Stage-specific treatment of colorectal cancer:A microRNA-nanocomposite approach
2023-12-14AewaleOluwaseunFaakaTaiwoAkinsojiAshwilKleinAramMaimaeMaieheMervinMeyerMarshallKeysterLukyMashuuSikhwivhiluNioleRemaliahSamanthaSiuyi
Aewale Oluwaseun Faaka ,Taiwo Akinsoji ,Ashwil Klein ,Aram Maimae Maiehe ,Mervin Meyer ,Marshall Keyster ,Luky Mashuu Sikhwivhilu ,Niole Remaliah Samantha Siuyi ,g,**
a Department of Anesthesia, Division of Pain Management, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, 45229, USA
b Department of Science and Innovation/Mintek Nanotechnology Innovation Centre, Biolabels Node, Department of Biotechnology, Faculty of Natural Sciences, University of the Western Cape, Bellville, 7535, South Africa
c School of Medicine, Southern Illinois University, Springfield, IL, 62702, USA
d Plant Omics Laboratory, Department of Biotechnology, Faculty of Natural Sciences, University of the Western Cape, Bellville, 7535, South Africa
e Nanobiotechnology Research Group,Department of Biotechnology,Faculty of Natural Sciences,University of the Western Cape,Bellville,7535,South Africa
f Environmental Biotechnology Laboratory,Department of Biotechnology,Faculty of Natural Sciences,University of the Western Cape,Bellville,7535,South Africa
g Department of Science and Innovation/Mintek Nanotechnology Innovation Centre,Advanced Materials Division,Mintek,Johannesburg,2125,South Africa
h Department of Chemistry, Faculty of Science, Engineering and Agriculture, University of Venda, Thohoyandou, 0950, South Africa
Keywords:Colorectal cancer microRNA Nanotechnology Nanocarriers OncomiRs TSmiRs
ABSTRACT Colorectal cancer (CRC) is among the leading causes of cancer mortality.The lifetime risk of developing CRC is about 5% in adult males and females.CRC is usually diagnosed at an advanced stage,and at this point therapy has a limited impact on cure rates and long-term survival.Novel and/or improved CRC therapeutic options are needed.The involvement of microRNAs (miRNAs) in cancer development has been reported,and their regulation in many oncogenic pathways suggests their potent tumor suppressor action.Although miRNAs provide a promising therapeutic approach for cancer,challenges such as biodegradation,specificity,stability and toxicity,impede their progression into clinical trials.Nanotechnology strategies offer diverse advantages for the use of miRNAs for CRC-targeted delivery and therapy.The merits of using nanocarriers for targeted delivery of miRNA-formulations are presented herein to highlight the role they can play in miRNA-based CRC therapy by targeting different stages of the disease.
1.Introduction
Colorectal cancer (CRC) is one of the most diagnosed types of cancers with over 600,000 global CRC-related mortality rates reported per annum [1].Like all cancers,CRC development is a multicomplex stage that involves genetic and epigenetic changes[2].CRC is a commonly used term to describe the combination of cancers of the colon and rectum.Globally,colon and rectal cancers accounted for approximately 1.14 million and 732,210 new cases;as well as 576,000 and 339,022 deaths in 2020,respectively [3].Despite an improved outcome with the current treatments,a large number of patients relapse and develop drug resistant CRC [4].Although treatment options such as surgical,chemotherapeutic,and immunotherapeutic approaches have advanced the treatment of CRC,the clinical prognosis of patients with CRC remains poor.Thus,improved or novel treatment strategies are required to prevent the challenges posed by the aforementioned approaches [5].As such,the importance and use of CRC-specific biomarkers for the early diagnosis and clinical treatment of CRC cannot be overemphasized.
Studies have elaborated on the use of oligonucleotides as CRC biomarkers and identified that noncoding RNAs play a key role in cancer development and progression [6].Regulatory noncoding RNAs include small nuclear RNAs,small interfering RNAs(siRNAs),and long noncoding RNAs.microRNAs(miRNAs)belong to the small noncoding RNA family and are involved in the regulation of gene expression at posttranscriptional level.Research advancements on these endogenous RNAs have been associated with their ability to alter gene expression,and they can be used as biomarkers for various diseases [7].miRNA mimics and inhibitors in preclinical development have shown potential as therapeutic agents[8].These RNAs were reported to play crucial roles in the regulation of cancers including CRC [9],and their dysregulation in CRC and specific expression signatures in different stages of the disease favor tumor stratifications.Thus,miRNAs can be employed in CRC management or therapy.
miRNAs have shown specific characteristics that impede drug design and efficacy.Factors,such as nuclease degradation [10],membrane permeability [11],endosome entrapment [12],binding specificity[12,13],cellular toxicity,immune system activation[14],and inability to exert desirable therapeutic efficacy in biological systems,have all been reported as limitations against the transition of miRNA-based therapies into clinical trials and their approval for use.Since the delivery of miRNA into target sites is inarguably a novel therapeutic strategy,chemical modifications and the use of delivery systems have been used to address their challenges [15].Chemical modifications of the sugar moiety and the backbone of the miRNA were shown to enhance the stability and efficacy of the miRNA.Replacement or modification of the 2′carbon of the ribose sugar by locked nucleic acid(LNA),2′-O-methyl,2′-fluoro and 2′-Omethyoxyethyl or modification of the miRNA backbone with phosphorodiamidate morpholino oligonucleotides and peptide nucleic acids has demonstrated varying degrees of improvement in their pharmacokinetics [14].Alternatively,lentiviruses and associated vectors have been used to successfully deliver miRNA into target cells due to their ability to efficiently express miRNA genes and incur low toxicity.However,their high production cost,antigenicity,low packaging efficiency,and safety of genomic integration are major concerns [16].The use of nonviral delivery systems such as nanocarriers can prevent these challenges and accelerate the use of miRNA in clinical trials.Nanocarriers have shown great and reliable potential for use in drug design for effective cellular delivery.Owing to the unique features of nanocarriers such as size,charge,surface composition and easily modifiable surface[17],they are best suited as drug delivery vehicles for the development of effective disease treatment with minimal or no side effects [18].Nanobased therapies can be tailored to be tissue-specific by attaching targeting moieties or be responsive to the tumor microenvironment.In this way,the biodistribution,uptake,drug release and drug delivery can be controlled through endogenous (pH,enzymes,and redox) or exogenous (light and temperature) stimuli[19].Efforts have also been made to assemble nucleic acids (DNA/RNA) into more efficient and biocompatible nanocarriers for RNAbased therapy[20].Nucleic acid technology has been demonstrated to have potential for miRNA-based therapy as delivery vehicles.However,the technology is still in its infancy,and more research into optimization for large-scale production and impact on human health is warranted[20].Therefore,insights into the use of miRNAbased CRC treatment and the design of miRNA-nanoformulations based on organic,inorganic,carbon-based and hybrid nanocarriers are summarized herein.We further review the delivery strategies and discuss specific examples of the preclinical development of miRNA-nanobased targeted therapeutics.By focusing on their role in CRC,we will demonstrate how this approach can improve miRNA-based therapeutics to accelerate their inclusion in clinical trials for potential use in disease treatment.
2.Role of miRNAs in cancer therapy
miRNAs are classified as mimics and inhibitors (antimiRs),and both antimiRs and mimics have been considered for miRNA-based therapeutics.AntimiRs bind to the complementary miRNA and repress its activity,while the mimics typically downregulate its target or cancer causing genes by overexpressing the miRNA [21].The potential use of miRNAs in cancer therapy as either oncogenes(oncomiRs) or tumor suppressors (TSmiRs) has been reported and discussed in detail previously [22].For instance,Lu et al.[23]identified the oncomiR role of miR-10b,which promotes cell migration and invasion in oral cancer [23].Similarly,the overexpression of miR-21 was correlated with tumor initiation and stimulation of prostate cancer invasion and metastasis [24,25].Evidence from a clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 deletion study suggested that miR-23b and miR-27b,through their suppressive activities under certain conditions,are oncomiRs in breast cancer [26].In addition,elevated levels of miR-23b have been implicated in a number of cancers including ovarian cancer[27],gastric cancer[28],and glioblastoma[29],as oncogenes by promoting tumor growth,proliferation,and metastasis.Other oncomiRs,such as miR-155,the miR-200 family,miR-495,and the miR-106b-25 cluster,have been well documented in various cancers [30].On the other hand,TSmiRs prevent tumorigenesis mainly through the inhibition of oncogenes including genes controlling differentiation and apoptosis[31].miR-32-3p is one example of TSmiRs,and its regulatory effect on theAurora Kinase Agene has been shown in different cancer types[32].TSmiR-15/16 clusters initiate the suppression of B-cell lymphoma 2,leading to cancer cell death,and further target specific cancercausing genes involved in cancer progression,including cluster of differentiation 1 and JUN [33].miR-19 inhibits gastric cancer cell proliferation by targeting myocyte enhancer factor 2D [34].Other TSmiRs include miR-133a-5p,which inhibits gastric cancer proliferation by targeting T-cell factor [35],whereas miR-34 inhibits tumorigenesis by targeting tumor protein p53 (TP53) in most cancers[36].The inhibition of oncomiRs by either miRNA inhibitors or TSmiR replacement with miRNA mimics suggests a valuable approach to cancer treatment [31].
Similarly,studies have reported the association between CRC and miRNA alterations [37].From our previous studies,we have extensively reviewed the modulatory effect of dietary miRNAs in CRC.Among others,the biosynthesis and function of miRNAs were discussed along with their tumor-specific metabolic reprogramming[38].In another study,we identified specific miRNAs that are differentially expressed in different stages of CRC[39].The pattern of expression along with their target genes was hypothesized to aid in the selection of targets for the diagnosis,drug development,and management of CRC [40].We also investigated the genomic profiling of miRNA target genes in CRC using computational approaches.This study revealed that miRNA genes with protein tyrosine kinases could be frequently altered in CRC[41].To further understand the mechanism of miRNA regulation in CRC,we explored the concept of miRNA-mRNA duplexes with Argonaut protein using in silico and molecular docking approaches.The observed interactions at the molecular level are important in protein folding,structural stability and in mediating the binding of the protein to their targets[42].Based on these findings,miRNA clearly presents an attractive target for the design of novel therapeutic strategies for CRC [43].
2.1.miRNA-based therapy for CRC
The expression of miRNA has a regulatory role in colorectal carcinogenesis,progression,and metastasis as highlighted in Fig.1.Depending on the cellular or tumor environment,miRNAs can act as TSmiRs or oncogenes.miRNA expression is frequently altered in various stages of CRC,and their expression patterns could be used in the diagnosis,prognosis and therapeutic outcome of CRC [44].Studies have investigated the involvement of miRNAs at each stage of CRC and their potential in the management of the disease.CRC represents a significant burden,globally.Early diagnosis and the identification of effective therapeutic strategies are pivotal for its management [45].As shown in Fig.1A,changes in normal cells resulting from DNA damage,mutation of specific genes or carcinogens underline colorectal carcinogenesis.These changes are correlated with dysregulation of different signaling pathways at various stages of CRC.During the progression stages (stages II and III),the PI3k and transforming growth factor (TGF)-β signaling pathways are frequently altered and this is the penultimate stage to neoplastic transformation.Gene alteration,phenotypic changes,cell proliferation,increased tumor size,invasion and cancer metastatic ability are evident at this stage.At stages 0-III,therapeutic agents should potentially alter the initiation and progression of CRC.In stage IV,tumor movement from the original site to other sites through blood circulation and the lymph system occurs.Thus,therapeutic agents with the potential to suppress angiogenesis and invasion of primary tumors can be used at stage IV.Therapeutic miRNAs for CRC have been identified,and some were reported to kill cancer cells through apoptosis with no significant effect on normal cells or tissues(Fig.1B).TSmiR exerts its function by binding to the RNA induced silencing complex (RISC) to carry out its suppressive role in various stages of CRC [22] (Fig.1C).
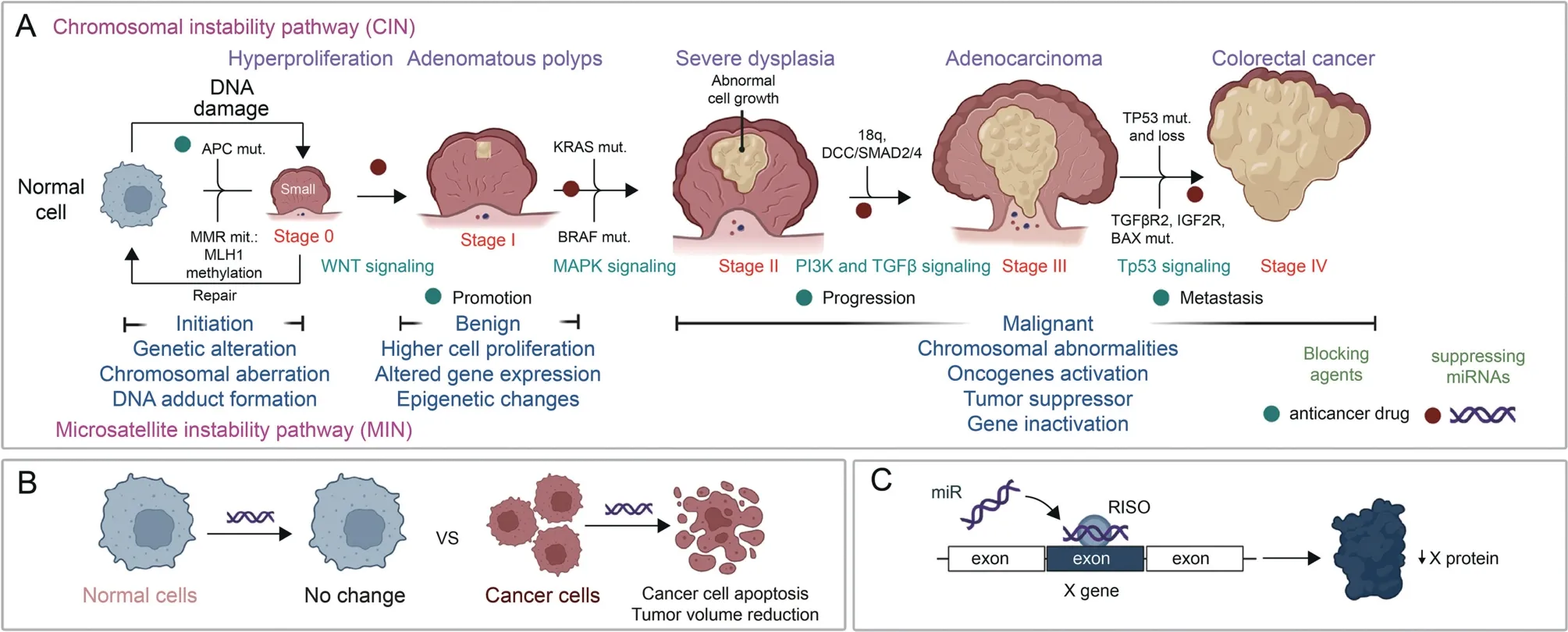
Fig.1.Colorectal cancer (CRC) development and progression,and the role of microRNA (miRNA) in its treatment.(A) Stages of colorectal carcinogenesis and the mechanism of tumor initiation,progression,and metastasis.(B) Differential effects of miRNA on tumor and normal cells.(C) The mechanism of action of tumor suppressor miRNA (TSmiR).
2.2.CRC stage-specific therapeutic miRNAs
The role of miRNA in CRC therapy has been widely investigated,identifying several miRNA candidates that are involved in CRC development,progression,metastasis,and drug response,as reviewed elsewhere [46].miR-196 emerged as a candidate therapeutic target and biomarker in CRC.miR-196 targets genes with oncogenic and/or tumor suppressor activities,such asHomeobox,Suppressor of cytokine signaling(SOCS)1,SOCS3,Annexin A1,DNA fragmentation factor subunit alpha,programmed cell death 4andinhibitor of growth family member 5.Alteration in the expression of these genes by miRNA could possibly reverse CRC by interfering with tumor development,progression,and response to therapy.miR-196 and its targets could then serve as novel biomarkers for early detection and prediction of prognosis in CRC patients[47].Some of the miRNAs have been associated with CRC resistance to radio-and chemo-therapy.Therefore,suppressing the expression of their gene targets could sensitize tumors to chemotherapeutic drugs such as oxaliplatin,doxorubicin,cisplatin,cetuximab,and 5-fluorouracil(5-FU)[46].The chemoresistance role of miR-27b-3p was investigated in CRC cells and xenograft models.The miR-27b-3 expression between oxaliplatin-resistant and the parental cells was found to be significantly reduced in oxaliplatin-resistant (SW480-OxR and HCT116-OxR)cells.In addition,miR-27b-3 expression was positively related to disease-free survival in CRC patients.Overexpression of miR-27b-3p in oxaliplatin-resistant cells increased their response to oxaliplatin,while inhibiting their expression in parental cells reduced the drug's activity.miR-27b-3p caused drug resistance by suppressing the expression of autophagy related (ATG) 10 at the posttranscriptional level,which in turn inhibited autophagy.c-Myc can potentially halt miR-27b-3p expression by suppressing its gene target,thereby enhancing the expression of ATG10 and activating autophagy.Thus,the therapeutic effect of miR-27b-3p is regulated through the c-Myc/miR-27b-3p/ATG10 signaling pathway which regulates CRC chemoresistance.miR-27b-3p can potentially be used in a clinical setting to evaluate how patients respond to oxaliplatin[48] and possibly other chemotherapies.
miRNAs implicated in various stages of CRC and their targets have been identified,as shown in Table 1 [48-69],and they can serve as theranostic biomarkers for CRC.These miRNAs target various molecular targets to either enhance or suppress their activities,and counteractive strategies against these actions can be used in cancer drug design and therapeutic intervention.
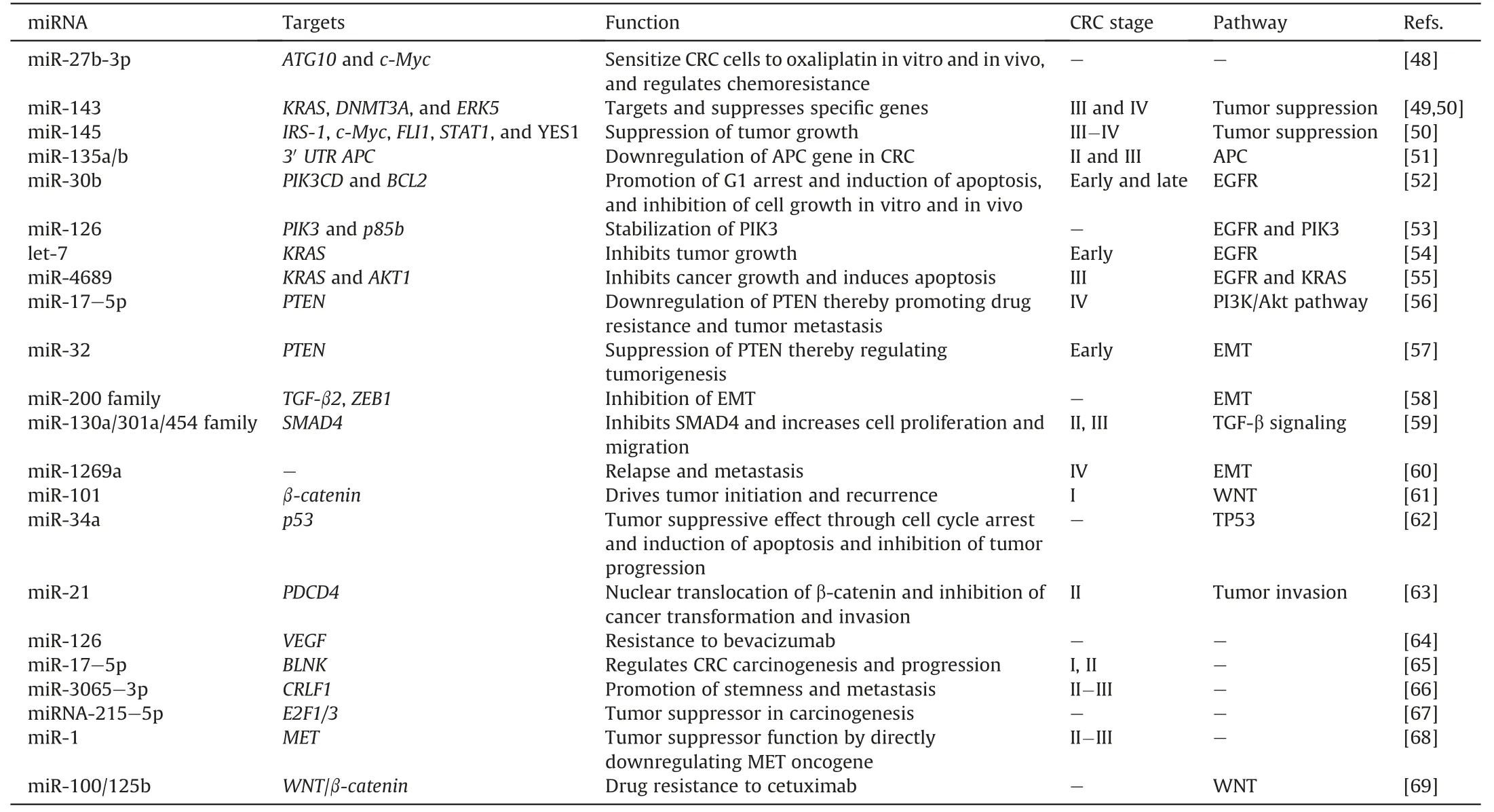
Table 1 Stage-specific microRNAs (miRNAs) with therapeutic activities in colorectal cancer (CRC).
The existing pool of knowledge suggests the multifunctional role of miRNAs as biomarkers and attractive drug targets for the management of nearly all human diseases,including CRC,as drug modulators and therapeutic agents [70].The approval of siRNA for clinical trials in 2018 was crucial to miRNA therapeutic advancement[71].Approximately eleven studies are registered with the U.S.National Library of Medicine (https://www.clinicaltrials.gov) that are undergoing various phases of miRNA clinical trials of colon cancerrelated therapy,as summarized in Table 2.As with other clinical studies,some therapeutic miRNAs have shown promising outcomes,while others have been discontinued due to adverse effects.MRX34(NCT01829971)is one of the miRNA therapies that was discontinued after presenting detrimental health effects in its phase I clinical trials for the treatment of solid and metastatic cancers[72].
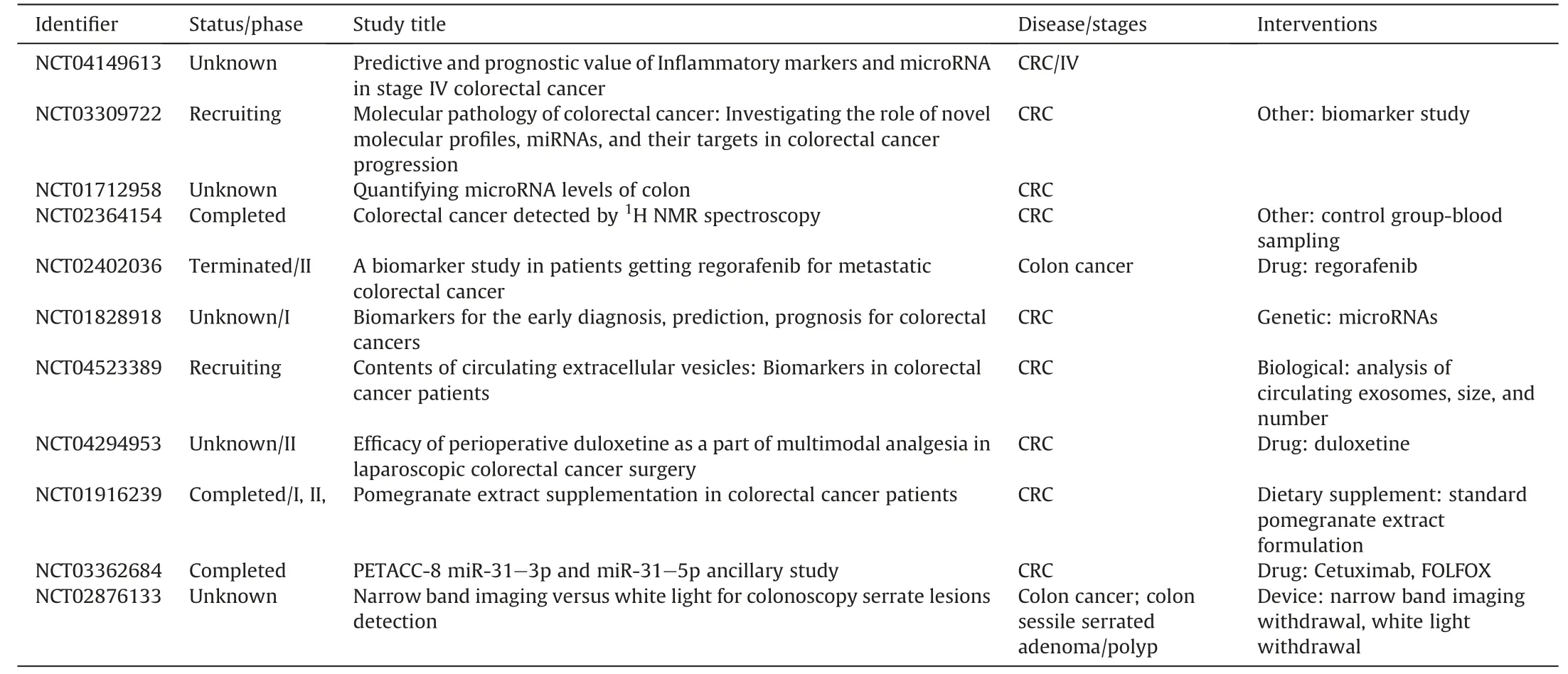
Table 2 microRNAs (miRNAs) associated with colorectal cancer (CRC) currently in clinical trials.
Challenges mitigating the efficacy and translation of miRNA therapeutics into clinical trials for cancer have been emphasized by experts in the field of drug discovery[73].Although some of these highlighted miRNAs might not be CRC-specific or have multiple targets,miRNA therapy is also limited by the factors shown in Fig.2,especially degradation by nucleases.The improvement or enhancement of miRNA stability,specificity and the design of excellent delivery systems that are disease-specific with minimal toxicity must be ensured for the effective use of miRNA in disease therapy [8].Chemical modification and miRNA delivery systems have been devised to overcome drawbacks of miRNAs.Chemical modification of the ribose sugar,nucleobases and the backbone of the miRNA may confer resistance to nuclease degradation while improving the thermal stability,binding affinity and potency of the oligonucleotides[14].The use of LNA,2‘-O-methyl,2’-fluoro and 2′-O-methyoxyethyl to replace the 2′carbon of the ribose sugar or modification of the oligonucleotide backbone with phosphodiester,phosphorothioate,phosphonoacetate,phosphorodiamidate morpholino oligomers,and peptide nucleic acid [74] all improved the nucleotide stability and activity.Detailed discussions on the RNA chemistries that can be used to modify the miRNA have been reviewed and reported elsewhere [74].Delivery vehicles are another strategy that has been successful in improving drug stability and biocompatibility,and can be achieved by attaching targeting moieties on miRNAs or using drug carriers such as exosomes,viral vectors or nanocarriers.In this review,various types of nanocarriers that can be used in miRNA delivery for various diseases with the focus on CRC,for sustained release,reduced toxicity,and targeted delivery will be discussed.Various organic,inorganic and carbon-based nanocarriers have all been proposed to ameliorate miRNA limitations.The specificity of the nanocarriers can be enhanced using targeting molecules(i.e.,antibodies and aptamers)or making them responsive to external (charge,heat,and light) or internal (pH,enzymes,and temperature)cellular stimuli.
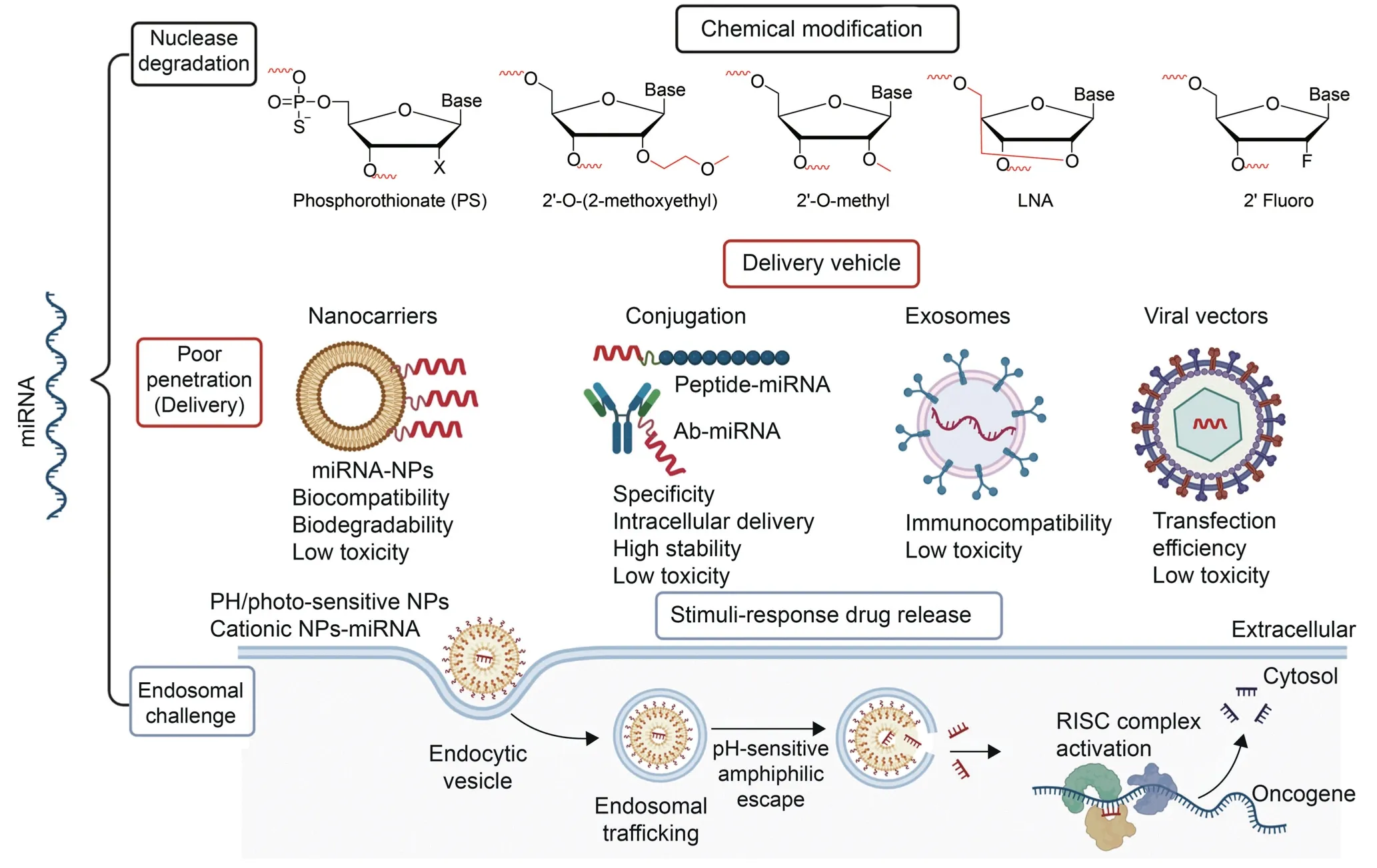
Fig.2.Challenges faced by microRNA (miRNA) therapeutics and the proposed solutions that can be used to overcome them.
3.miRNA nanocarriers for CRC management
The benefits of miRNAs in gene manipulation and cancer therapy have been increasingly recognized for developing miRNAbased therapies.However,controlled delivery of miRNAs into specific cells is a challenge.This section describes nanocarriers that might be useful for miRNA and further proposes two models using hybrid nanocarriers for miRNA delivery to eradicate CRC cells and tumors.Drug delivery systems or hybrid nanocarriers that can deliver chemotherapeutic drugs and oligonucleotides to specific tissue sites may present a crucial approach for cancer therapy,as discussed below.
Nanocarriers are referred to as any material typically within a size range of <500 nm and have shown potential for transporting substances such as drugs,proteins,and other molecules to specified targets[75].Owing to their physicochemical properties,especially their size,drug loading capacity,surface composition,etc.,these nanomaterials have been used to circumvent the challenges associated with drug transportation across biological systems [75].Their continuous investigation over the last decade is also attributed to their potential or great promise as drug delivery agents.Many cancer drug carriers or delivery vehicles present cumbersome limitations,such as cellular toxicity,induction of drug resistance and poor drug specificity which decrease the therapeutic effects of the drugs [76].Nanocarriers have enabled effective delivery of therapeutic agents into the tumor site by exploiting the characteristics of the tumor microenvironment,and significantly improved the patient's response to drugs.Nanocarriers can be broadly classified into organic,inorganic,carbon-based and hybrid nanocarriers depending on the materials from which they were synthesized.Some of the well documented nanocarriers are depicted in Fig.3.All these nanomaterials follow a similar mechanism of cellular uptake and internalization which is based on their size,charge and surface composition.Their small size allows them to passively or actively pass through cellular barriers and shuttle their cargoes.Passive targeting is made possible by the enhanced permeability and retention (EPR) effect on the tumor vasculature and microenvironment,while active targeting takes advantage of the differentially expressed disease-specific biomarkers [77].Biomarkers associated with tumor or diseased cells can be targeted by conjugating targeting ligands or molecules on nanocarriers and selectively be delivered to cells that express receptors on the cell surface [78].The design of multisystem (hybrid) nanocarriers consisting of organic and inorganic nanomaterials can enhance the properties of such nanosystems.
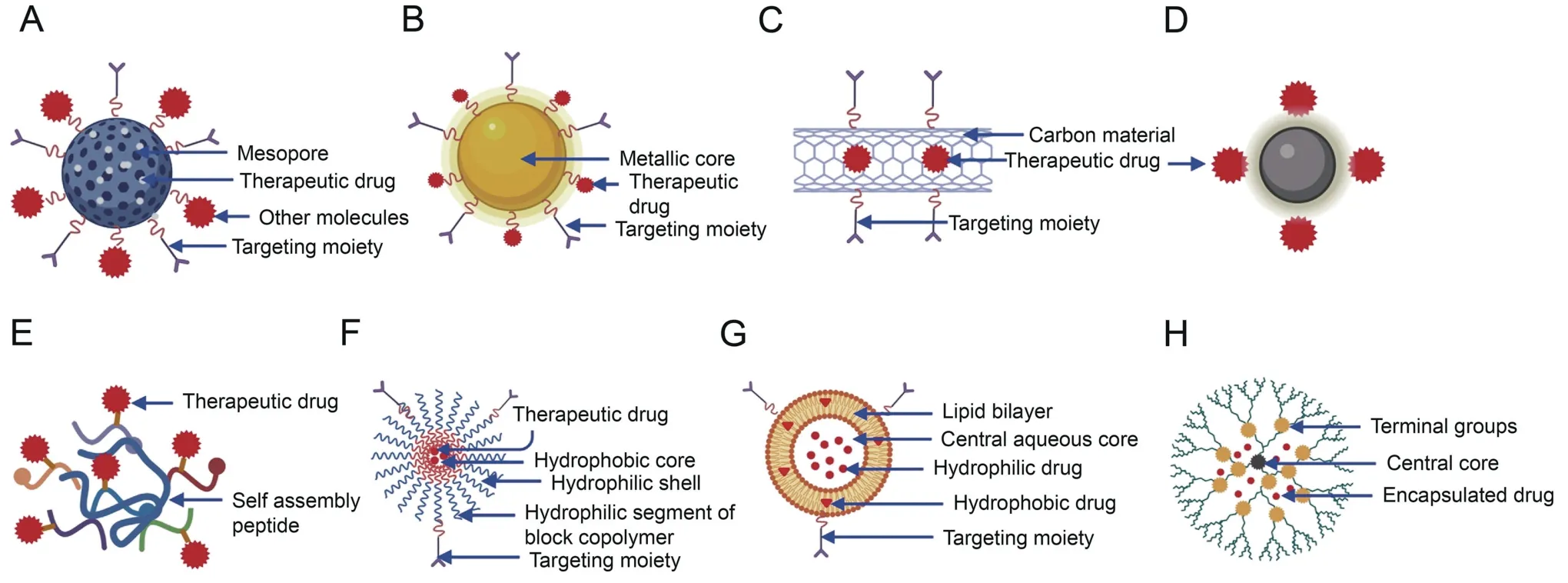
Fig.3.Various types of nanocarriers used in drug delivery.Drugs and targeting moieties are conjugated or encapsulated on nanocarriers to create disease-specific therapies,such as the(A) mesoporous silica nanocarriers (MSNs),(B)metallic nanoparticles (MNPs)including gold nanoparticles (AuNPs),(C)carbon nanotubes(CNTs),(D)quantum dots(QDs),(E)peptides nanocarriers,(F) micelles,(G) liposomes,and (H) dendrimers.
3.1.Organic nanocarriers in drug delivery
Organic nanocarriers are generally characterized by their biodegradability,biocompatibility,and improved drug loading capacity.Chemotherapeutic agents can be trapped or bound within their matrix.Common forms of this class of nanocarriers include dendrimers,liposomes,peptides,and micelles [79].
3.1.1.Dendrimers
Dendrimers are composed of a central core,a hyperbranched mantle,and a corona with peripheral reactive functional groups.These distinct domains allow for the ramification of subunits and the manipulation of their physical and chemical properties [80].Dendrimers possess enhanced properties compared to conventional polymers.In addition to their application as delivery systems,dendrimers also possess therapeutic properties such as antimicrobial and anticancer activities [80].They have been used as carriers to improve the issues that impede clinical applications of therapeutic agents by ameliorating drug solubility and stability,as well as reducing toxicity [81].Although several dendrimers have been identified and successfully developed as vehicles for drugs,only polyamidoamine (PAMAM) and poly-propylene imine (PPI)are widely used for clinical applications.The benefits of these nanocarriers can be recognized by their potential to penetrate biological barriers through active and passive targeting and are presently exploited for various medical applications as drug delivery agents [82].
The benefits of dendrimers include efficient loading capacity of the therapeutic molecules via surface functional groups and internal cavities,high bioavailability,and high permeability through biological barriers [83].The biomedical uses of dendrimers have attracted much attention,particularly due to their potential for use as tailored therapeutic molecules [81],gene delivery systems [84]and imaging-based diagnosis[85],along with the improvement in disease therapy such as cancer [86],cardiovascular diseases [87],inflammatory diseases [86],and viral and bacterial infections[86].Although this special class of nanoparticles(NPs)has a wide range of applications in medical fields,their toxicity is well documented as a limitation for their application [88].To increase their biocompatibility,dendrimers can be functionalized with biological molecules (i.e.,dendriplexes) for therapeutic purposes [82].The dendriplex formulation is favored by the opposing charges between the dendrimer surface and its conjugates,where positively charged dendrimers can bind to negatively charged biomolecules such as oligonucleotides[89].
Several anticancer drugs,such as doxorubicin have been formulated with dendrimers to achieve enhanced anticancer effects [90].Cationic dendrimers are a good choice as miRNA drug delivery systems because they can readily interact with negatively charged nucleic acids.Duan et al.[91] produced dendriplexes by coating PAMAM with chondroitin sulfate (CS) for the delivery of miR-34a into pancreatic cancer cells.The effect of CS-PAMAM/miR-34a was investigated on a human pancreatic cancer (MiaPaCa-2) cells.The study demonstrated the uptake and delivery of miR-34a within the cells.In addition,scratch and transwell migration assays confirmed their suppressive activity against tumor cell growth [91].Similarly,folic acid(FA)was conjugated to PAMAM to deliver miR-7a to U251 glioma cells.FA-PAMAM/miR7a bound to the FA receptors that are overexpressed on the cell surface of cancer cells,penetrated the cells and efficiently silenced the expression of EGFR,PI3K,and AKT2 to achieve favorable effects in the treatment of glioma[92].
Protein-protein interaction(PPI)dendrimers have also attracted enormous attention in gene therapy and have mostly been studied for their siRNA delivery potential.Protonation of the amino groups of PPI allows for interaction with negatively charged oligonucleotides and thus loading and transportation of multiple biomolecules [93].PPI dendrimers were modified with siRNA(siRNA-PPI NPs)and further conjugated with luteinizing hormonereleasing hormone(LHRH)peptide,which targets cancer receptors to improve biocompatibility and promote target specificity.This strategy enhanced tumor targeting and uptake of the siRNA,efficient gene silencing,and successfully increased serum siRNA stability.In vivo data confirmed that targeted siRNA-PPI-LHRH accumulated at the tumor site,while nontargeted siRNA-PPI was detected in other organs,and only trace amounts reached the tumor site [94].To improve the detection of tumor progression and therapeutic responses in situ,Taratula et al.[95]further conjugated superparamagnetic iron oxide (SPIO) to siRNA-PPI-LHRH.This system could simultaneously deliver siRNA to tumor cells while using SPIO for MRI imaging of the primary tumor and metastases.This system could effectively deliver siRNA therapeutics for cancer treatment and monitor therapeutic responses in situ with reduced side effects on healthy organs [95].Unfortunately,PPI dendrimers could alter the expression of endogenous genes primarily involved in immune defense responses,cell proliferation and apoptosis[96].These findings indicate that PPI dendrimers may have an intrinsic effect on human gene expression,which may restrict their clinical use.
3.1.2.Peptide nanocarriers
Peptide nanocarriers are self-assembling peptides rationally folded into supramolecular structures at a nanoscale size/diameter[97].These self-assembled nanostructures have been exploited for drug delivery purposes owing to their binding affinity and target propensity.Well-defined nanostructures are produced through the interactions of the peptide residues or by microphase separation[98],and assembled into micelles,vesicles,nanofibers,nanoribbons or nanotubes.In addition to their biocompatibility and biodegradability,peptide nanocarriers also allow attachment of multiple molecules,have high body retention time,and can easily penetrate cellular barriers [99].Several peptide-based nanocarriers (peptiplexes)have been developed for the delivery of nucleic acid-based therapeutics[100].However,purely peptide-based nanoconstructs for nucleic acid delivery in vivo are considerably lower due to their chemical characteristics.Limitations such as their instability and susceptibility to biodegradation in vivo can be improved through PEGylation [101].
3.1.3.Liposomes
Research into liposomes as nanocarriers for bioactive molecules is rapidly expanding because of their excellent biodegradability,biocompatibility,low tissue toxicity,low immunogenicity,and fair cost of synthesis[102].Lipid-based nanostructures are composed of amphipathic phospholipids and colipid sterols.Liposomes can transport molecules by entrapping hydrophobic and hydrophilic bioactive compounds within their bilayer and aqueous center[103].A phase II study of sterically stabilized liposomal cisplatin(SPI-77)was carried out in advanced non-small cell lung cancer (NSCLC)[104].The efficacy and tolerability of SPI-77 was assessed in 26 participants who were divided into three groups.Each group received different concentrations of SPI-77 (100 mg/m2,200 mg/m2,and 260 mg/m2).At the end of the study,drug response,toxicity,and disease status,among others,were assessed.Modest activity of SPI-77 was observed in patients with NSCLC.The study further concluded that even though SPI-77 did not demonstrate sufficient activity to warrant additional investigation,large doses of liposomal cisplatin could be administered safely with less toxicity[104].Consequently,this application can be extended to other cancers.In another phases I and II clinical trials,cisplatin was encapsulated in PEGylated liposomes (SPI-077),and the formulation was investigated against 18 participants with inoperable head and neck cancer.SPI-077 was well tolerated with no toxicity but the study concluded that SPI-077 was not active against squamous cell carcinoma of the head and neck and that SPI-077 could continue as chemotherapy [105].Similar results were obtained from the treatment of ovarian cancer patients with SPI-77 in a phase II clinical trial.The study reported no side effects despite a large cumulative dose of the formulation.However,concerns associated with large lipid load and prolonged persistence of residual platinum in body stores were detected [106].These results concluded that the significant toxicity of cisplatin was successfully prevented when it was entrapped or formulated within the liposomes.Of note,liposomes were the first nanocarriers to enter clinical trials in 1995 for the delivery of doxorubicin under the tradename Doxil.To date,there are a number of liposome-based nanocarriers on the market that are used for the delivery of fungal antibiotics,analgesics,vaccines,etc.[107].These systems have also shown promise for the delivery of miRNA-based therapeutics for cancer treatment [108].
3.1.4.Micelles
Polymeric micelles are self-assembling nanoconstructs with a core-shell structure capable of transporting poorly soluble drugs[109].These amphiphilic copolymers showed extended circulation time in the blood and increased drug accumulation at the pathological site [110].Cellular toxicity,poor solubility and poor biodistribution associated with therapeutic molecules and other drug delivery systems are some of the drawbacks that can be easily averted by the use of micelles [111].Ribociclib,a poorly soluble anticancer drug,demonstrated improved solubility when encapsulated in dodecylphosphocholine (DPC) micelle nanocarrier through molecular dynamics simulation.DPC micelles spontaneously encapsulated the hydrophobic ribociclib molecules in aqueous environments through hydrophobic and van der Waals interactions.The solvent accessible surface area and radial distribution function in this study showed that the nanocarrier can improve the aqueous solubility of any hydrophobic drug [112].Many micelle nanocarriers have been developed for siRNA delivery over the years[113].Micelles are modified by conjugating targeting molecules or by attaching environmentally sensitive block copolymers with a clear focus of preventing siRNA degradation within systemic circulation until it reaches the RNAi machinery in the cytoplasm [114].
It is evident that the use of organic nanocarriers offers distinct benefits over free drugs.Such benefits include 1) drug encapsulation/entrapment which can protect biomolecules from nuclease or proteolytic biodegradation,2)sustained or stimuli-responsive drug release that allows delivery of the drugs at a desired target and rate,3)prolonged drug circulation,and 4)active and passive targeting of the diseased sites for optimal therapeutic effects.
3.2.Inorganic nanocarriers in drug delivery
Inorganic nanocarriers have been reported as excellent drug carriers and effective drug delivery systems due to their unique physicochemical properties which include biocompatibility,nontoxicity,ease of surface manipulation,high surface-to-volume ratio,high stability,imaging ability,and hydrophilicity [115].Inorganic NPs are divided into metallic,ceramic,and semiconductor NPs.Although NPs made from noble metals(e.g.,silver NPs(AgNPs)and gold NPs (AuNPs)) and metal oxides (e.g.,magnetic (Fe3O4),zinc oxide,and copper oxide (CuO) NPs) are widely studied [89],other inorganic NPs exist.Only the most researched inorganic NPs used in the delivery of drugs and miRNAs will be discussed in this section.
3.2.1.Mesoporous silica nanocarriers(MSNs)
The important parameters to be considered in the development of effective drug carriers are their loading capacity and drug release profiles.Silica NPs appear to be perfect candidates because they are eco-friendly,less toxic at 100-200 nm,and have a higher loading capacity and controllable drug release profile,and their synthesis involves non-metallic materials [116].Due to their unique properties such as size,shape,morphology,extremely high surface area and uniformly sized mesopores,they have been used in different fields of research for diverse applications,including drug delivery purposes.Because dispersibility is one of the major limitations of this material,surface manipulation can be carried out to compensate for the poor dispersion and to improve biocompatibility[117].Silica material has been approved for phase I clinical trials in humans [118].This advancement has propelled the use of this material for drug delivery development.MSNs are ideal for drug delivery,taking advantage of their pore channels.The process of drug loading and release from MSNs can be divided into two categories,namely,the use of organic or inorganic materials as a“gate keeper” or surface coating with biodegradable polymers or biomolecules as shown in Fig.4.Surface functionalization can be achieved by the addition of targeting moieties,stabilizing agents(PEG),peptides,proteins,and gatekeepers.Cargo loading also includes nucleic acids (DNA,RNA,siRNA,and miRNA),imaging agents,and bioactive molecules.Different chemotherapeutic drugs have been loaded onto MSNs to treat different cancers [119].Similarly,these strategies can be employed in the development of miRNA-nanocarriers for CRC.
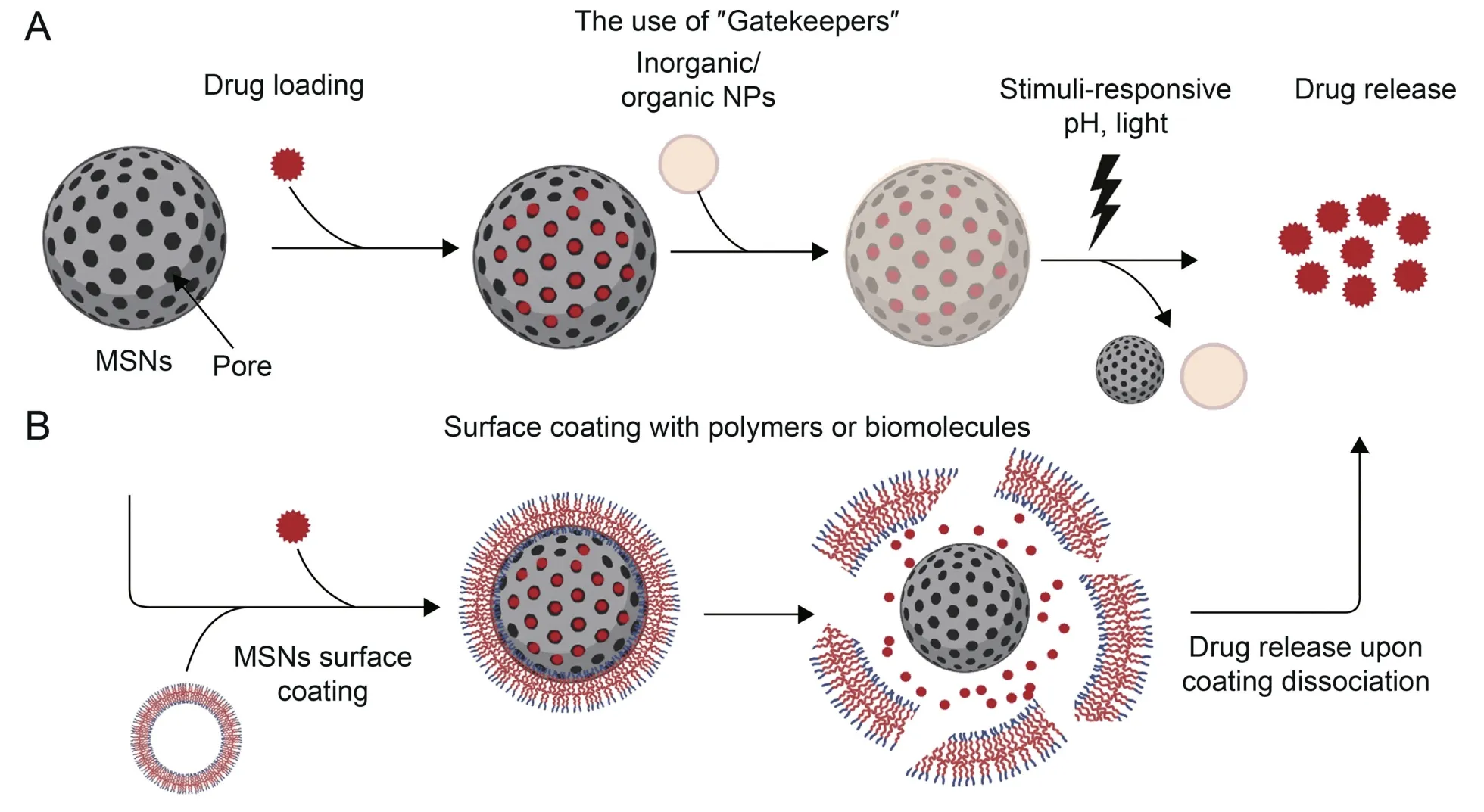
Fig.4.Schematic illustration of the drug loading and release activities of mesoporous silica nanocarriers(MSNs).Drugs can be loaded directly into the pores.(A)Different materials can act as gatekeepers to control the release of drugs,or (B) surface coating can be achieved using biodegradable polymers or macromolecules.
3.2.2.Metallic nanoparticles(MNPs)
MNPs possess remarkable properties leading to their use in different research fields.The most notable MNPs used in the field of drug delivery include gold,palladium,silver,copper,titanium,nickel,zinc,iron,and their oxides.The ease of synthesis and surface modification of these NPs with various bioactive molecules,such as contrast agents,antibodies,and therapeutic drugs,make them a valuable tool for theranostic purposes.Studies have extensively reviewed the synthesis,physicochemical properties,applications,and drawbacks of AgNPs [120,121],AuNPs [122],Cu/CuO [123],Fe2O3[124],and other metal-based NPs [125,126].The AuNPs and AgNPs illustrated in Fig.5 are some of the examples of metal-based NPs used for oligonucleotide delivery.
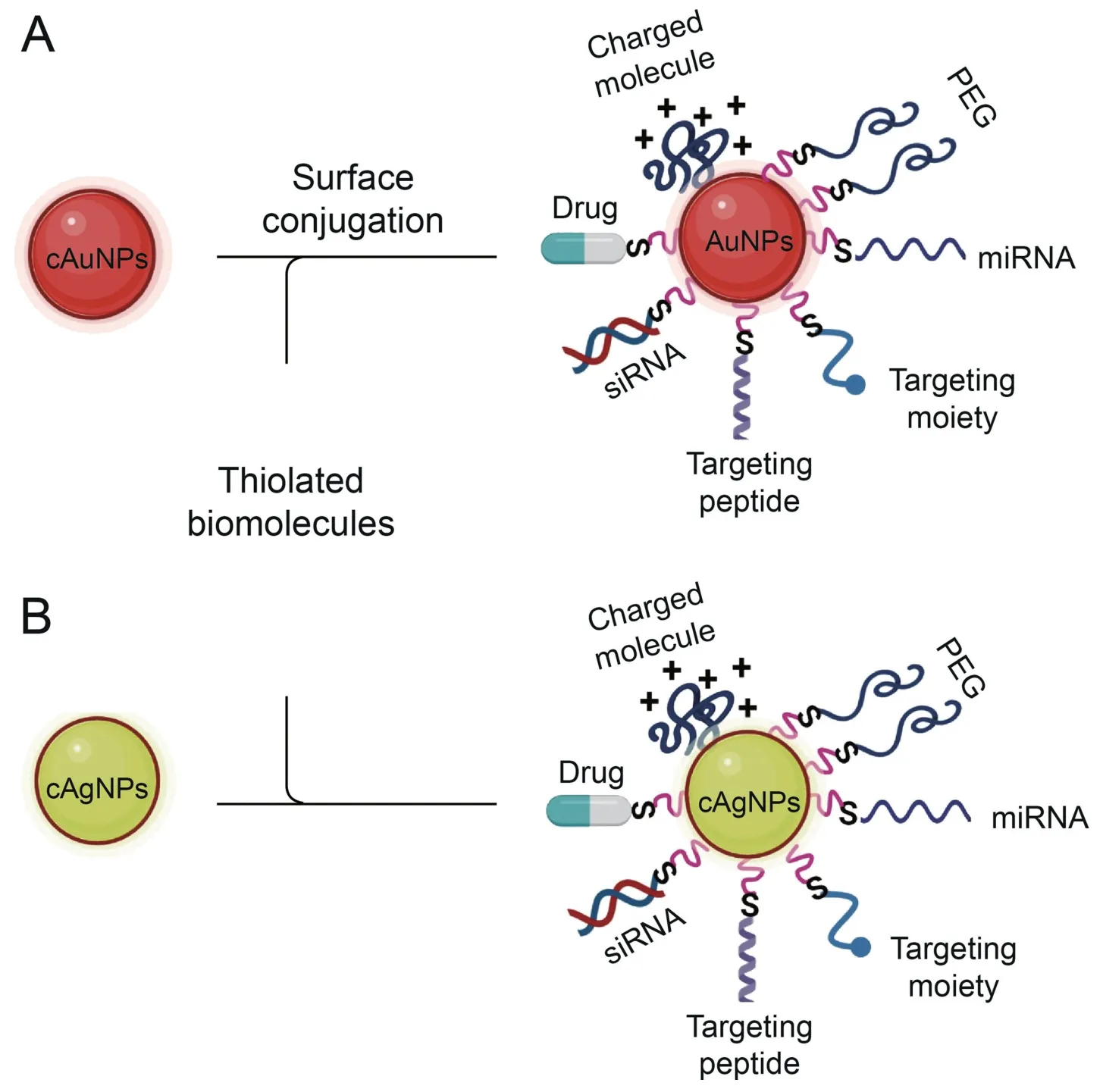
Fig.5.Schematic illustration of inorganic nanocarrier surface functionalization for microRNA(miRNA)delivery.Surface conjugation of(A)gold nanoparticles(AuNPs)and(B)silver nanoparticles (AgNPs) for delivery of miRNA.
Stabilization and further manipulation of MNPs can be achieved by surface functionalization with different molecules through different chemistries.MNPs have high affinity for thiolated molecules,and any molecules modified with a thiol group can easily adsorb on their surfaces.Nucleic acids and antibodies can be conjugated on the surface of MNPs by first introducing thiolated linkers followed by chemistries such as electrostatic interactions or covalent conjugation through 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC)/N-hydroxysuccinimide (NHS) coupling chemistry[127].MNPs,especially AuNPs,have been used in gene therapy as vehicles and transfection agents[115].DNA can be immobilized on AuNPs and still retain its bioactivity.The DNA can stably attach to the AuNPs and remain bound in the presence of other biomolecules(proteins).In addition,stabilization of the AuNPs with proteins does not mask their activity,as confirmed by bovine serum albumin(BSA)-stabilized DNA-AuNP conjugates,which were able to hybridize with their complementary DNA [128].
The transfection efficiency and efficacy of oligonucleotide-AuNP conjugates were demonstrated to be time-dependent and effective at lower doses.The AuNPs served as biosensing or targeting probes against multiple myeloma cells.The conjugation of Cy5 and miR-130b on the AuNPs was achieved by first treating AuNPs with diethyl pyrocarbonate,followed by chemisorption of miR-130b duplexes and oligoethylene glycol through a thiol group.Although 4%coverage of miR-130b was achieved,the miRNA-AuNP conjugate was able to produce significant gene knockdown efficiency [129].
In an independent study,the therapeutic ability of miRNA mimic(miR-206)was queried in breast cancer(MCF-7)cells using AuNPs as delivery systems.PEGylated AuNPs were stabilized by the addition of NH2-PEG-SH which facilitated attachment of the miRNA-206 mimic.The miRNA-206-AuNP conjugate was successfully delivered into MCF-7 cells and induced cell death by arresting cells in the G0-G1phase and downregulating the NOTCH-3 pathway which subsequently induced apoptosis [130].Similarly,AgNPs were also used as miRNA carriers as demonstrated in Figs.5B and 6.In Fig.6 [131],the AgNPs were functionalized with fluorescein amidine-tagged miR-148b and 5’ amine miR-148b.The delivery of miR-148b by light-inducible AgNPs selectively induced apoptosis in Ras-expressing keratinocytes in vitro and suppressed tumor growth in vivo.AgNPs-miR-148b achieved precise spatiotemporal control,high cellular uptake,and low cytotoxicity,escaped from endosomes and released a functional miRNA into the cytosol[131].
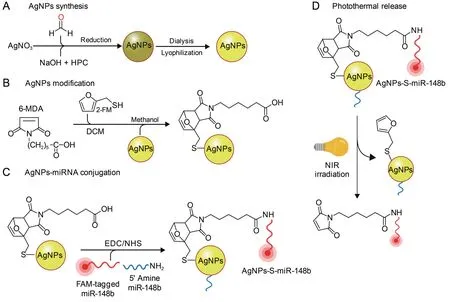
Fig.6.Photothermal release of microRNA(miRNA)-silver nanoparticles(AgNPs)nanocarriers for cancer treatment.(A)Chemical reduction of AgNO3 was achieved by formaldehyde and hydroxypropyl cellulose (HPC).(B) AgNPs were further modified by 6-maleimidohexanoic acid (6-MDA) and 2-furanmethanethiol (2-FM).(C) Conjugation with miRNA was further carried out using EDC/NHS chemistry.The miRNA release rate was measured upon irradiation with 415 nm wavelength light.FAM:fluorescein amidites;EDC:(1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride;NHS: N-hydroxysuccinimide;DCM: dichloromethane;NIR: near-infrared.
3.3.Carbon-based nanostructures as drug carriers
Carbon-based nanocarriers(CBNs)are highly recommended for biomedical applications,as they are perceived to be biocompatible.CBNs have exceptional chemical and physical properties that have generated much interest in theranostics,and their photostable fluorescent properties allow for deep tissue imaging and tracking in real-time.Carbon nanotubes (CNTs),graphene-based nanomaterials and carbon dots(CDs)are some examples of CBNs being explored in disease diagnosis and therapy as drug delivery,bioimaging and therapeutic agents.Limitations of this class of CBNs include low solubility,rapid excretion from the body,low biocompatibility,and low targeting[132].Possible toxicity of CBNs has been reported,where a nontoxic dose of these materials can alter cellular morphology and certain genetic profiles[133].Surface modification of the CBNs was shown to improve their biocompatibility and therapeutic efficacy.Their potential use in gene delivery is highlighted below.
3.3.1.CNTs
CNTs,produced from graphene sheets,are classified into single walled (SW) and multiwalled (MW) CNTs.Although considered safe for biomedical applications,studies revealed that the residual of the heavy metals used in their synthesis could potentially be cytotoxic [89],resulting in the use of polyamine polymers (polyethyleneimine (PEI) and PAMAM) to reduce toxicity and ease of functionalization [134].SWCNTs proved to be biofriendly than MWCNTs and were successfully used in drug delivery.Potent gene silencing and siRNA delivery were achieved by SWCNTs modified with cleavable disulfide bonds for controlled release of their cargo within the cells [135].The SWCNT-PL-PEG-S-S-DNA-Cy5 and SWCNT-PL-PEG-S-S-siRNA complexes achieved a more desirable effect than the SWNT systems without the disulfide linkage.The DNA-Cy5/siRNA was released by the pH-dependent lysosomal enzyme from the SWCNTs into the cytosol and translocated into the nucleus,while the control accumulated in the perinuclear region and was unable to penetrate the nuclear membrane [135].The major limitation for CNTs as delivery vehicles is their hydrophobicity,and changing the surface composition through surface modification can alter their properties.Moreover,the aspect ratio also plays a role in the efficiency of CNTs as miRNA delivery systems.Polyamine-coated MWCNTs demonstrated differential transfection efficiency of miRNA mimics in human embryonic kidney 293 cells [134].
3.3.2.Graphene-based nanocarriers
Graphene derivatives such as graphene oxide (GO),reduced graphene oxide (rGO),nanographene oxide (NGO),graphene quantum dots (GQDs) and graphene nanoribbons have improved solubility and have been widely used in biomedical applications.GO,GQDs,and rGO are frequently studied as nanocarriers for genes and therapeutic molecules[136].Specifically,GO and GQDs contain oxygen-active groups on their surface,have high solubility and high drug loading capacity and are thus more efficient drug delivery systems [137].In addition,the size and fluorescence properties of GQDs make these nanographenes suitable probes for simultaneous drug delivery and imaging [138].The use of nanographenes as carriers for therapeutic molecules including miRNA has been investigated and well documented [139].Graphene-based nanocarriers have been reported for miRNA delivery in different cancers including lung cancer[140],breast cancer[141],liver cancer[142],and colorectal adenocarcinoma [143].
Graphene-based complexes increased the transfection efficiency of mimic and antisense miRNAs into glioblastoma cancer cells.The delivery of GO and rGO complexed with either miRNA mimics(miRNA-124 and miRNA-137)or antisense oligonucleotides(miRNA-21,miRNA-221,and miRNA-222) was investigated in various glioblastoma (U87,U118,U251,and T98) and human fetal foreskin fibroblast (HFFF) 2 cells.The GO-miRNA complexes reduced cell viability by activating apoptosis in all glioblastoma cells and not HFFF2 cells,showing their potential as a promising delivery system in cancer therapy [144].Graphene nanocarriers could be designed to achieve controlled drug release by making them responsive to external stimuli such as pH or temperature[145].Temperature changes exerted by infrared light,mostly used to induce the photothermal effect,can be exploited for regulated drug release[146],while acidic environments (pH)can trigger the release of therapeutic molecules such as doxorubicin [147],and 5-FU [143].GO-nanosystems can act as alternative delivery vehicles for orally administered drugs to prevent degradation by digestive enzymes and low pH in the stomach.GO-loaded with 5-FU when capped with alginate (ALG,GO-ALG-5-FU) prevented drug release until it reached the colon where ALG was dissolved in the alkaline environment and released the 5-FU.GO-ALG-5-FU also showed significant inhibition of colon cancer cell viability and liver metastasis in mice,thus making these nanosystems a promising drug carriers for CRC therapy[143].As such,graphene nanosystems hold potential as efficient nanocarriers for therapeutic drugs,including miRNA delivery,for CRC treatment.
3.3.3.CDs
CDs are another class of CBNs with unique attributes such as biocompatibility,optical properties,high stability,low cytotoxicity,and low cost [148].CDs have attracted considerable interest in different fields,including biological sensing,drug delivery,photodynamic therapy,photocatalysis,and solar cells in recent years[149].Different methods of synthesis,applications,merits,and demerits of CDs have been systematically summarized elsewhere[150];hence,only the use of CDs as nanocarriers for miRNA delivery will be highlighted herein.There are no reports on CDs as miRNA carriers for CRC yet;however,CDs have been used as fluorescent probes for the detection of miRNA[151]and as transfection agents[152].CDs can combine their imaging properties when used as delivery systems for therapeutic molecules or genes to form imaging-guided nanosystems that can track drug mobility,targeting,delivery and therapeutic response [153].For instance,DNA probes conjugated to CDs were able to detect miRNA-21 in human serum and MCF-7 cells through the fluorescent signal of the CDs[151].As a nanocarrier,CDs loaded with miRNA combinations(miRNA-1,miRNA-133,miRNA-208,and miRNA-499) were delivered into mouse hearts and improved cardiac function.The CDmiRNA complex showed improved cellular uptake and had low toxicity[152].CDs stand out among other CBNs with high aqueous solubility and photostable fluorescent intensity that can be exploited for the development of smart traceable delivery systems for miRNA-based therapy for CRC.
3.4.Hybrid nanocarriers
Hybrid nanocarriers combine properties from different materials to create drug delivery systems with improved and effective potency.Organic NPs are generally less stable than inorganic NPs.Therefore,combination of organic and inorganic materialss can produce stable,biodegradable,biocompatible,selective,and efficient nanodelivery systems.This can be achieved by exploiting the easily modifiable surface of inorganic NPs by conjugating desired bioactive molecules,to assist in their delivery to the target site without being degraded or losing their therapeutic activity.For example,surface coating of MSNs with PEI facilitated binding of the negatively charged siRNA due to PEI cationic charge and improved cellular uptake and siRNA delivery[154].Prabhakar et al.[155]have also demonstrated a stimuli-response release of siRNA in MDA cancer cells using MSN-PEI hybrid nanocarriers.The MSN-PEI hybrid nanocarrier had a payload of 120 mg/g siRNA,and PEI promoted endosomal escape and triggered release of the siRNA through redox-responsive actions.The hybrid nanocarriers were more efficient in transfection and delivery of the siRNA and had enhanced activity when compared to lipofectamine [155].A redox and pH-responsive lipid-capped MSN hybrid nanocarrier loaded with doxorubicin was constructed to overcome drug resistance in breast cancer.Doxorubicin was successfully transported in the MSN-lipid hybrid nanocarriers,and its release was triggered by redox and pH when internalized by MCF-7 cells [156].
The hybrid nanocarriers also showed great potential in the treatment of CRC in vitro and in vivo.Xu et al.[157] developed a codelivery strategy to transport the first-line therapeutic drug (5-FU) for CRC and a miRNA (miR-34a) mimic using a QD-β-cyclodextrin (CD) nanocomposite in CRC patient-derived tumor xenografts [157].Briefly,the CD-modified QDs were produced by first synthesizing cadmium telluride(CdTe)QDs that were stabilized by 3-mercaptopropyl acid,as depicted in Fig.7 [157].The TCP1 targeting peptide was conjugated to the QDs for target specificity.5-FU and miR-34a were loaded into the TCP1-CD-QD nanosystem and subsequently codelivered into DLD1 CRC cells.The drugs suppressed the proliferation of CRC cells and inhibited tumor growth in a CRC cell-derived tumor xenograft [157].
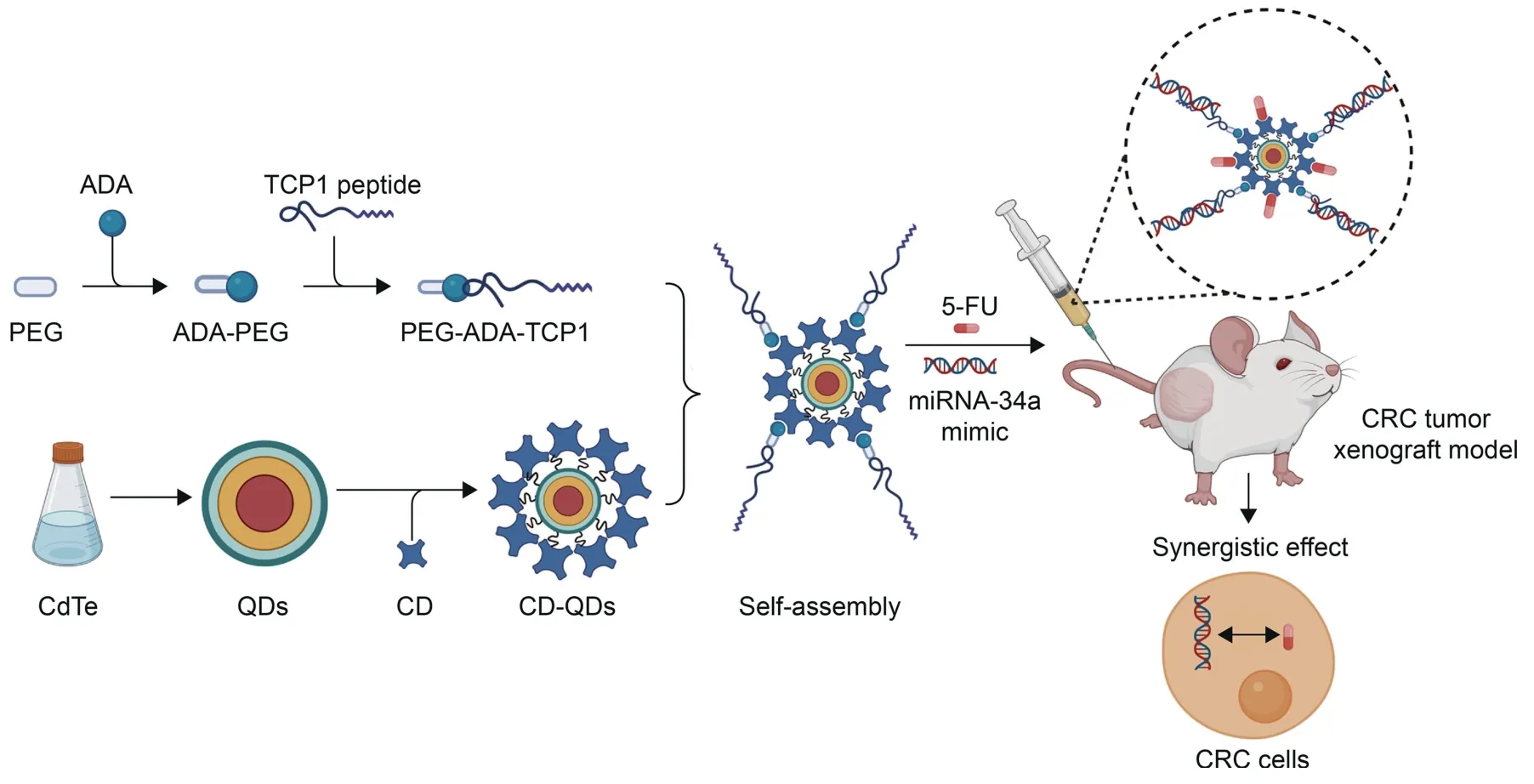
Fig.7.Stepwise synthesis and administration of 5-fluorouracil(5-FU)-miR-34a-nanocarrier(codelivery system)for synergistic therapy of colorectal cancer(CRC).ADA:adamantine;PEG: polyethylene glycol;TCP1: T-complex protein 1;CD: cyclodextrin;CdTe: cadmium telluride;QDs: quantum dots.Reprinted from Ref.[157] with permission.
Similarly,a polymeric layer-by-layer (LbL) assembly of silicasupported mesoporous titania (MTNst) hybrid nanocarriers was constructed for the codelivery of miR-708 and paclitaxel (PTX) to HCT-116 and DLD-1 CRC cells [158].The hybrid nanosystem was loaded with PTX and a TSmiR (miR-708),as illustrated in Fig.8[158].Overexpression of cellular Fas-associated protein with death domain-like interleukin-1β-converting enzyme-inhibitory protein (c-FLIP) is associated with apoptosis and chemotherapy resistance in cancer,including CRC;therefore,miR-708 was used in the cotreatment to suppress c-FLIP and sensitize the cells to PTX function.Evidently,LbL miR708/PTX-MTNst showed enhanced and dose dependent activity on CRC cells compared to the vehicle and free drug.The nanosystem suppressed c-FLIP expression and resulted in increased expression of proapoptotic proteins.Furthermore,the nanocarrier accumulated in DLD-1 xenograft tumor-bearing mice and showed a significant inhibitory effect when compared to free PTX and miR-708.This hybrid nanocarrier was biocompatible and showed no cytotoxicity up to 1 mg/mL of the drug-free nanocarrier.Importantly,these nanocarriers could accumulate and be retained in the tumors and showed no apparent toxicity in vivo as the mice retained their body weights [158].
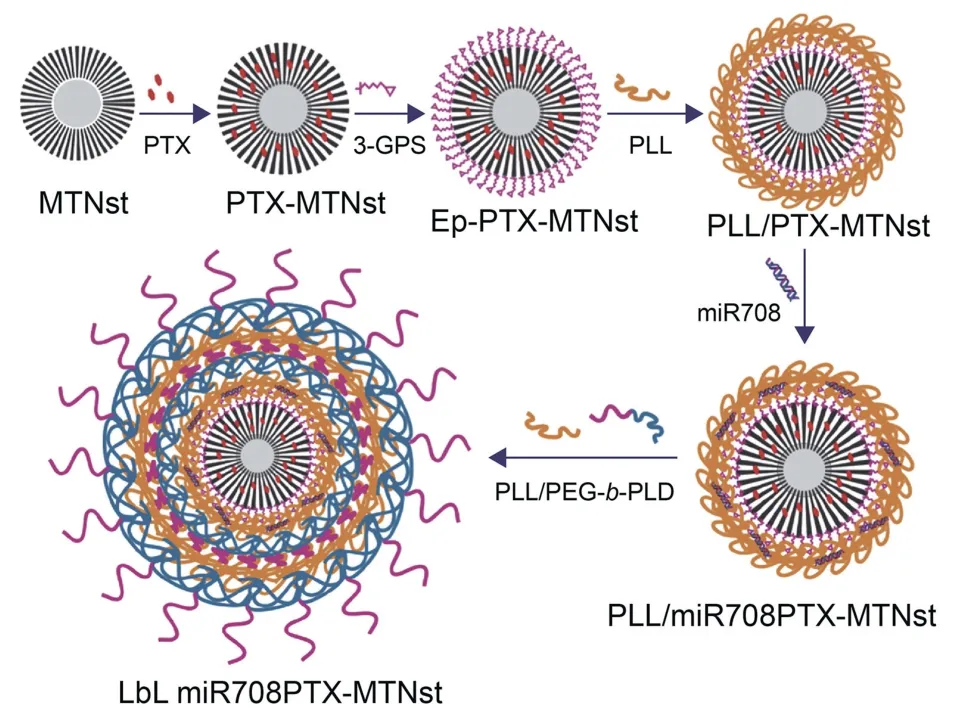
Fig.8.Synthesis and drug-loading of miR708/PTX-MTNst nanohybrid.PTX:paclitaxel;3-GPS: (3-glycidyloxypropyl)trimethoxysilane;PEG-b-PLD: methoxy-poly (ethylene glycol)-block-poly (L-aspartic acid);MTNst: silica-supported mesoporous titania NPs;LbL: layer-by-layer.Reprinted from Ref.[158] with permission.
Several other nanohybrid combinations provided similar effects in CRC models.miR-145 encapsulated in disulfide cross-linking micelles (DCM) and the miR-145-DCM nanocomposite resulted in selective delivery of miR-145 to colon cancer cells in vitro and in vivo.The miR-145-DCM nanocomposite promoted cellular uptake and significantly increased miR-145 expression by colon cancer cells.Accordingly,cell growth was halted at the G1phase.The mechanism by which the nanosystem inhibited apoptosis was through the suppression of MYC and FSCN1,which could serve as miR-145 targets.The DCM nanocarriers could also assist in further investigation of the role of miRNA in cancer management [159].Additionally,systemic miRNA-655-3p delivery was achieved by a platinum-based nanoconstruct (PTEN/miR-655-3p) in HCT116 cell-mouse xenografts.Suppression of proliferation and inhibition of epithelial transition to mesenchymal was achieved by halting the nuclear translocation of β-catenin,with no indication of immunoor hepa-toxicity [160].
Dysregulation of the polyamine pathway is common to cancers [161],and targeting this pathway may be important for cancer regulation.A codelivery procedure involving a bisethylnorspermine (BENSpm)-based biodegradable polycation(DSS-BEN/miR-34a) nanocomposite was shown to deliver miR-34a and target this pathway.After release,BENSpm mediated the depletion of polyamine levels,while enhanced miR-34a levels inhibited the expression of Bcl-2 in HCT116 cells [162].Other notable miRNA nanodelivery systems in CRC were the use of FDA approved PEGylated poly(lactic-co-glycolic acid)NPs(PLGA-PEGNPs) to deliver miR-122 into human colon cancer xenografts in mice [163].Similarly,PLGA/PEI/HA encapsulated miR-145 [164]and PEG-poly (amino acid)-miRNA-139-5p nanosystems [165]were effectively delivered in HCT-116 cells and tumor-bearing mouse xenografts and suppressed tumor growth and migration.These studies demonstrated the significance and importance of nanocarriers as useful tools for the effective delivery of therapeutic miRNAs,suggesting that miRNA-based therapy is a promising strategy for the treatment of cancers,including CRC.Using hybrid nanocarriers is a promising therapeutic strategy that could enhance CRC targeting,miRNA delivery and efficacy for effective CRC therapy.
4.Metal organic frameworks (MOFs): upcoming materials with drug delivery capabilities
MOFs or porous coordination polymers are currently exploited for the delivery of gene therapy.MOFs are unconventional synthetic polymers that are considered for drug delivery due to their welldefined structure,ultrahigh surface area,porosity,tunable pore size,and easy chemical functionalization [166].They are a unique class of crystalline solids composed of cations and coordination organic linkers with promising applications in miRNA delivery.Literature has reported the applications of MOFs as drug delivery agents[167].Zhao et al.[168]recently studied the dual role of this class of carriers for miRNA delivery and adjuvants for chemodynamic therapy.miR-34a-m was conjugated to MOF ZIF-8,and the miR-34a-m@ZIF-8 formulation demonstrated enhanced cellular uptake and lysosomal stimuli-responsive miRNA release in triple negative breast cancer (MDA-MB-231) cells and MDA-MB-231 tumor-bearing mice.Zn2+triggered the generation of ROS,which consequently induced apoptosis of the tumor cells.miR-34a-m release was associated with reduced Bcl-2 expression and efficient cancer cell death through apoptosis.In vivo assays showed high efficacy using the nanosystem to suppress tumor growth through gene and chemodynamic therapy in the studied model[168].Other MOFs used for disease diagnosis and therapy include Ni-IRMOF-74-II for miRNA delivery and detection [169],Ca-MOF@miR-124 nanoformulation for the assessment of neural stem cell therapy for ischemic stroke [170],miRNA-21 detection and imaging in living cells by DNA-MOF nanosystem [171],and fluorescein amidites(FAM)-probe21-NMOF-FA complex for miR-21 biosensor in prostate cancer cells [172].Several therapeutic molecules have been recently delivered through MOFs due to their unique properties and significant advantages.Despite the achievements made through the applications of MOFs,several challenges limit their full potential.The major concern in the clinical application of MOFs is their potential toxicity associated with the degradation and metabolism of MOFs inside the body [173].
5.Model representation of miRNA-nanohybrid for effective CRC treatment
The role of miRNAs in the development and progression of CRC is evident,and their therapeutic potential is quite fascinating and warrants further clinical exploration [46].With the use of various nanomaterials as delivery agents,the efficacy of miRNA in both in vitro and in vivo studies could be enhanced[130].The bottleneck in these strategies is the fact that cancer hallmarks are similar in all types,and one strategy may not specifically apply to a specific cancer.Similarly,miRNA discussed in this review might not be CRCspecific but also affected in other cancers.Of interest,NPs can be tailored to deliver their cargoes to a specific site,thereby increasing the specificity of the drug of choice.Currently,MSNs are inarguably the most researched inorganic NPs used for nanohybrid construction and were selected in this study to simulate a miRNA-nanohybrid for CRC treatment following two strategies that are highlighted in Fig.9.MSNs have a large surface area and pore volume,which allows for loading and attachment of multiple bioactive molecules[117].Moreover,the addition of gatekeepers or capping agents to MSNs could allow for the development of stimuli responsive drug release nanohybrids with high payload [156].
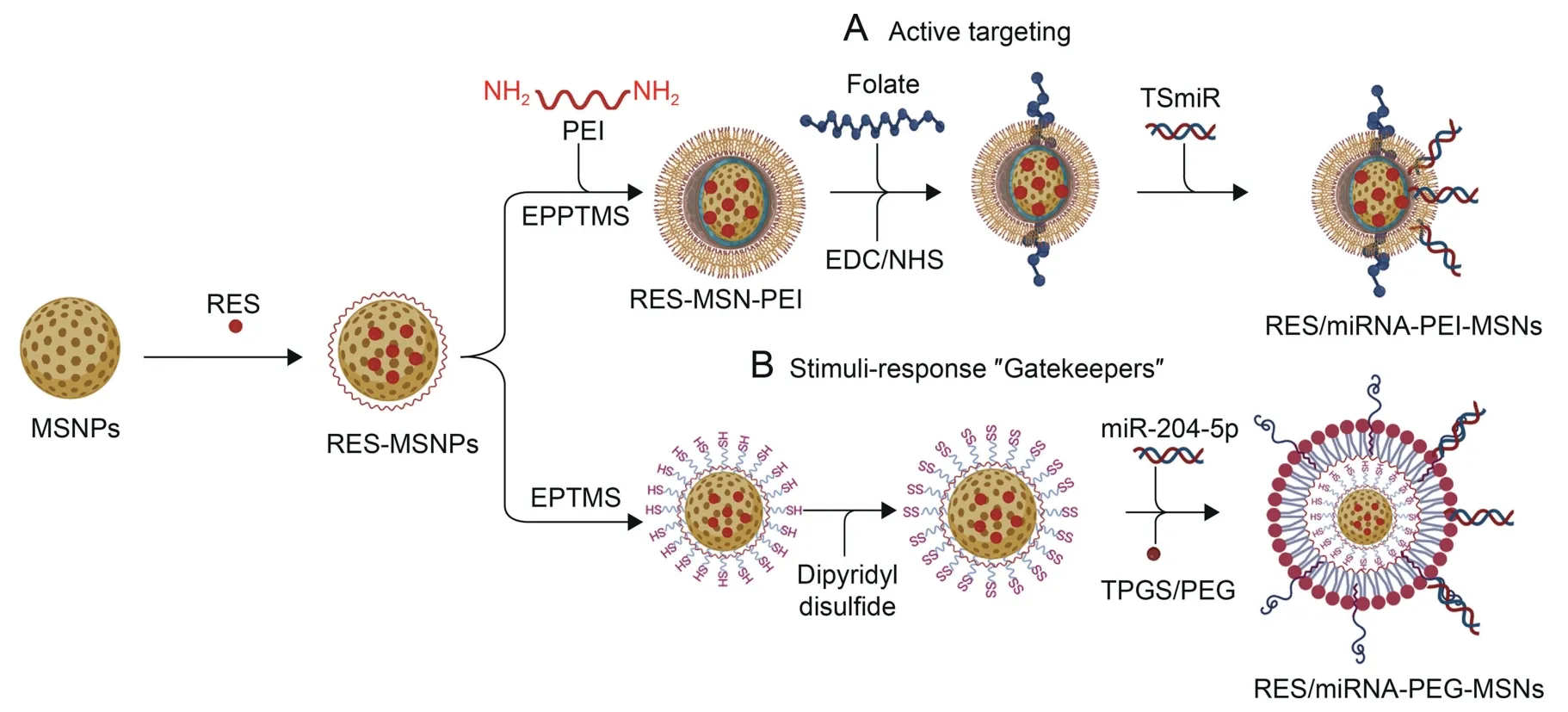
Fig.9.Nanohybrid system for codelivery of resveratrol(RES)and microRNA(miRNA)for colorectal cancer(CRC)treatment.Mesoporous silica nanocarriers(MSNs)loaded with RES and used for either (A) targeted delivery of tumor suppressor miRNA (TSmiR) or (B) stimuli-responsive delivery of miRNA to the tumor environment.EPPTMS: epoxypropoxypropyl-trimethoxysilane;EPTMS: epoxypropoxy-trimethoxysilane;PEI: polyethyleneimine;EDC: (1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride;NHS:N-hydroxysuccinimide;PEG: polyethylene glycol;TPGS: D-α-tocopheryl polyethylene glycol succinate.
In the proposed CRC codelivery nanosystem,resveratrol (RES),an anticancer drug,was coloaded with TSmiR on MSNs to create a RES-TSmiR-MSN nanohybrid,while organic biomaterials were added as gatekeepers.RES serves as a model for drugs with poor solubility,poor oral bioavailability and high membrane permeability [174].Its poor oral bioavailability [175] is due to partial intestinal absorption,rapid intestinal metabolism and efflux into the intestinal lumen by ATP-binding cassette (ABC) transporters[176].All these limitations can be overcome using nanocarriers and ensure that the drug reaches and accumulates in the target site for effective treatment.The RES-TSmiR-MSN nanohybrid was tailored to function in one of the two proposed strategies,via active targeting of the CRC folate receptors (Fig.9A) or through stimuliresponsive drug release(Fig.9B).Active targeting is one of the most successful treatment strategies in cancer and will only be effective against cells that express target receptors on the cell surface.Folate receptors unfortunately are among the universal biomarkers that are expressed by most cancers,including CRC,and are only used as an example.Similarly,VEGF and EGFR,which were shown to be essential for CRC progression and promising targets for CRC stages II and III,are also involved in other cancers[177].Thus,therapeutic miRNAs that target these genes might be crucial for the treatment of cancers and not exclusively for CRC.
The second strategy,unlike the first strategy,will rely on the size-dependent properties of the nanomaterials and the EPR effect on the tumor microenvironment for uptake.The drugs (RES and TSmir)will only be released from the nanocarriers when they reach the tumor site under tumor-specific stimuli,such as low pH or in response to oxidative stress.In the two strategies,drug delivery and release in the cytoplasm will activate RES and TSmir activities resulting in cancer cell death.These strategies can increase the concentration of RES reaching the targeted tissue,which will in turn confer its chemopreventive effect in CRC.
An independent study demonstrated the feasibility of a hybrid nanocarrier composed of lignin (AL) and Fe3O4magnetic (AL/RES/Fe3O4)NPs for the delivery of RES in murine Lewis lung carcinoma xenografts.The AL/RES/Fe3O4NPs showed significant anticancer effects,enhanced in vitro drug release,stability,drug accumulation,and reduction of tumor size with lower adverse effects than free RES.This study suggested the efficient delivery of poorly soluble and less stable drugs[178],which might also be a possibility for CRC treatment.Moreover,nanocarriers can bypass P-glycoproteinmediated drug resistance and prevent drug extrusion [179].As such,nanobased drug delivery strategy might present high potency and minimal drug side effects.The proposed CRC treatment strategy was motivated by the efflux pump inhibitory effect and enhanced drug activities demonstrated by PEGylated derivatives such as polyoxyethylated stearates,D-α-tocopheryl PEG 1000 succinate,and thiolated PEG-g-PEI in cancer cells [180].Although the effects of these delivery systems were selective and presented no toxicity to normal cells[181],immunogenicity is a major hurdle for polymer-based delivery systems.Most importantly,in nucleic acid-based therapies,only polycation polymers will be able to shuttle oligonucleotides across the negatively charged cell membrane.Because some of cationic polymers are not biodegradable,they tend to be toxic [182].Thus,a hybrid nanocarrier for the codelivery and dual therapeutic activities of chemotherapeutic drugs and miRNAs targeted at stage-specific CRC signaling pathways may be effective against CRC.The feasibility of the proposed strategy in Fig.9 is yet to be tested,but has potential for the delivery of cancer miRNA therapeutics that are not limited to CRC.
6.Conclusion and future perspectives
CRC remains a global public health challenge.The chemotherapeutic drugs against this disease are limited by their various side effects,especially their nonspecificity to both cancer and healthy cells.These side effects not only reduce the quality of life but may also compromise the drug's efficacy by necessitating dosage reductions or interruptions of the therapy window.
Dysregulation of miRNAs has been observed in different diseases including cancer.Dysregulated expression of miRNAs has been shown to mediate the signaling pathways important in various stages of CRC carcinogenesis and metastasis.Studies suggest that these class of noncoding RNAs possess oncogenic or tumor suppressing activities in CRC.Supporting evidence has provided a distinct role of miRNAs at each stage of CRC:initiation,progression,and development.Therefore,miRNA can be used as a tool for CRC classification apart from its diagnostic,prognostic,predictive and therapeutic activities.This suggests that miRNA-based therapy targeted at a specific stage of CRC could be an efficient strategy in the treatment of this disease.However,this class of RNAs requires a delivery system either alone or in combination with existing CRC drugs to prevent their degradation by proteolytic enzymes,reduce their off-target effects,and enhance their stability and efficacy at the tumor site.
Generally,nanocarriers have shown superiority over other drug delivery systems and have been proposed as suitable delivery systems to transport miRNAs.The review highlighted the feasibility of various nanomaterials as miRNA nanocarriers for cancer,and possibly CRC treatment.The ultimate choice for bioapplication is based on various factors,and the most important aspects are the safety and performance of these nanomaterials.Organic NPs are biodegradable and considered to be biocompatible,while inorganic NPs provide stability and can be easily modified with miRNAs.Despite these advantages,numerous challenges still limit their individual transition into clinical trials.A notable drawback is the regulatory mechanisms available for nanomedicines,safety and toxicity assessments are required for their optimal clinical applications.Liposomes have been in clinical trials for about 28 years,and other organic nanomaterials (lipid-based,polymeric NPs,and micelles) have followed suit in several formulations for drug delivery due to their perceived safety.However,emerging evidence raises concerns,as lipid loads and drug residuals could be detrimental to human health.Inorganic nanomaterials such as AuNPs and MSNs are less progressive in clinical trials,as the fate of these nonbiodegradable materials is unclear.Regardless of these shortfalls,NP-based therapies have shown merits as gene delivery agents and have been clinically proven as mRNA,RNAi,and siRNA delivery agents using lipid-based NPs.
To date,no research has reported the use of miRNA-nanocarrier delivery systems in humans or clinical trials for CRC.This could be associated with the combined challenges of these systems.Animal studies using miRNA-nanocarriers have provided a positive impact toward the advancement of nanosystems in humans and potential FDA approval for clinical trials.The miRNAnanocarriers can increase the stability of miRNA against nucleases,enhance cellular uptake,facilitate endosomal escape,and reduce toxicity.Moreover,these nanocarriers can serve multiple purposes as diagnostic and therapeutic agents.The two proposed hybrid nanosystems might potentially be effective in experimental trials (in vitro and in vivo) against CRC and postulate that nanobased miRNA strategies can target specific signaling pathways involved in various stages of CRC development and progression,thus representing an effective therapeutic strategy for CRC.Although advances have been made to obtain safe and efficacious nanobased miRNA therapeutics,further optimization of miRNA-nanosystems,reproducible synthesis methods,conjugation and understanding how the nanoformulations interact with biological systems,are imperative to ensure that these delivery systems meet medical needs and market standards.If these conditions are met,then miRNA-nanosystems will become promising candidates for further exploitation in drug discovery and clinical applications.
CrediT author statement
Adewale Oluwaseun Fadaka:Conceptualization,Methodology,Investigation,Writing -Original draft preparation,Reviewing and Editing,Data curation,Project administration;Taiwo Akinsoji:Investigation,Writing -Original draft preparation;Ashwil Klein:Methodology,Investigation,Writing -Original draft preparation,Funding acquisition,Resources;Abram Madimabe MadieheandMervin Meyer:Supervision,Methodology,Funding acquisition,Writing -Reviewing and Editing,Resources;Marshall Keyster:Methodology,Investigation,Writing -Original draft preparation;Lucky Mashudu Sikhwivhilu:Supervision,Funding acquisition,Writing -Reviewing and Editing,Resources;Nicole RemaliahSamantha Sibuyi:Conceptualization,Methodology,Investigation,Writing-Original draft preparation,Reviewing and Editing,Project administration.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
The study was funded by the Department of Science and Innovation/Mintek Nanotechnology Innovation Centre.
杂志排行
Journal of Pharmaceutical Analysis的其它文章
- Biosensors for waterborne virus detection: Challenges and strategies
- Development status of novel spectral imaging techniques and application to traditional Chinese medicine
- Oridonin restores hepatic lipid homeostasis in an LXRα-ATGL/EPT1 axis-dependent manner
- Ginsenoside Rg5 enhances the radiosensitivity of lung adenocarcinoma via reducing HSP90-CDC37 interaction and promoting client protein degradation
- Canonical transient receptor potential channel 1 aggravates myocardial ischemia-and-reperfusion injury by upregulating reactive oxygen species
- Nanoscale coordination polymer Fe-DMY downregulatingPoldip2-Nox4-H2O2 pathway and alleviating diabetic retinopathy
