile phone application with participants engaged in completing daily electronic diaries.
Participants enrolled into the intervention trial period received sixteen 16-ounce bottles of enterade®IBS-D and were instructed to drink 8-ounces once in the morning (30 min prior to eating) and 8-ounces once in the evening (30 min prior to eating) for 14 d.Each day during the intervention period,participants filled out daily symptom diaries with outcome measures described below.Additionally,validated surveys from the Rome Foundation were used to monitor patient reported IBS-D associated symptoms,including the IBS-Severity Scoring System (IBS-SSS)[20] and the Global Improvement Survey (GIS)[21].Participants filled out the IBS-SSS on days 0 and 14,and the GIS on day 14 as a final questionnaire about the overall study experience.Day 0 was the last day (day 14) of the run-in period,whereas day 14 was the last day of the intervention period.
Study product
The amino acid based medical food beverage used in this study was enterade®IBS-D,which contains a patented blend of plant-based amino acids (valine,aspartic acid,serine,threonine,tyrosine),electrolytes (sodium,potassium,chloride),vitamins (ascorbic acid),and minerals (zinc,copper,calcium) carefully selected to help replenish the loss of water,electrolytes and other nutrients that occur secondary to frequent diarrhea and help to support and maintain normal digestive function in IBS-D.The liquid product (mixed berry lot IBSD-0001;orange lot IBSD-0002) is specially manufactured and processed in compliance with medical food regulations in the United States and designed to be consumed on a partial basis in patients exclusively with the specific medical condition of IBS-D.It is for the partial oral feeding and hydration of an individual with IBS-D and is formulated based upon research demonstrating the unique nutritional needs and requirements of those with the disease,including hydration,select vitamins,minerals and electrolytes which differ from the nutritional needs of healthy normal individuals.The proper use of the product as part of the management of the disease incorporated in the medical nutrition therapy,is by the guidance of the health care practitioner.
Outcome measures
The primary outcome,measure of tolerability,was assessed by the number of Adverse Events (AEs) spontaneously reported by participants for the duration of the study.Any event occurring after obtaining participant consent was recorded as an AE,which was defined as any unfavorable and unintended diagnosis,symptom,sign,syndrome,or disease which either occurred during the study,having been absent at baseline,or if present at baseline,appeared to worsen.All AEs were recorded according to the common terminology criteria for AEs (CTCAE Version 6.0),regardless of their relationship to the study intervention.
The Food and Drug Administration emphasizes the difference between statistical significance and clinical relevance in their IBS guidance for industry[22].While our product is a medical food,where relevant we examined potential product benefits for secondary outcomes considering clinically based rules,including ‘responder’ analyses[22] and minimally clinically important differences (MCID)[23].Stool consistency was assessedviathe BSFS,a validated ordinal scale of stool types ranging from 1 through 7,with types 6-7 being indicative of diarrhea.BSFS responders were defined as those with a 50% decrease in the number of days per week with at least one type 6-7 bowel movement relative to baseline which meets the level of a “responder”.Baseline is defined here as the 2-week run-in period.The number of days were included for the baseline across both weeks of the run-in,and a similar approach was taken for week 2 of the trial period.
Abdominal pain and discomfort severity was assessedviaan 11-point numeric rating scale (APS-NRS)[23],with 10 representing most severe pain and 0 representing no pain.Pain responders and discomfort responders were defined as those participants recording a 30% decrease in,respectively,the mean weekly pain and discomfort score on the NRS.The average pain/discomfort was calculated across the 2-week run-in period and compared to the average pain/discomfort scores in week 2 of the trial period.
Urgency or severity of urgency was recorded on a 4-point Likert scale from 0 (None) to 4 (Severe urgency).Improvement and/or worsening on the urgency scale was defined as the difference between the maximum urgency score at baseline (2-week run-up) and during the trial period.Category changes were derived (maximum urgency trial period minus maximum urgency at baseline) and designated as follows: “Significant improvement/worsening”,a 3-category change;“moderate improvement/worsening”,2-category change;“improvement/worsening”,and 0,"No change”.
The change in IBS symptom severity,assessedviathe IBS-SSS total score[20] was assessed from baseline to day 14.Scores on the IBS-SSS range from 0 to 500,with five domains related to abdominal pain severity and frequency,abdominal distention,dissatisfaction with bowel habits,and interference with quality of life.A decrease of 95 points has been associated with a clinically meaningful improvement[23] and was used for classification of IBS-SSS responders.
Statistical analyses
Study data are summarized using means (SD).The denominator to determine the percentage of participants in each category is based on the number of participants with complete paired data unless otherwise noted.A descriptive analysis was used for the primary,secondary,and exploratory outcomes.The IBS-SSS efficacy analysis evaluated the changes (day 0vsday 14) using a pairedt-test.All statistical analyses were performed using R package 1.3-0 or GraphPad Prism v 9.4.0.
RESULTS
A total of 987 screened patients who participated in the 2-week run were assessed for eligibility and 100 participants were enrolled into the study intervention period (Figure 1).Enrollment and intervention occurred continuously from October 2022 through May 2023.All 100 participants who participated in the 2-week intervention period completed the study.

Figure 1 Participant flow chart.
Baseline characteristics are presented in Table 1.The mean age of participants were 39.7 (SD 10.6),with 67% being female.All patients met the ROME IV criteria for IBS-D and had baseline mean IBS-SSS and FBDSI scores of 314 (77) and 195 (52.6) respectively.The average BSFS and APS-NRS scores were 6 (0.1) and 6 (0.2) respectively.The average IBS-SSS,BSFS and APS-NRS scores indicate moderate to severe IBS-D symptomology at study outset.A summary of the standard of care therapies is provided in Table 2.

Table 1 Baseline socio-demographic details
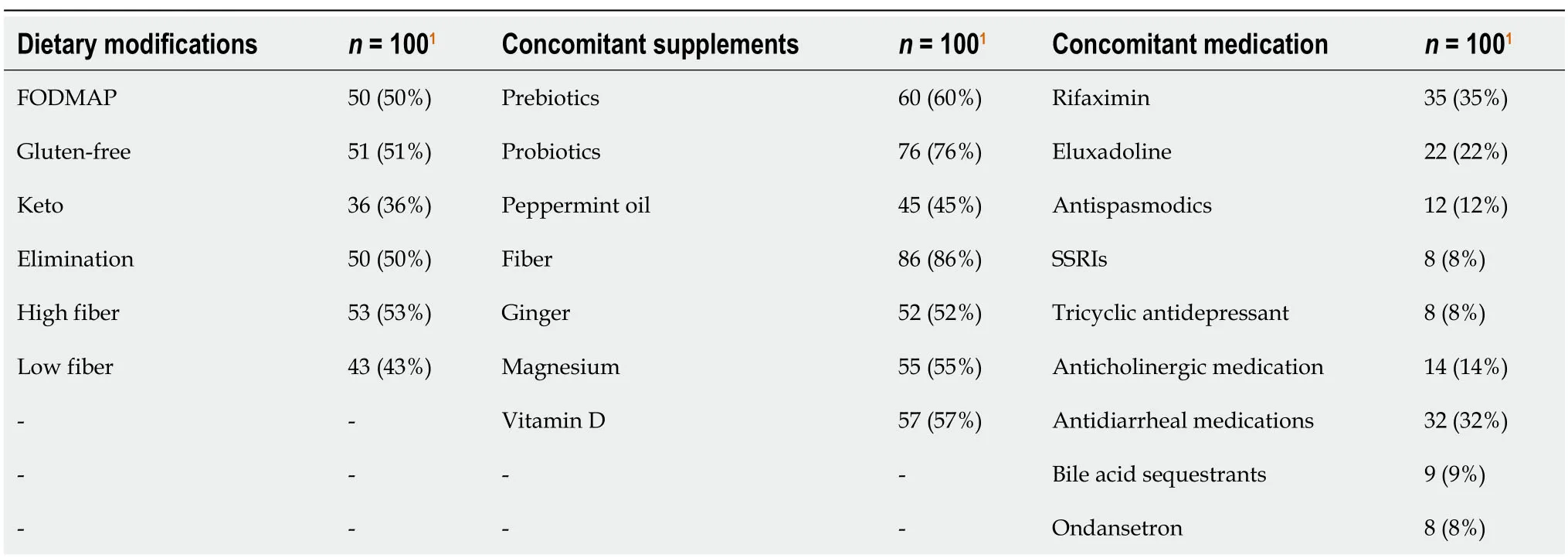
Table 2 Overview of standard of care practices
Tolerance to the amino acid-based medical food,taken twice daily for a period of 14 d,was excellent.Every participant successfully completed the full 14-day trial,and there were no instances of dropouts or discontinuation of the study product reported.Only two Treatment Emergent AEs were spontaneously reported over the course of the study.Both events were reported by the same participant,who experienced a bout of abdominal bloating followed by a temporary increase in abdominal discomfort.The AEs were characterized as mild,and they were self-limiting,with no need for discontinuation of the test product.Importantly,neither of these events was deemed to be a serious adverse event.
Table 3 shows the responder analysis for the secondary outcomes of the study.The number of IBS-D stool consistency responders,based on a decrease of 50% or more in the number of days per week with at least on type 6-7 bowel movement,as compared to baseline was 40 (40%).The number of IBS-D pain and discomfort responders who exceeded a clinically meaningful threshold with a reduction of 30% mean weekly pain and discomfort were 51 (53%) and 53 (55%)respectively.The number of participants who achieved both clinically meaningful responses in pain/stool consistency or discomfort/stool consistency were 24 (25%) for both composite endpoints.Table 4 presents the overall improvement in the amount of urgency experienced by participants,with 58% of participants achieving some level of improvement.
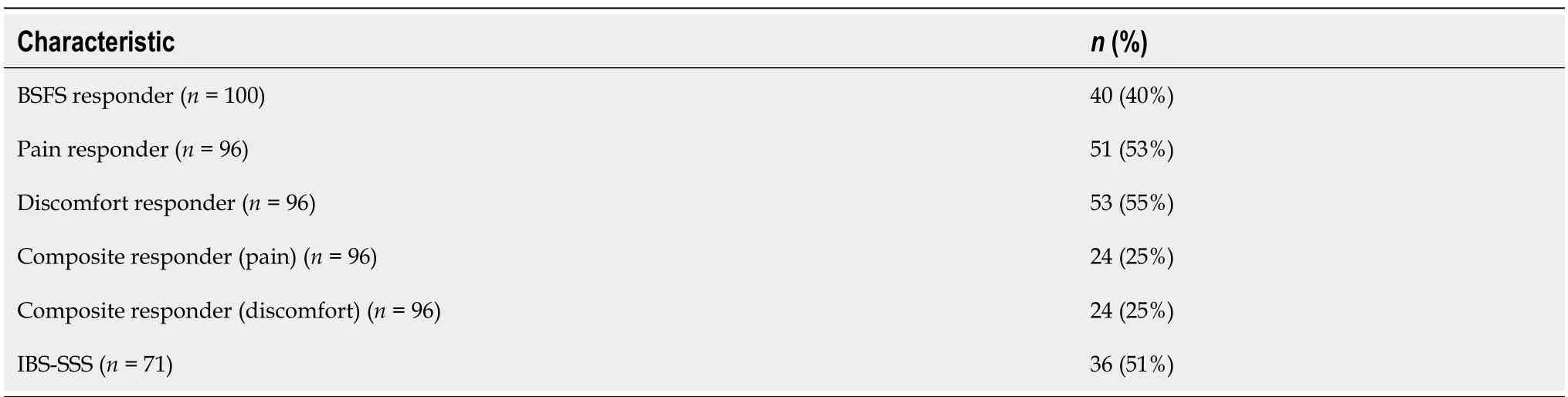
Table 3 Diarrhea predominant irritable bowel syndrome responder analysis

Table 4 Overview of maximum weekly urgency experienced between baseline and intervention period
Seventy-one participants provided complete IBS-SSS data on days 0 and 14 for pairwise comparison.Figure 2 illustrates the differences in each of the 5-item IBS-SSS endpoints and their significant improvement over 14 d.Individual and significant average changes in the aggregate IBS-SSS experienced by participants are shown in Figure 2.Three participants began the study with mild scores (75 to 175;4%),n=29 began with moderate scores (176 to 300;41%),andn=39 began with severe scores (301 to 500;55%).Overall,75% (53/71) of participants experienced a reliable clinical scoreimprovement (Figure 2).In addition,51% of participants experienced ≥ 95-point score improvement,often considered the MCID[23].The mean score reduction (-109.4,95% confidence interval: -130.1,-88.8) exceeded both thresholds[20,23].A category shift from severe to moderate or mild occurred in 69% of participants (27/39) and a shift from moderate to mild categories occurred in 52% of participants (15/29),such that 62% (42/68) of the total moderate to severe IBS-SSS sufferers experienced a full IBS-SSS category improvement.Seventy-six percent of participants (54/71) reported relief on the GIS after 14 d of amino acid beverage consumption.
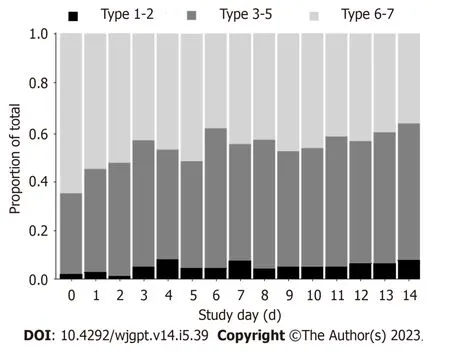
Figure 2 Change in proportion of grouped Bristol Stool Form Scale types during 14 d of amino acid beverage consumption. Sample size varies across days.
DISCUSSION
The purpose of this open-labeled,home-use study was to evaluate the tolerability and functional impact of a select amino acid blended medical food in 100 confirmed IBS-D participants using validated survey instruments from the Rome Foundation.The study design was employed to evaluate the effectiveness of an amino acid-based medical food in a pragmatic setting.This approach employed a decentralized methodology to enroll a diverse range of participants while minimizing disruptions to routine care,thereby ensuring that the study findings are relevant to real-world clinical practice.Strict enrollment criteria ensured that participants had recurring watery diarrhea.Further,the 2-weeks of daily consumption of an amino acid-based medical food demonstrated that the product was safe and tolerable in 100% of the population tested and had a significant positive impact on the exploratory variables assessed.
The daily consumption of approximately 474 mL (16 fl.oz.) of an amino acid-based medical food beverage for 2-weeks drastically reduced the group percentage of BSFS 6-7 from 65% to 36% (Figure 2).Chronic diarrhea in IBS-D may double or triple ordinary stool frequency[3].In conjunction with stool quality of BSFS 6-7[7],IBS-D may increase daily bowel water losses to 500 mL[6,24] along with accompanying salt losses[8,25].Although no measures of hydration status were made in this study,the complement of approximately 474 mL of fluid with approximately 30 mmol/L of key electrolytes consumed daily,alongside a significant reduction in BSFS from liquid to solid,makes plausible an improvement in whole body hydration status over the 2-weeks.The improvements in BSFS over the 14-day product intervention period are entirely consistent with the reduced stool frequency benefits reported in previous studies consuming amino acid-based beverages with ancillary compositional differences to the product tested herein[26-29].Hypotonic oral rehydration therapy is widely recognized as the preferred intervention for diarrhea and dehydration independent of etiology[13,30,31].However,a dual action product that rehydrates while reducing gastrointestinal symptoms (e.g.diarrhea),as reported in this study,has not been previously demonstrated[32].
The IBS-SSS is a widely used consensus-based measurement of IBS-D severity[20].Consumption of an amino acid beverage for 2-weeks resulted in improvements on all 5-IBS-SSS survey items (Figure 3A).The mean overall improvement on the IBS-SSS was 109 points (Figure 3B).Seventy-five percent of participants improved by a clinically meaningful score of at least 50 points[20],improvements similar for IBS-D patients on a starch and sucrose restricted diet for 4-weeks (82.3%)[33].The overall improvement reported on the GIS in this study (76%) was comparable to the 80%improvement reported in the initial 2-weeks of a pharmaceutical IBS-D intervention trial of Solifenacin[34].
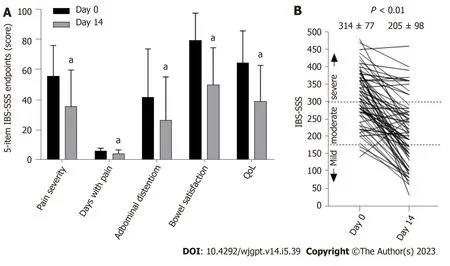
Figure 3 Change in irritable bowel syndrome severity scoring system survey items and overall improvement on the irritable bowel syndrome severity scoring system. A: Change in individual 5-item irritable bowel syndrome severity scoring system (IBS-SSS) scores following 14-days of amino acid beverage consumption (n=71 pairs).Lower scores indicate improvement.aP <0.05;B: Change in global IBS-SSS following 14 d of amino acid beverage consumption.The mean difference was significant by paired t-test (n=71 pairs).
While an open label study improves ecological validity,there are some inherent limitations to this study design.First,evaluations were based exclusively on patient reported outcomes and the possible involvement of the placebo effect in the results cannot be excluded.Since pooled estimates of placebo response are estimated[35-37] to range from 22% for composite pain and stool assessments,39.6% for pain and discomfort,33% stool consistency,and 43% for IBS-SSS it is acknowledged that placebo response rate is quite high and inconsistent across studies.Even when taking this into consideration,the present study verified that an amino acid based medical food can confer additional health benefits when used as an adjunct to the standard of care (Table 3) in line with clinically meaningful improvements beyond common placebo effects recorded in the above cited literature.
Second,the short duration of this pilot trial can be viewed as both a limitation and a benefit.The limitation resides in the fact that IBS-D is a lifelong,chronic condition so the durability of benefit observed after 2 weeks remains unknown.The benefit is the observed significance and magnitude of effects on par with other dietary interventions used for much longer time periods.Of note,low FODMAP diets are recommended by consensus for IBS[10] and often produce excellent outcomes (e.g.,Russoet al[38]).But unlike a daily amino acid beverage,low FODMAP diets can become impractical,tiresome,and even unhealthy as a long-term solution for IBS-D.
Finally,the study was structured intentionally to provide real-world patient experiences (open-label,standard of care)within a controlled clinical trial framework.This approach yields valuable insights into the practical use,benefits,and risks of using a medical food as an adjunct (rather than a replacement) to existing care,demonstrating its effectiveness in broader healthcare settings.However,while the use of patient reported outcomes in IBS research is essential[39],future studies that include direct blood and fecal markers will complement and add strength to the conclusions drawn from pragmatic studies like this one.
Importantly,this study addresses the replenishment of water and nutrients lost due to diarrhea in IBS-D patients,which is a nutritional complement to the gut benefits afforded by the amino acids.Until now,there has not been a specifically formulated medical food available to address the nutritional deficits experienced by IBS-D patients[11,12,24].As previously mentioned,the most recent guidelines for managing patients with IBS emphasize the importance of tailoring treatments to individual needs and adopting a holistic approach that encompasses dietary,lifestyle,medication,and behavioral interventions[10].The open label study design in the present study exemplifies this approach,as participants were employing all the previously mentioned modalities and practices.It is also important to note that despite the use of these modalities and practices (Table 2),participants in the study did exhibit recurring watery diarrhea to the degree that met inclusion criteria for participation in the study. However,the positive improvements in gastrointestinal symptoms reported in the present study demonstrate that this specifically formulated medical food can be used in conjunction with other approaches.
CONCLUSION
Patient reported outcomes collected in this pragmatic study provide evidence that the integration of an amino acid-based medical food as an adjunct to the standard care for patients with IBS-D is both feasible and well-tolerated.Furthermore,the improvements in gastrointestinal symptoms demonstrated statistically significant benefits with an order of magnitude on par with longer nutrition intervention trials (e.g.,Gayosoet al[33];Russoet al[38]).Lastly,a specifically formulated amino acid beverage appears a promising adjunct approach for individuals suffering from IBS-D in relieving gastrointestinal symptoms.
ARTICLE HIGHLIGHTS
Research background
Irritable bowel syndrome (IBS) is a common gastrointestinal disorder characterized by recurrent abdominal pain and changes in bowel habits.Diarrhea predominant IBS (IBS-D) is the most prevalent subtype,causing chronic and debilitating diarrhea.This study explores the potential benefits of an amino acid based medical food,enterade®IBS-D,to address nutritional deficits in IBS-D patients and improve their gastrointestinal symptoms,aiming to provide an additional means to management.
Research motivation
The motivation to perform this research was to discover additional therapies to aid in the management of chronic diarrhea and associated symptomology resulting from IBS-D.
Research objectives
The study primarily focused on the tolerability of the amino acid based medical food by monitoring adverse events (AEs)reported by participants.Secondary outcomes were assessed using a number of clinical criteria,including changes in stool consistency [Bristol Stool Form Scale (BSFS)],reduction in abdominal pain and discomfort,improvement in bowel urgency,and changes in the overall IBS-Severity Scoring System.The study considered both statistical significance and clinical relevance as recommended by the Food and Drug Administration for IBS-related assessments.
Research methods
The study used a decentralized,open-label,single-arm design,with data collected electronically through the Laina Clinical Research Platform during a 2-week baseline assessment and a subsequent 2-week intervention period,where participants consumed enterade®IBS-D.The study included adults aged 18-65,diagnosed with IBS-D in the United States.Participants were evaluated during a 2-week run-in period to confirm their diagnosis using Rome IV criteria.Eligible participants needed to experience diarrhea at least four times weekly (BSFS types 6 and 7),along with abdominal pain/discomfort and a Functional Bowel Disorder Severity Index score above 60.Exclusion criteria included other IBS subtypes,gastrointestinal disorders,pregnancy/breastfeeding,allergies to the test product components,and anticipated medication changes that could affect bowel habits.Outcome measures included daily symptom diaries,the IBS-Severity Scoring System,and the Global Improvement Survey (GIS) from the Rome Foundation.
Research results
The study involved 100 participants with IBS-D who completed a 2-week intervention period.All participants met ROME IV criteria for IBS-D,and their baseline IBS severity scoring system (IBS-SSS) score and FBDSI scores indicated moderate to severe symptomology.Tolerance to the amino acid-based medical food was exceptional,with no dropouts or discontinuations,and only two mild AEs reported.Responder analysis for secondary outcomes revealed that 40% of participants achieved a 50% or more decrease in days with type 6-7 bowel movements.Additionally,53% and 55%experienced clinically meaningful reductions in pain and discomfort,respectively.About 25% of participants achieved meaningful responses in both pain/stool consistency or discomfort/stool consistency.Fifty-eight percent of participants showed improvements in urgency.IBS-SSS data demonstrated significant improvement,with 75% experiencing a reliable clinical score improvement,51% achieving a ≥ 95-point score improvement,and a category shift from severe to moderate or mild in 69% of participants.Furthermore,76% reported relief on the GIS after 14 d of amino acid beverage consumption.
Research conclusions
The study suggests that adding an amino acid-based medical food to the standard care for IBS-D patients is practical and well-tolerated.The improvements in gastrointestinal symptoms were statistically significant and comparable to longer nutrition intervention trials,making this amino acid beverage a promising adjunct for relieving IBS-D symptoms.
Research perspectives
While patient reported outcomes in IBS research are crucial endpoints,future studies that include collection of biological samples,such as direct blood and fecal markers,will supplement and strengthen the conclusions that have been drawn from this pragmatic study.
FOOTNOTES
Author contributions:Niles SE,Cheuvront SN,Kenefick RW,and Fawkes N designed the research study;Niles SE,Blazy P,Cheuvront SN,Kenefick RW,Vidyasagar S,Smith AB,Fawkes N,and Denman W analyzed the data and wrote the manuscript;Denman W acted as the Principal Investigator;all authors have read and approve the final manuscript.
Institutional review board statement:The study was reviewed and approved by the Advarra Institutional Review Board (Approval No.Pro00065894).
Informed consent statement:All study participants or their legal guardian provided informed written consent about personal and medical data collection prior to study enrolment.
Conflict-of-interest statement:All authors are employees of Entrinsic Bioscience LLC (EBS) and EBS was the research sponsor.Patent 174821-011205/PCT (PCT/US11/53265) is issued to EBS Holdings,and patent 174821-012001/PCT (PCT/US13/25294) is issued to EBS Holding.
Data sharing statement:All analyzed data are included in this published article.The original data are available upon reasonable request to the corresponding author.
STROBE statement:The authors have read the STROBE Statement—checklist of items,and the manuscript was prepared and revised according to the STROBE Statement—checklist of items.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:United States
ORCID number:Samantha E Niles 0000-0002-1826-1802;Neil Fawkes 0000-0002-0261-7341.
S-Editor:Lin C
L-Editor:A
P-Editor:Lin C
