基于苯四酸和双咪唑甲基苯构筑的Co(Ⅱ)和Ni(Ⅱ)配合物的合成、晶体结构及光催化活性
2023-12-21符钰培董露露陈小莉崔华莉王记江
符钰培 杨 华 乔 丹 董露露 陈小莉 崔华莉 刘 琳 王记江
(延安大学化学与化工学院,陕西省反应工程重点实验室,新能源新功能材料实验室,延安 716000)
0 Introduction
In recent years, the rapid development of the printing and dyeing industry has brought huge profits to many developing countries, but a large amount of wastewater polluted by dyes has caused serious environmental problems. The discharge of large amounts of dye can change the pH of the water, and affect the organic carbon level and gas solubility[1]. On the other hand, dyes have complex aromatic structures that result in greater stability and difficulty to be degraded,releasing carcinogenic compounds in the process[2].Therefore, we urgently need a green method for wastewater treatment. Compared with other mature technologies such as adsorption and biological and electrochemical treatment, the economical and environmentally friendly solar - driven photocatalytic technology has great potential in treating organic pollutants[3-4].
Metal-organic frameworks (MOFs) are 3D supramolecular structures that are composed of metal ions or clusters as secondary building units (SBUs) and organic molecules as pillars (Struts). In recent years, the design and synthesis of MOFs have attracted intensive interest due to their fascinating structures and multifield potential applications in magnetism[5-7], molecular adsorption[8-9], molecular recognition[10-12], asymmetric catalysis[13-14], biological medicine[15], electric conductivity[16]and photoluminescence[17-18].
It is well known that the critical factors for constructing novel structures are metal ions, organic ligands, synthetic methods, reaction conditions, and pH[19-25]. The selection of appropriate organic ligands still plays a crucial role in achieving the expected coordination polymers. Imidazole derivatives and polycarboxylate ligands possess a variety of structural features and thus have been considered ideal organic building blocks for constructing metal - organic frameworks(MOFs)[26-30]. Moreover, the carboxyl group can be partially deprotonated to form hydrogen bonds in the form of donors and acceptors and then to form supramolecules by self-assembly.
1,2,4,5-Benzene tetracarboxylic acid (H4L, Fig.1)is a rigid aromatic tetracarboxylic acid,and 1,3-bis((1Himidazole-1-yl) methyl) benzene (1,3-bib, Fig.2a) and 1,4-bis((1H-imidazole-1-yl) methyl) benzene (1,4-bib,Fig. 2b) are flexible imidazole derivatives. The two imidazole rings can rotate freely through the C—C bond, which provides the possibility for the construction of novel functional complexes.
Fig.1 Structure of H4L
Fig.2 Structures of 1,3-bib(a)and 1,4-bib(b)
Taking into the above-mentioned consideration,we chose H4L as the first ligand, 1,3-bib, and 1,4-bib as the auxiliary ligand. Two new coordination polymers[Co(L)0.5(1,3-bib)](1)and[Ni2(L)(1,4-bib)3(H2O)2]·2H2O(2), were synthesized by using the hydrothermal method. The structures of 1 and 2 were confirmed by elemental analysis, FTIR spectroscopy, and single-crystal X-ray diffraction.In addition,the activities and mechanisms for the photocatalytic degradation of dyes by 1 and 2 were investigated in the presence of H2O2.
1 Experimental
1.1 Reagents and physical measurements
The solvents and reagents were purchased from the chemical companies and used without further purification. The C, H, and N elemental analyses were performed on a Perkin-Elmer 2400 elemental analyzer.The IR spectra were recorded in the 4 000-400 cm-1region with a Shimadzu FTIR - 8400s spectrometer using a KBr pellet. A Netzsch STA 449C thermogravimetric analyzer was used for the thermogravimetric analysis (TGA) from 30 to 800 ℃at a heating rate of 10 ℃·min-1in a nitrogen atmosphere. Powder X-ray diffraction (PXRD) patterns were recorded with a Rigaku D/Max Ⅲdiffractometer operating at 40 kV and 30 mA using CuKαradiation (λ=0.154 18 nm) at a scanning rate of 2 (°)·min-1from 5° to 50°. The fluorescence spectra were obtained with a Hitachi F-7100 fluorescence spectrophotometer at room temperature.
1.2 Synthesis of complex 1
A mixture of Co(NO3)2·6H2O(0.029 1 g,0.1 mmol),H4L(0.025 4 g,0.1 mmol),1,3-bib(0.047 7 g,0.2 mmol)and H2O-DMF (7 mL and 3 mL, respectively) was sealed in a Teflon-lined stainless steel vessel and heated to 160 ℃for 96 h. The reaction system was then cooled to room temperature at a rate of 5 ℃·h-1. Lightpink crystals were obtained in ca. 53% yield based on Co. Anal. Calcd. for C19H15CoN4O4(% ): C 54.04, H 3.59, N 13.26; Found(%): C 54.06, H 3.54, N 13.24.IR (KBr,cm-1): 3 516(w), 3 138(w), 2 974(w), 2 372(w),1 550(s), 1 379(s), 1 219(w), 1 083(s), 950(w), 819(s),738(m),640(m).
1.3 Synthesis of complex 2
A mixture of Ni(NO3)2·6H2O(0.058 2 g,0.2 mmol),H4L(0.025 4 g,0.1 mmol),1,4-bib(0.023 8 g,0.1 mmol)and H2O (10 mL) was stirred, and the pH value of it was adjusted to 6.5 with 0.5 mol·L-1NaOH solution,then sealed in a 25 mL Telfon-lined stainless steel container, which was heated to 160 ℃for 96 h. The reaction system was cooled to room temperature at a rate of 5 ℃·h-1.Light-green crystals were obtained in ca.53% yield based on Ni. Anal. Calcd. for C52H52N12Ni2O12(%):C 54.05,H 4.50,N 14.55;Found(%):C 54.16,H 4.63,N 14.26. IR (KBr, cm-1): 3 608(w), 3 540(w), 3 418(m),3 130(w), 2 361(w), 1 611(s), 1 390(s), 1 285(w), 1 242(w), 1 098(m), 1 029(m), 812(m), 734(m), 668(w), 612(w),556(w).
1.4 Photocatalytic degradation experiment
To verify the photocatalytic degradation activity of complex 1, we mixed 1 (20 mg) with MO (methyl orange)/MB (methylene blue) (20 mL, 10 mg·L-1)under stirring to obtain stable suspensions. After the dark reaction for 20 min, the suspensions reached adsorption equilibrium and then were irradiated under visible light (500 W Xenon lamp). At 30-minute intervals, a certain volume (1 mL) of sample was removed from the photocatalytic reactor and the concentration of dyes was analyzed by UV-Vis absorption spectrometry.To verify the photocatalytic degradation activity of complex 2, the photocatalytic degradation experiment of MB/RhB (rhodamine B) by 2 was carried out similarly to 1.
1.5 Structure refinement
Diffraction intensity data for complexes 1 and 2 were collected at room temperature on a Bruker Smart APEX Ⅱ CCD diffractometer employing graphitemonochromatized MoKαradiation (λ=0.071 073 nm).The structures were solved by direct methods and refined by full-matrix least-squares onF2using the SHELXTL-2014 program. Non-hydrogen atoms were refined anisotropically and hydrogen atoms of organic ligands were placed in calculated positions. Crystal data and structure refinement parameters for 1 and 2 are summarized in Table 1. Selected bond distances and bond angles are listed in Table 2 and 3.
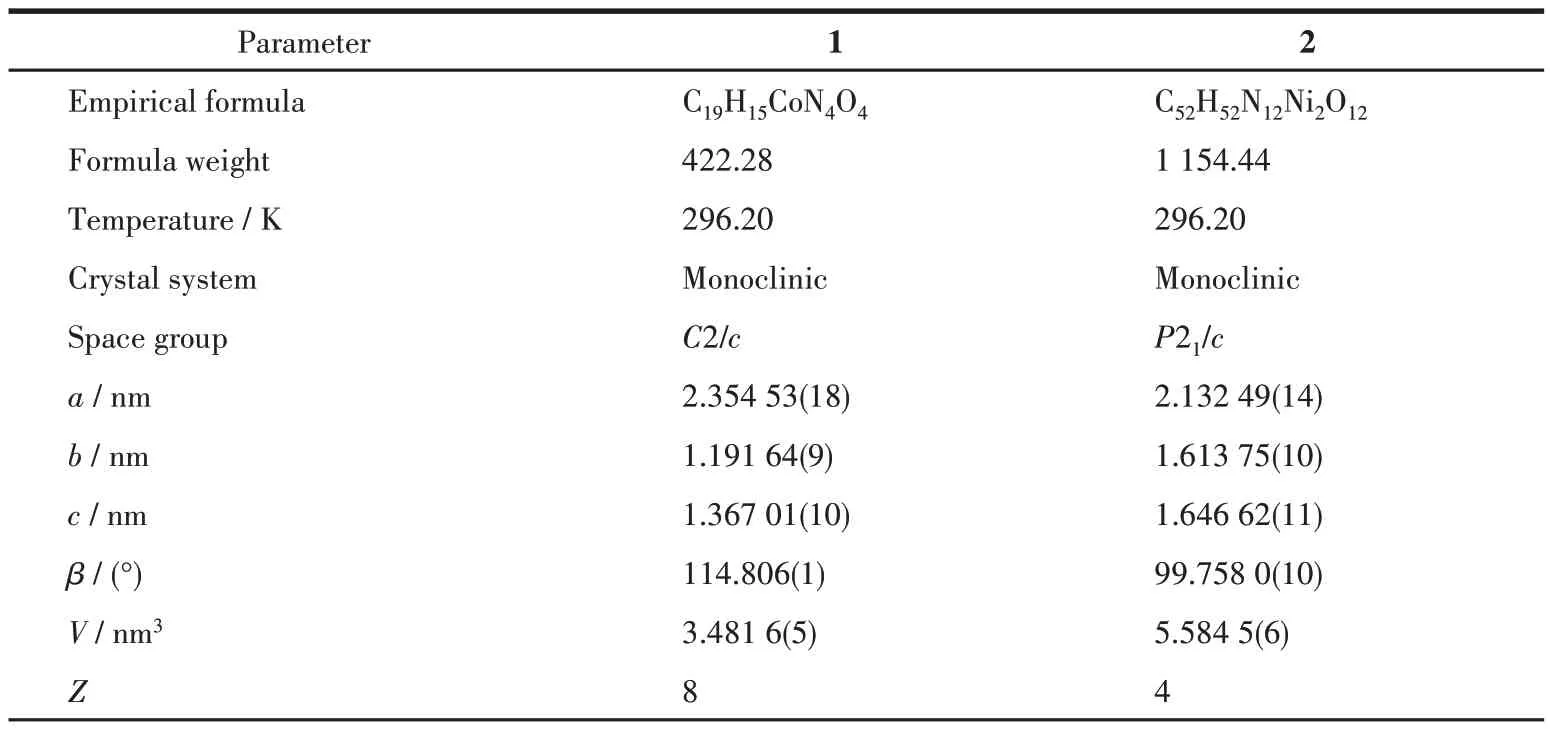
Table 1 Crystallography and structural parameters of complexes 1 and 2
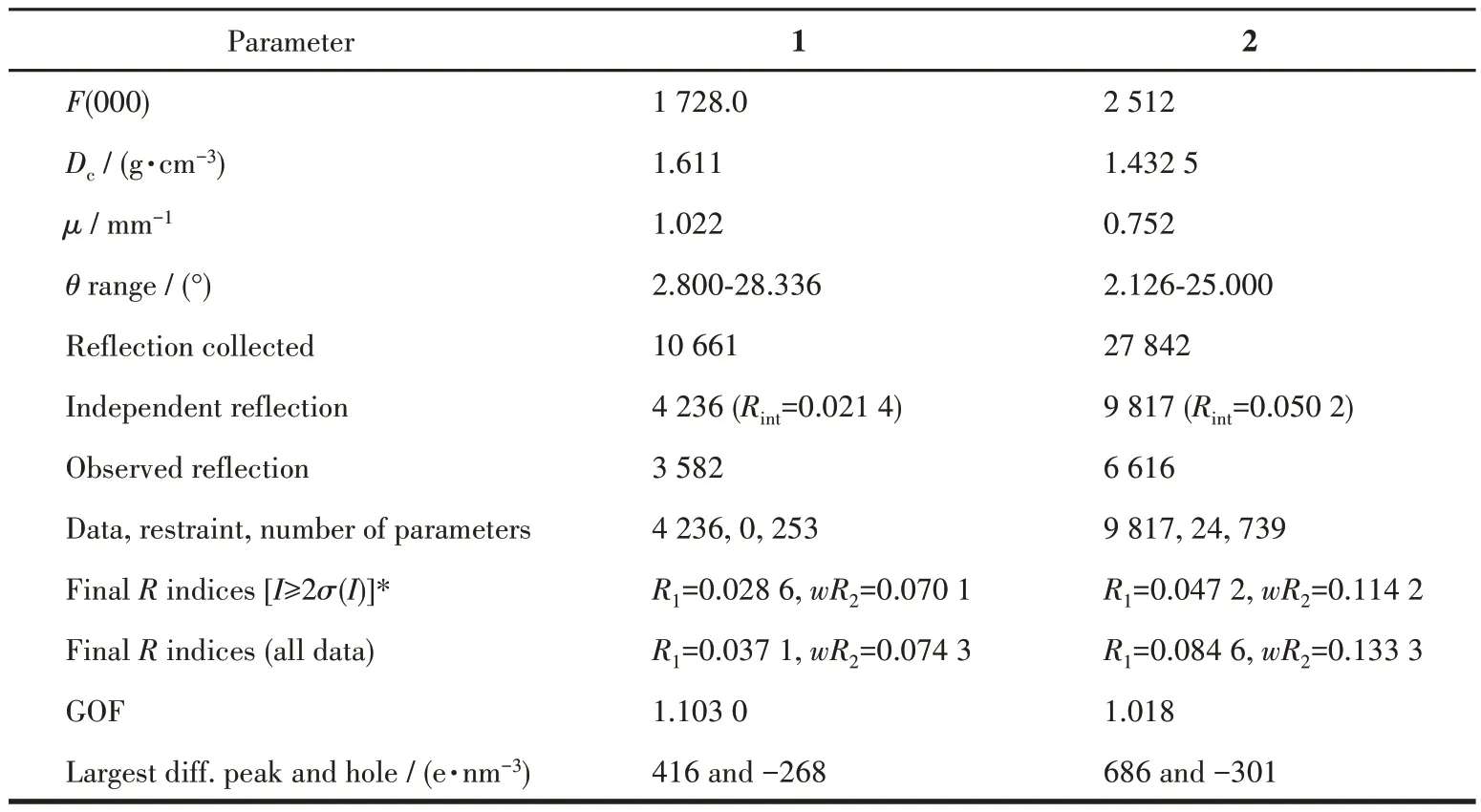
Continued Table 1
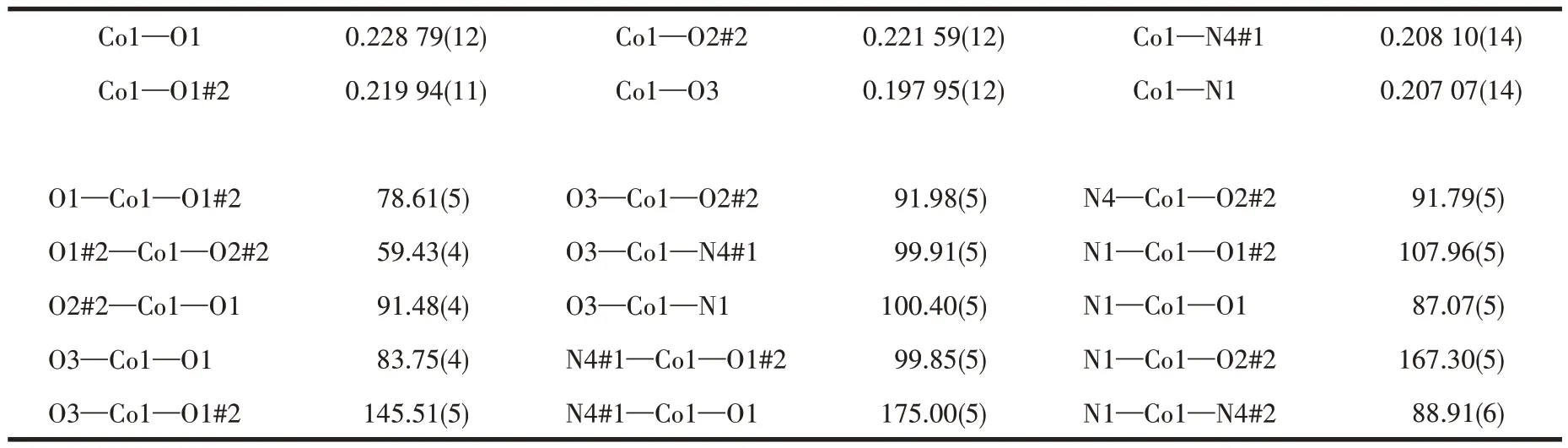
Table 2 Selected bond lengths(nm)and bond angles(°)of complex 1
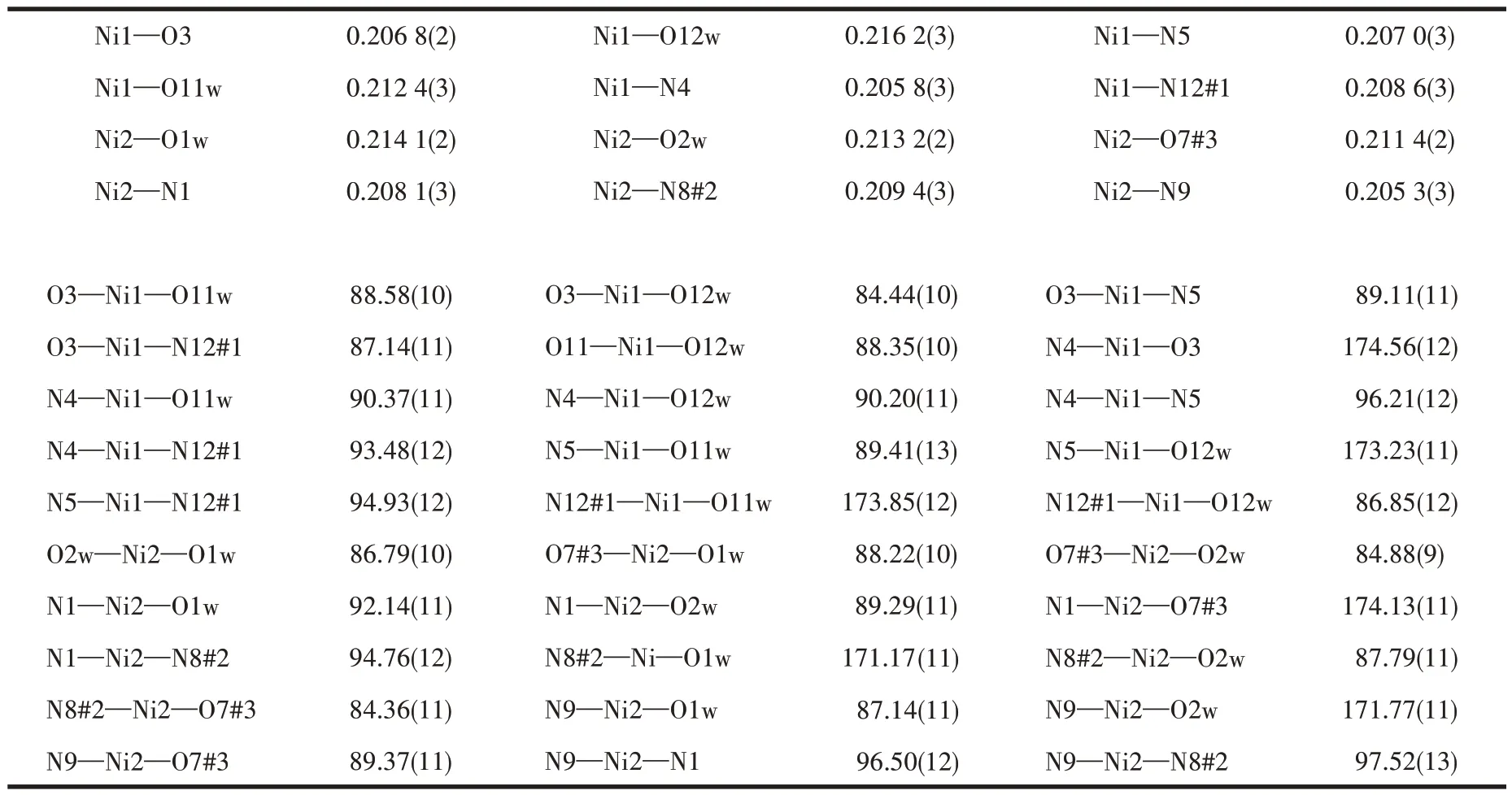
Table 3 Selected bond lengths(nm)and bond angles(°)of complex 2
CCDC:2219951,1;2219953,2.
2 Results and discussion
2.1 Molecular structure of complex 1
Complex 1 crystallizes in the monoclinic space groupC2/c.In the asymmetric unit,there is one crystallographically unique Co(Ⅱ)ion, half an L4-ligand, and one 1,3-bib molecule. As shown in Fig.3a, the Co1 ion is coordinated by four oxygens (O1, O3, O1#2, O2#2)from two completely deprotonated L4-ligands and two nitrogens (N1,N4#1)from two 1,3-bib molecules.Thus the Co2+ion displays a distorted octahedral geometry.Two oxygen atoms (O1#2, O2#2) of the same carboxyl group adopt a chelate coordination mode, and two oxygen atoms (O1, O3) of different carboxyl groups show a monodentate coordination mode. O2#2, O1#2, O3, and N1 atoms are located on the equatorial plane while N4#1 and O1 occupy the axial positions with the N4#1—Co—O1 angle of 174.99°. The Co(Ⅱ)to O/N distances fall in the ranges of 0.197 95(12)-0.228 79(12) nm and 0.207 07(14)-0.208 10(14) nm, respectively, similar to those found in other Co-MOFs[31-32]. The L4-ligand was linked to four Co(Ⅱ)ions by theμ4-к2∶к1∶к2∶к1mode.
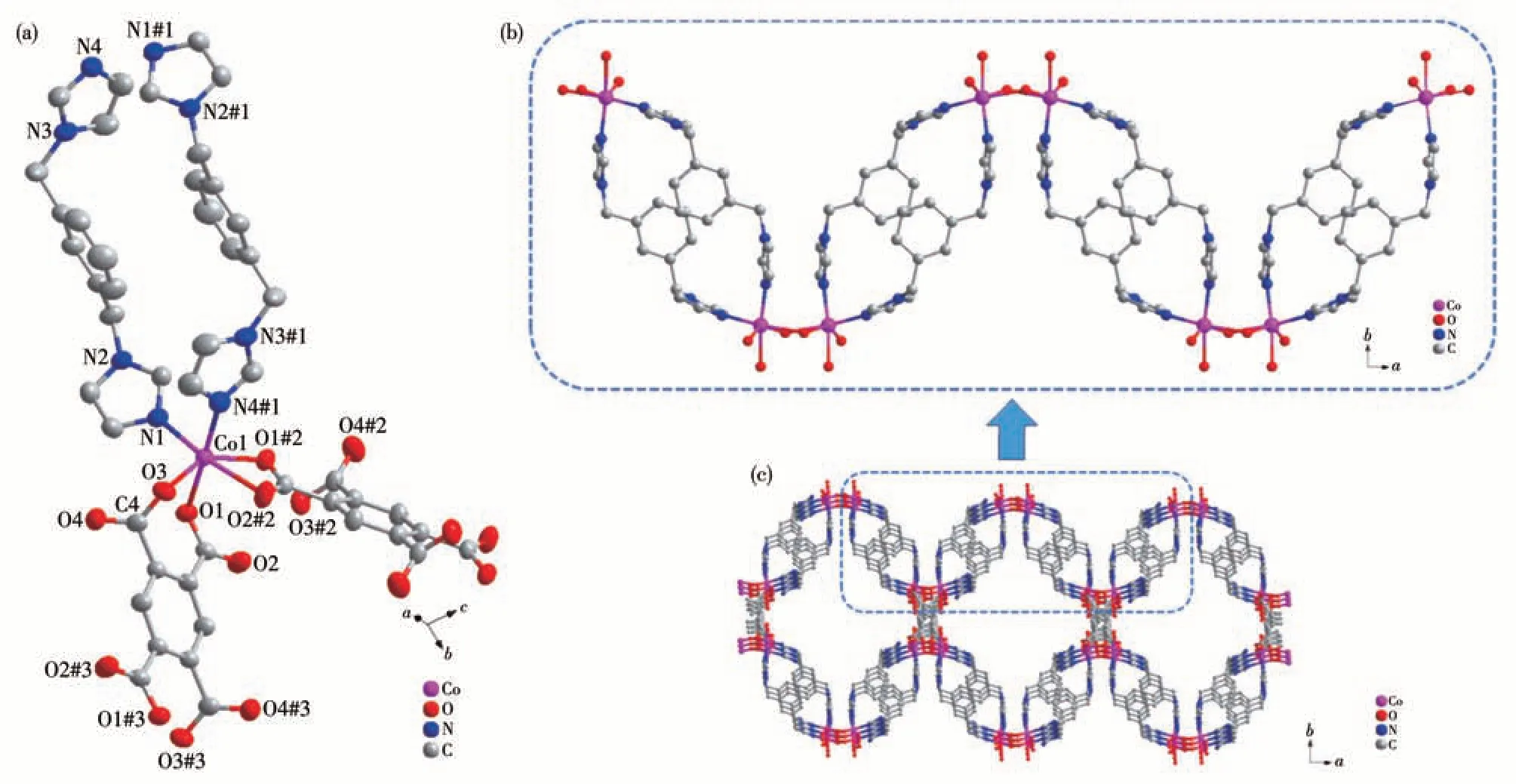
Fig.3 (a)Coordination environment of Co(Ⅱ)center in complex 1(Ellipsoid probability:70%);(b)1D chain in 1;(c)3D network structure of 1
In the 1, two 1,3-bib molecules connect to two Co(Ⅱ)ions to form an asymmetric unit. The asymmetric units are connected with two O1 atoms of L4-to generate a 1D chain (Fig.3b). The chain structure extends in two different directions via the O2 and O3 atoms of L4-,forming a 3D network structure(Fig.3c).
2.2 Molecular structure of complex 2
Complex 2 crystallizes in a monoclinic space groupP21/c. The asymmetric unit of 2 contains two independent Ni2+ions, one completely deprotonated L4-ligand, three 1,4-bib molecules, two coordinated water molecules, and two lattice water molecules. As shown in Fig.4a, the Ni1 ion is coordinated by three oxygens(O3, O11W, O12W) from one completely deprotonated L4-ligand and two coordinated water molecules, and three nitrogens (N12#1, N5, N4) from three 1,4-bib molecules.The coordination geometry of the Ni1 center can be described as a distorted octahedron. Three oxygen atoms and three N atoms adopt a monodentate coordination mode. O3, O12W, N4, and N5 are located on the equatorial plane, while N12#1, Ni1, O11W atoms occupy the axial positions with the N4#1—Co—O1 angle of 173.85°. The Ni(Ⅱ)to O/N distances fall in the ranges of 0.206 8(2)-0.216 2(3) nm and 0.205 3(3)-0.208 94(3) nm, respectively, similar to those found in other Ni-MOFs[33].The L4-ligand was linked to two Ni(Ⅱ)ions by theμ2-к1∶к0∶к1∶к0mode.
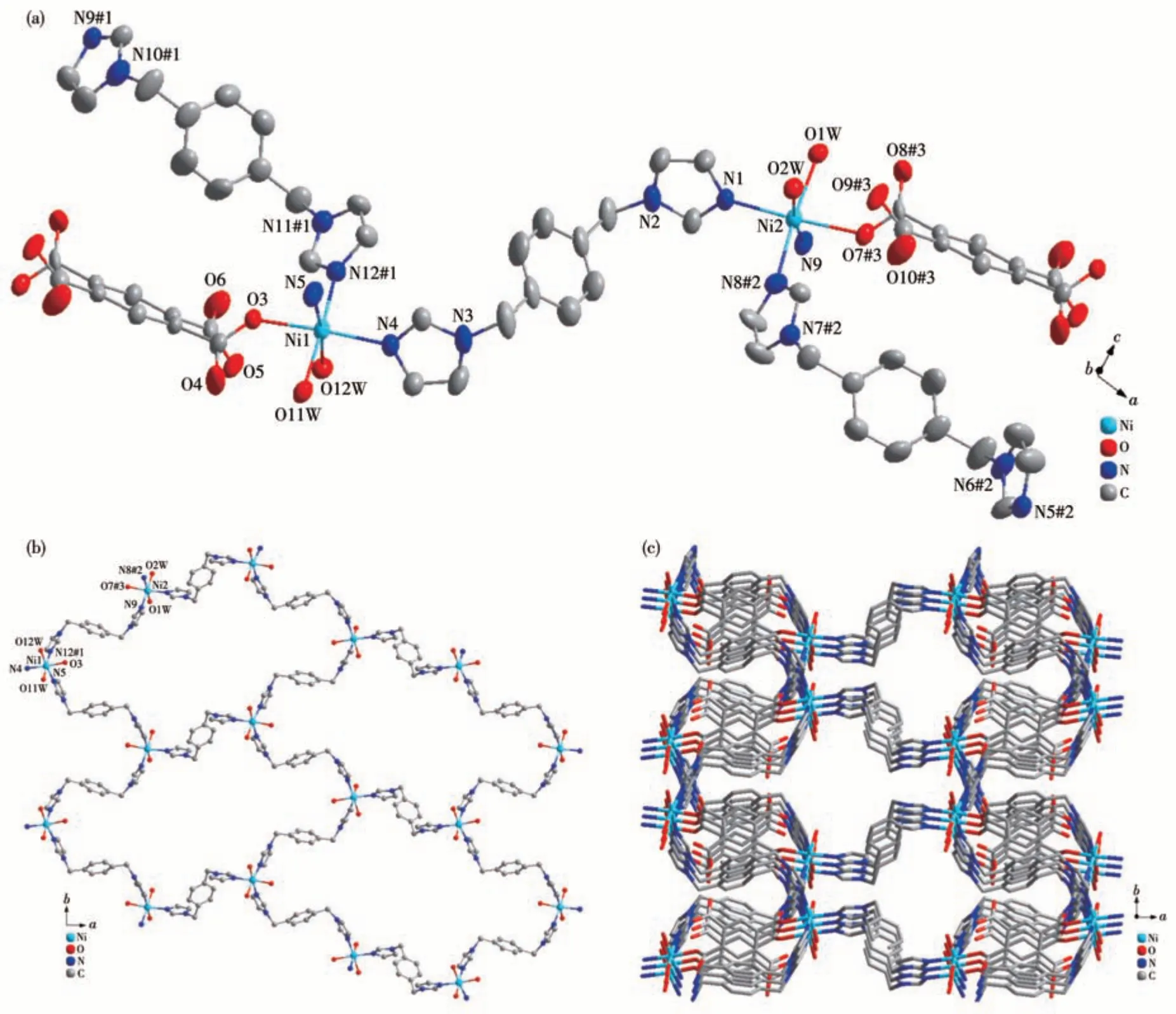
Fig.4 (a)Coordination environment of Ni(Ⅱ)center in complex 2(Ellipsoid probability:60%);(b)2D plane structure of 2;(c)3D plane structure of 2
In 2, each Ni2+ion is connected to three 1,4-bib molecules, and each 1,4-bib molecule is connected to two Ni atoms, resulting in a 2D plane structure(Fig. 4b). The 2D plane structure extends to a 3D network via two carboxyl oxygen atoms of L4-(Fig.4c).
2.3 PXRD and TGA of complexes 1 and 2
The PXRD analyses were performed at room temperature to confirm the phase purity of the bulk materials.The peaks of the simulated and experimental PXRD patterns (Fig.5a and 5b)were in good agreement with each other, confirming the phase purities of complexes 1 and 2.

Fig.5 Simulated and experimental PXRD patterns of complexes 1(a)and 2(b);TG curves of 1(c)and 2(d)
To study the thermal stabilities of 1 and 2, TGA was carried out from 20 to 800 ℃at a heating rate of 10 ℃·min-1under a nitrogen atmosphere. The TG curves of 1 and 2 are shown in Fig.5c and 5d.Complex 1 first showed a weight loss of free H2O below 150 ℃,corresponding to 10.8%. Then 1 was relatively stable up to 150-285 ℃. The second weight loss was 71.4% in a temperature range of 285-600 ℃,corresponding to the decomposition of the L4-and 1, 3 - bib ligands(Calcd. 71.9%). The remaining weight of 19.4% was CoO, which is consistent with the calculated value of 17.8%.
Complex 2 lost 6.5% of its weight between 0 and 100 ℃, probably due to the loss of coordinated H2O and crystalline H2O in the complex (Calcd. 6.2%). The complex was relatively stable between 100 and 250 ℃,and lost 80.4% of its weight between 250 and 650 ℃,probably due to the loss of the L4-ligand as well as the 1,4-bib ligand (Calcd. 83.6%). The last remaining 13.1% was probably NiO(Calcd.12.9%).
2.4 Photocatalytic properties
With the development of industry, a lot of organic dyes have been synthesized and applied in chemical engineering. Although organic dyes bring many benefits to our lives, organic dyes are difficult to degrade,resulting in serious pollution of our environment, especially water resources. How to deal with pollutants in water is an urgent problem.
Research has shown that MOFs play an important role in the degradation of dyes in water under UV irradiation[34-35]. For this reason, we used complexes 1 and 2 to test the degradation capacity for dyes. The photocatalytic ability of the complexes is affected by their band gap energy (Eg). Therefore, using the Kubelka-Munk function,we made the plot with(αhν)2as the vertical coordinate and hν as the horizontal coordinate.The band gap energies of 1 and 2 were calculated by the intersection of the tangent line andy=0 in it plot.As shown in Fig.6a and 6b, the band-gap energies of 1 and 2 were 3.09 and 3.17 eV, respectively. This confirms that 1 and 2 can be activated by radiation from ultraviolet to visible light regions, suggesting that they may be used as photocatalysts.
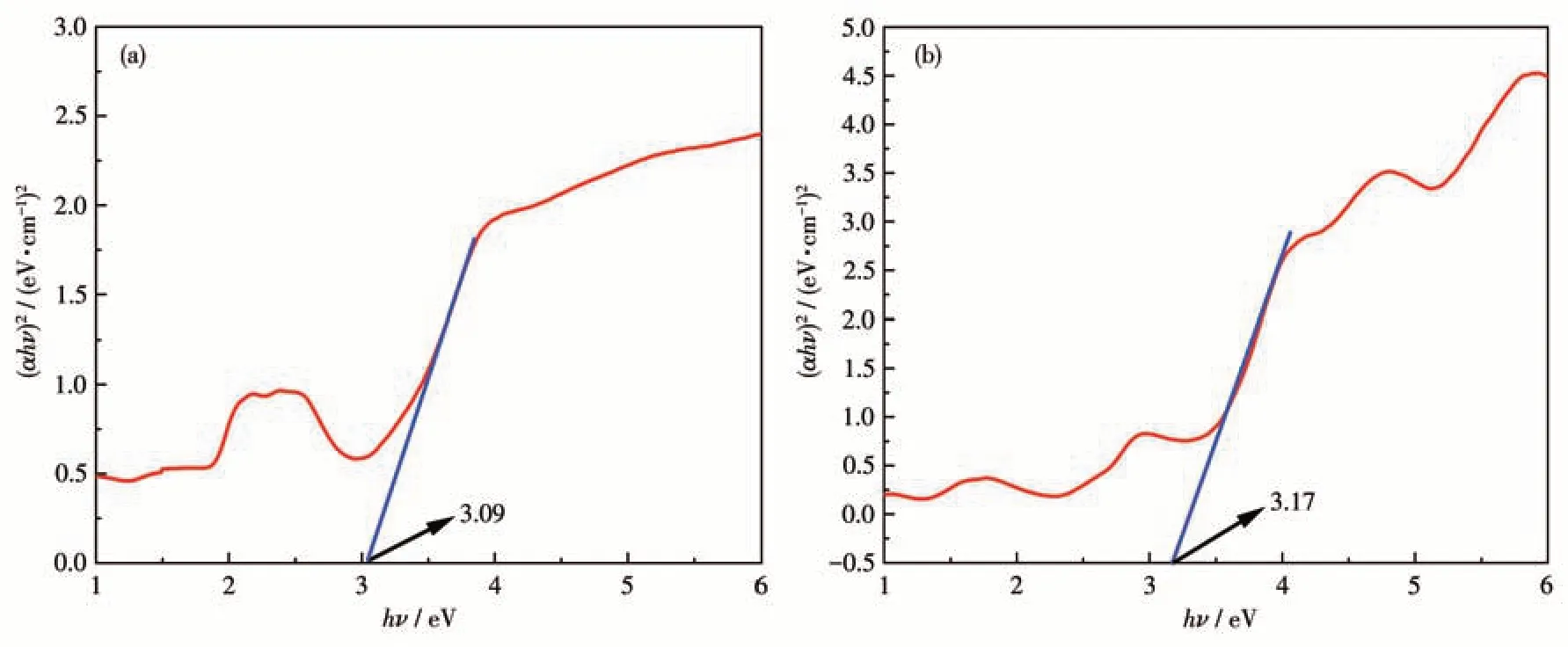
Fig.6 Plots of(αhν)2 vs hν for complexes 1(a)and 2(b)
2.4.1 UV-Vis absorption spectra and photocatalytic properties of complex 1
For a more comprehensive understanding of the degradation process, the photocatalytic degradation reaction of the dye was carried out under several different reaction conditions. Fig. 7 shows the UV-Vis absorption spectra of MB and MO solutions in the presence of H2O2or/and complex 1 under visible light irradiation with time. We can see that the degradation efficiency of dyes was not the same under different conditions.In the presence of H2O2,the degradation efficiencies of MO and MB had a smaller increase (Fig.7a and 7d) under visible light. In the presence of 1, the degradation efficiencies under visible light had a more considerable increase (Fig.7b and 7e). When 1 (20 mg)and H2O2(1 mL) coexisted,the degradation efficiencies were greatly improved, and the dyes were almost completely degraded (Fig.7c and 7f). The results show that 1 has degradation activity on MO and MB under visible light irradiation.
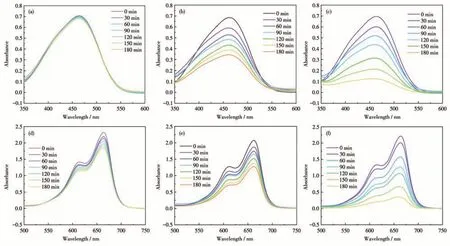
Fig.7 (a,d)UV-Vis absorption spectra of MO and MB solutions in the presence of H2O2,respectively;(b,e)UV-Vis absorption spectra of MO and MB solutions in the presence of complex 1,respectively;(c,f)UV-Vis absorption spectra of MO and MB solutions in the presence of 1 and H2O2,respectively
To further determine the photocatalytic degradation activity of 1,the degradation of the dyes was monitored using UV-Vis absorption spectroscopy, and the obtainedc/c0(c0: dye concentration at the beginning of irradiation;c: instantaneous concentration of dye) plots are shown in Fig.8a and 8d. From the obtainedc/c0plots it can be found that the degradation efficiencies of MO and MB were only 0.6% and 4.3%, respectively under visible light irradiation after 180 min. In the presence of H2O2, the degradation efficiencies of MO and MB were 12.2% and 20.8%, respectively, under visible light irradiation. In the presence of 1, the degradation efficiency could reach 49.9% and 38.8%,respectively, under visible light irradiation. However,when 1 (20 mg) and H2O2(1 mL) coexisted, the degradation efficiencies were greatly improved, reaching 83.2% (MO) and 84.5% (MB), respectively. Subsequently, we applied a pseudo-first-order kinetic model:-ln(c/c0)=kt(t: irradiation time;k: total reaction rate constant) to fit the degradation behavior of the dyes.The linear relationships are shown in Fig.8b and 8e,respectively. The results showed that the degradation of dyes follows pseudo-first-order kinetics.As shown in Fig.8c and 8f, in the presence of H2O2, the degradation rate constants for 1 were the highest, which could reach 0.009 6 min-1(MO)and 0.009 8 min-1(MB).
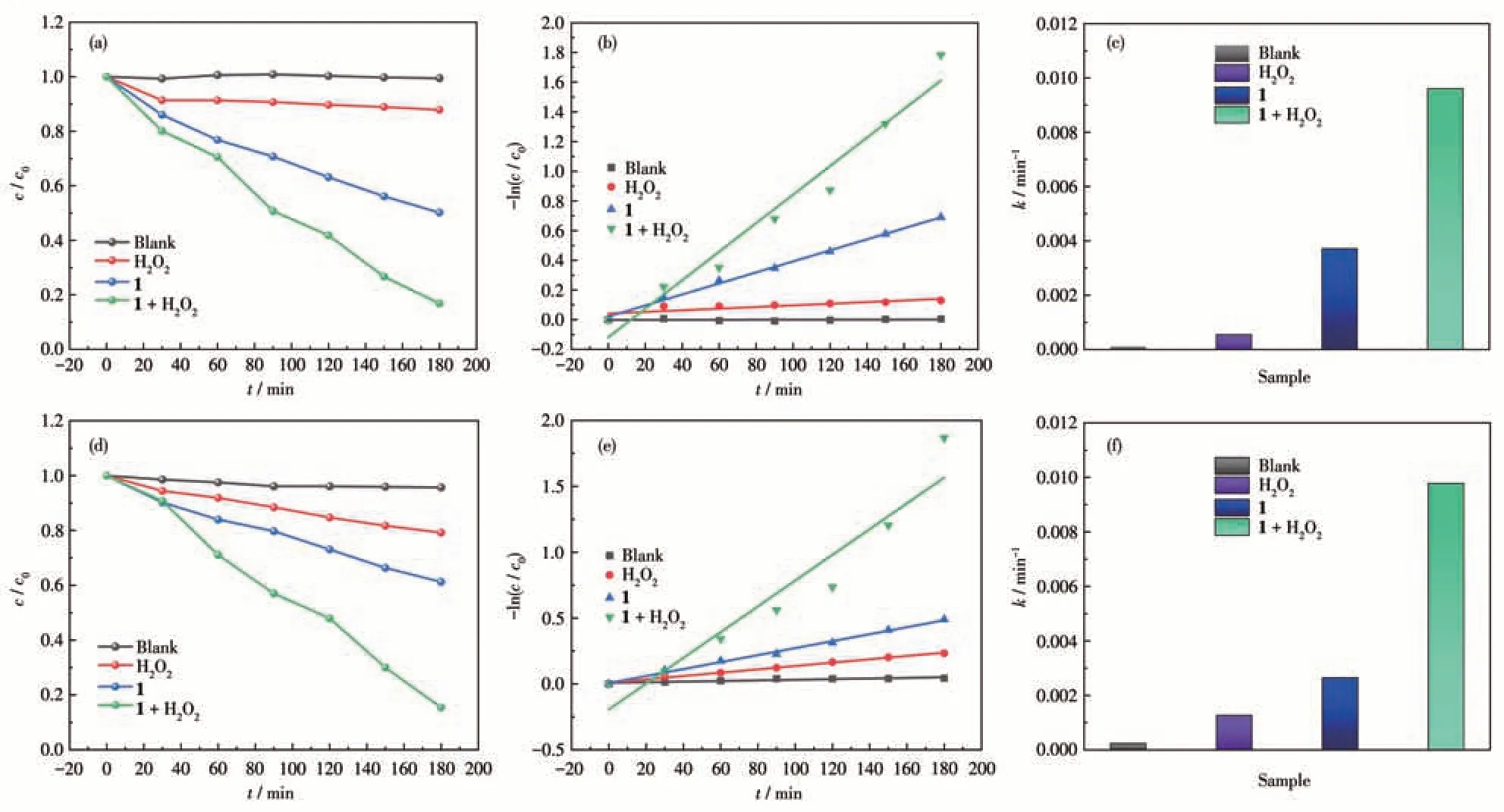
Fig.8 (a,d)Variation of photocatalytic degradation efficiencies of MO and MB with visible light irradiation time under different conditions;(b,e)Plots of-ln(c/c0)vs t and the corresponding linear fittings for the pseudo-first-order kinetic equation;(c,f)Reaction rate constants of MO and MB under different conditions
2.4.2 UV-Vis absorption spectra and photocatalytic properties of complex 2
Following the same method, the degradation experiments of complex 2 were also carried out under different conditions. Fig.9 shows the time-dependent UV-Vis absorption curves of MB and RhB solutions in the presence of H2O2or/and 2 under visible light irradiation. We can learn that the degradation efficiencies of the dyes were different under different conditions.Under visible light irradiation, MB and RhB were slightly degraded in the presence of H2O2(Fig.9a and 9d), MB and RhB were partially degraded in the presence of 2 (Fig.9b and 9e), while MB and RhB could be completely degraded in the coexistence of 2 and H2O2(Fig.9c and 9f).The results indicate that 2 has degradation activity against MB and RhB under visible light irradiation.
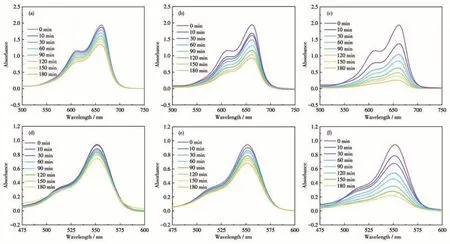
Fig.9 (a,d)UV-Vis absorption spectra of MB and RhB solutions in the presence of H2O2,respectively;(b,e)UV-Vis absorption spectra of MB and RhB solutions in the presence of complex 2,respectively;(c,f)UV-Vis absorption spectra of MB and RhB solutions in the presence of 2 and H2O2,respectively
The photocatalytic degradation ability of 2 was studied by the same method. The degradation efficiencies of the dyes were monitored using UV-Vis absorption spectroscopy,and the obtainedc/c0plots are shown in Fig.10a and 10d. From the obtainedc/c0plots, it can be found that the degradation efficiencies of MB and RhB were only 4.3% and 5.2% under visible light irradiation, and the degradation efficiencies of MB and RhB were 30.8% and 21.3% in the presence of H2O2,while 51.8% of MB and 27.8% of RhB were degraded in the presence of 2. However, when H2O2and 2 coexisted, the degradation efficiencies of MB and RhB could be greatly improved, reaching 87.0% (MB) and 77.4% (RhB).Subsequently,we applied a pseudo-firstorder kinetic model of-ln(c/c0)=ktto fit the degradation behavior of the dye. The linear relationships are shown in Fig.10b and 10e. The results showed that the degradation of dyes follows pseudo-first-order kinetics. As shown in Fig.10c and 10f, in the presence of H2O2, the rate constants were the highest for 2, which could reach 0.011 5 min-1(MB)and 0.007 8 min-1(RhB).
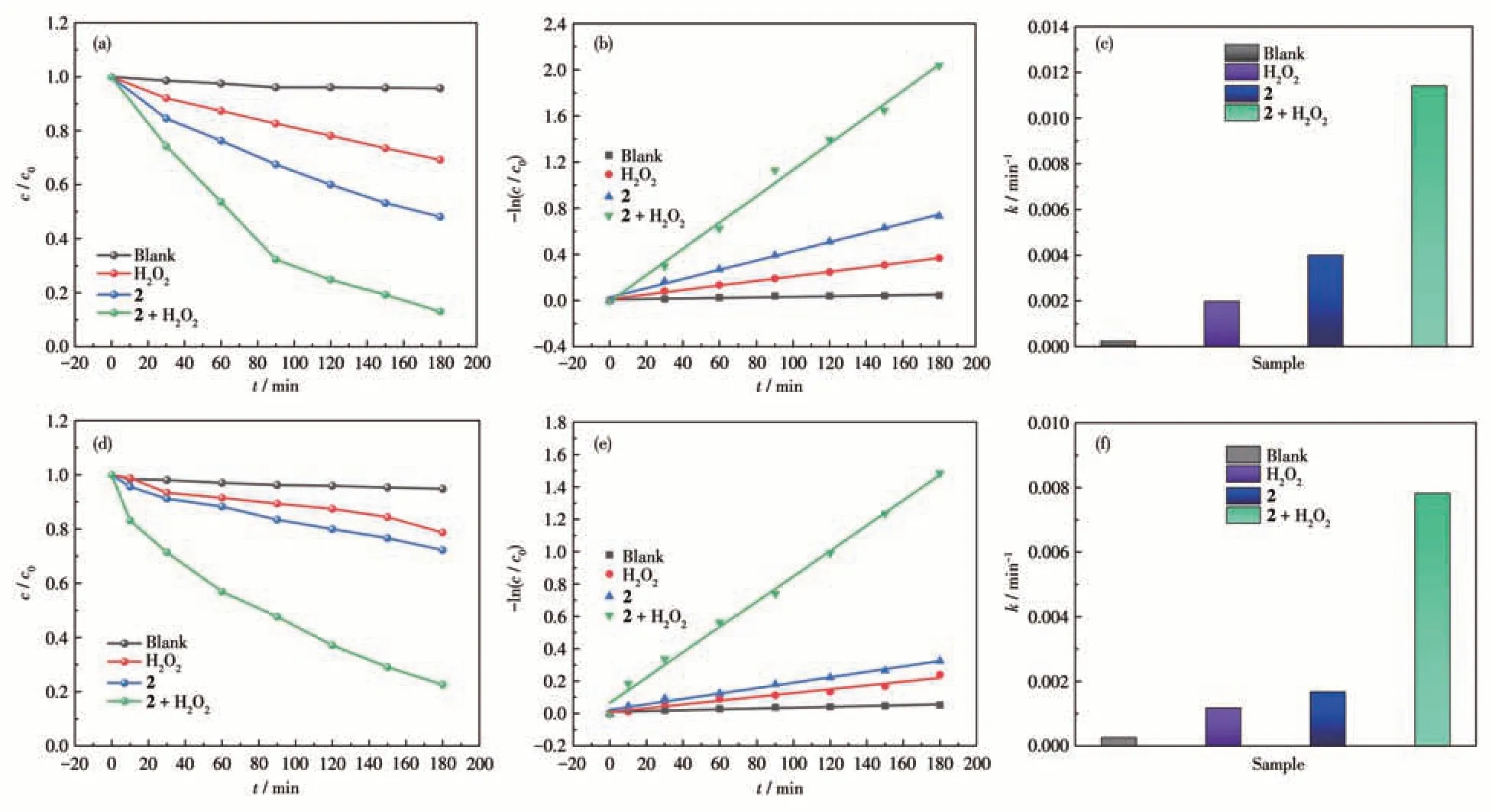
Fig.10 (a,d)Variation of photocatalytic degradation efficiencies of MB and RhB with visible light irradiation time under different conditions;(b,e)Plots of-ln(c/c0)vs t and the corresponding linear fittings for the pseudo-first-order kinetic equation;(c,f)Reaction rate constants of MB and RhB under different conditions
2.4.3 Photocatalytic properties of starting materials
To further demonstrate that complexes 1 and 2 play a major role in the photocatalytic degradation of dyes, subsequent experiments on the photocatalytic degradation of dyes were carried out in the presence of H2O2using metal salts (Co2+, Ni2+), auxiliary ligands(1,3-bib, 1,4-bib) and their mixtures, respectively, and the results are shown in Fig.11. In the presence of Co2+and 1,3-bib,there was only slight degradation of MO in 180 min,while there was more pronounced degradation in the presence of 1 (Fig.11a).MB was also only slightly degraded in the presence of 1,3-bib, 1,4-bib, Co2+,and Ni2+,while there was more pronounced degradation in the presence of 1 or 2 (Fig.11b). Finally, the same was true for RhB in the presence of 1,4-bib, H4L, Ni2+,and the mixture where there was only slight degradation, while in the presence of 2 there was more pronounced degradation(Fig.11c).
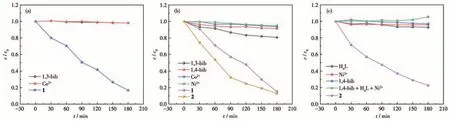
Fig.11 Degradation of MO(a),MB(b),and RhB(c)under different conditions
Why we did not degrade MO and MB in the presence of H4L? This is because H4L is acidic in an aqueous solution and will break the conjugate system of MO and MB, forming new substances, which does not serve the purpose of our initial degradation, so the photocatalytic degradation experiment of H4L was not conducted.
To prove the novelty of this work, a literature review was conducted. It is found that the auxiliary ligand used in this work has more advantages in the case of the same main ligand H4L. Compared to other works, the degradation efficiency (η) can still reach 70%-80% which is higher than that reported in the literature,as shown in Table 4.
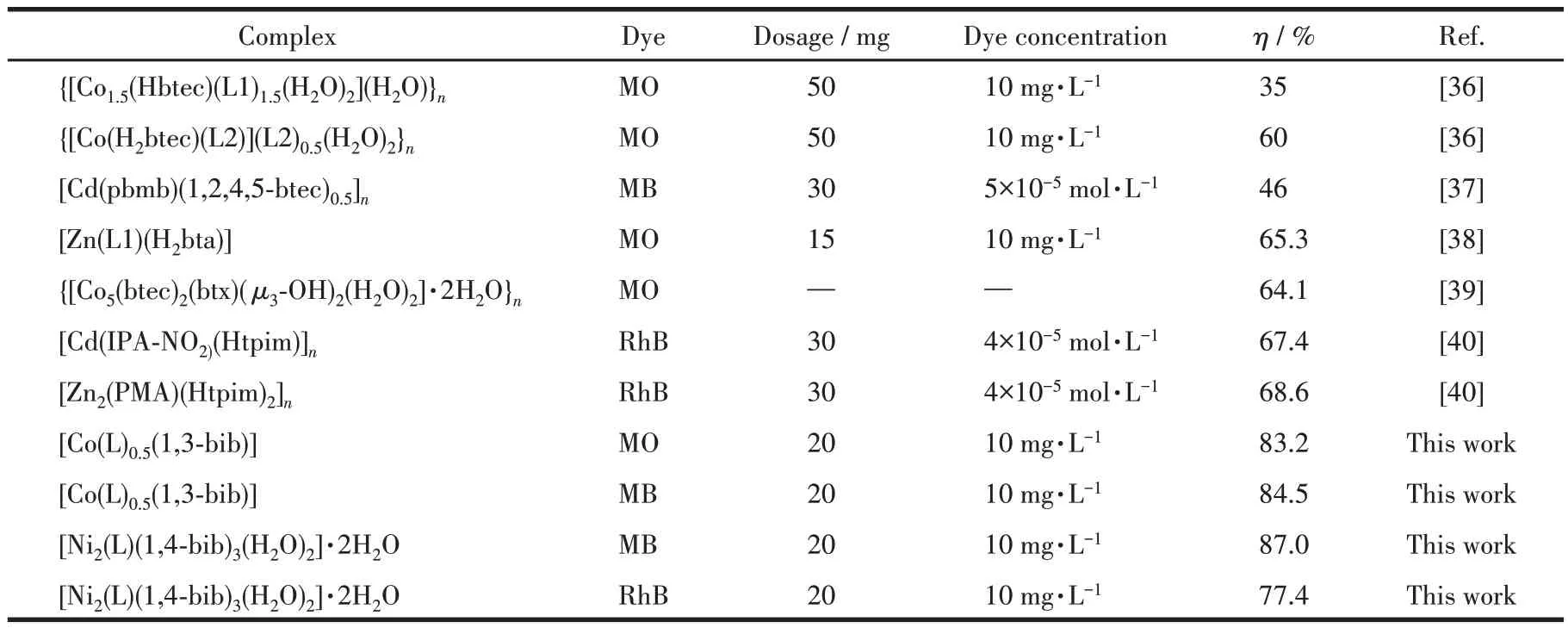
Table 4 Comparison of catalytic activity of MOFs-based photocatalysts
2.4.4 Photocatalytic degradation mechanisms
The photocatalytic mechanism of degradation of organic dyes is reported in the literature[41-44]. When semiconductor particles are excited by visible light irradiation, an electron from the highest occupied molecular orbital (HOMO) is stimulated to the lowest unoccupied molecular orbital (LUMO). Then, electron transfer from the VB (valence band)to the CB (conduction band) under irradiation of appropriate energy occurs, resulting in the formation of e-(CB)-h+(VB)pairs[45]. For the metal-organic frameworks, the process can be described as follows: electrons are transferred from the 2porbital of oxygen or nitrogen to the lowest empty orbital of the metal. Then, the electrons of the water molecule are trapped,leaving an equal amount of positive ·OH. Meanwhile, the e-in LUMO can react with oxygen which is adsorbed on the surface of MOFs,resulting in the generation of ·O2-. Hence, the dyes could be degraded effectively by oxidized substances and the photocatalytic process could be accelerated(Fig.12).
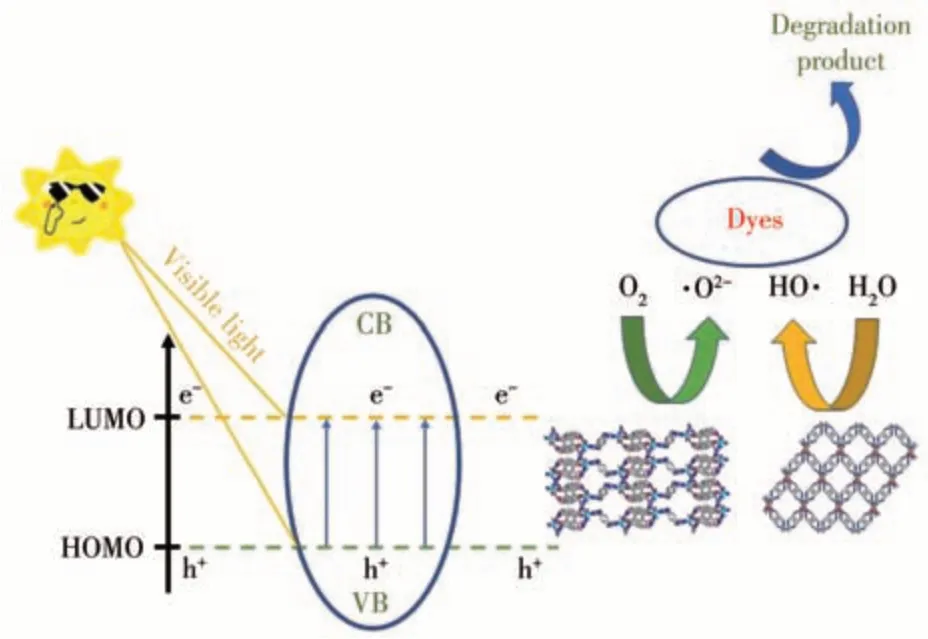
Fig.12 Mechanism of photocatalytic degradation of dyes by complexes 1 and 2 under visible light
To confirm the photocatalytic degradation process, we carried out a free radical trapping experiment in the process of reaction[46]. Then, BQ (benzoquinone,10.8mg)and TBA(tert-butyl alcohol,2 mL)were added to the solution containing dyes (MO, MB) and complex 1, and the solution containing dyes (MB, RhB) and complex 2 to capture the ·O2-and ·OH during the reaction,respectively.
Under the same experimental conditions, the degradation performances of complexes 1 and 2 in the presence of TBA and BQ were lower than those of the control groups without TBA and BQ (Fig.13), so we speculate that the intermediates may be ·OH and ·O2-species. They play an active role in the degradation process.
3 Conclusions
In summary, two new coordination polymers[Co(L)0.5(1,3-bib)](1)and[Ni2(L)(1,4-bib)3(H2O)2]·2H2O(2) were synthesized under hydrothermal conditions.The structures of complexes were confirmed by single crystal X-ray diffraction, IR spectra, elemental analysis, PXRD, and TGA. 1 shows a 3D network structure that is built from the Co unit interconnected by the L4-ligand.In 2,each Ni2+ion is connected to three 1,4-bib molecules, and each 1,4-bib molecule is connected to two Ni2+atoms, resulting in a 2D plane structure. The 2D plane structure extends to a 3D network via two carboxyl oxygen atoms of L4-. The photocatalytic studies show that 1 has good photocatalytic performance for MB/MO, and 2 can be used as an optional functional material to degrade MB/RhB in wastewater.
