Reappraisal of Bothriolepis sinensis Chi,1940 from the Tiaomachien Formation,Hunan,China
2023-12-08LUOYanChaoZHUMinLULiWuPANZhaoHui
LUO Yan-Chao ZHU Min LU Li-Wu PAN Zhao-Hui
(1 Key Laboratory of Vertebrate Evolution and Human Origins of Chinese Academy of Sciences,Institute of Vertebrate Paleontology and Paleoanthropology,Chinese Academy of Sciences Beijing 100044)
(2 University of Chinese Academy of Sciences Beijing 100049)
(3 Geological Museum of China Beijing 100034)
Abstract Bothriolepis sinensis Chi 1940,mainly based on anterior median dorsal plates from the Middle Devonian Tiaomachien Formation of Hunan,is the first Paleozoic vertebrate taxon erected in China.Although additional materials of B. sinensis from the type locality were described by Lu in 1988,its morphology and phylogeny remain poorly understood.In this study,we complemented the morphology of the skull and trunk armor of B. sinensis based on Chi’s specimens housed in the Institute of Vertebrate Paleontology and Paleoanthropology,Chinese Academy of Sciences,and several previously undescribed specimens in the Geological Museum of China.Bothriolepis sinensis differs from other Bothriolepis in the following combination of characteristics: enlarged supraotic thickening,length/width ratio of head shield 1.4-1.6,broad orbital fenestra (greater than 1/3 of the head shield width),and fan-shaped preorbital recess.The phylogenetic analysis did not place B. askinae in the most basal position of the genus and revealed that B. sinensis and B.kwangtungensis consistently from a monophyletic group characterized by their slender proximal segment of the pectoral fin (length/width ratio greater than 7).A majority of Chinese Bothriolepis species (B. niushoushanensis,B. lochangensis,B. tungseni,B. kwangtungensis and B. sinensis)were clustered in a clade characterized by the pectoral pit-line on the ventral central plate 1 extending to the ventral central plate 2.The paleogeographic reconstruction using the data from the DeepBone platform showed that Bothriolepis had its oldest occurrences in South China and East Gondwana in Eifelian,dispersed rapidly worldwide,and then diversified across the coasts of the Rheic Ocean.
Key words Hunan,Middle Devonian,Antiarcha,Bothriolepis,phylogeny,paleobiogeography,data visualization
1 Introduction
The Antiarcha is a group of placoderms that first appeared in the Ludlow (late Silurian)and became extinct in the Late Devonian (Moy-Thomas and Miles,1971;Wang,1991;Carr,1995;Janvier,1996;Zhao et al.,2016).As a diverse group in the Devonian,the Antiarcha is characterized by its highly specialized dermal armor pattern,especially the pectoral fin and the head shield (Goujet and Young,1995;Janvier,1996).Recent phylogenetic studies (Brazeau,2009;Davis et al.,2012;Zhu et al.,2013,2016;Dupret et al.,2014;Giles et al.,2015;Long et al.,2015;Qiao et al.,2016) placed the Antiarcha at or near the most basal position of placoderms and made it a keenly watched early vertebrate group (Zhu M et al.,2012;Giles et al.,2013;Long et al.,2015;Trinajstic et al.,2015;Charest et al.,2018;Wang and Zhu,2018,2021;Zhu Y A et al.,2021,2022).
Bothriolepis,one of the earliest known Paleozoic vertebrate taxa among placoderms(Eichwald,1840;Miller,1841),was distributed worldwide.Bothriolepissinensiswas the first Paleozoic vertebrate taxon erected in China (Chi,1940),mainly based on three specimens of the anterior median dorsal plate (AMD) found by Chi Y S in 1937.These specimens were collected from the Middle Devonian Tiaomachien Formation (also spelled as Tiaomajian Formation) in Changsha,Hunan (Chi,1940;Pan,1958,1959;Pan and Dineley,1988;Wang,1984;Zhao and Zhu,2010;Qie et al.,2019,Fig.1).Later,Pan and colleagues collected additional materials ofB.sinensisand made some complementary descriptions (Pan,1959;Lu,1988).However,these descriptions in Chinese were overlooked by other researchers for a long time,resulting in the phylogenetic affinity ofB.sinensisunsettled.Here we redescribe the holotype ofB.sinensisand other available specimens from Chi’s collection housed in IVPP,and additional specimens housed in GMC.We also established a relatively comprehensive matrix comprising 77 taxa (75Bothriolepisspecies and 2 outgroups) and 69 characters to discuss the interrelationships ofBothriolepis.Based on the data from DeepBone (www.deepbone.org,Pan and Zhu,2019) and Scotese’s paleogeographic reconstructions (Scotese et al.,2021),we also explore the temporal and spatial distribution ofBothriolepis.
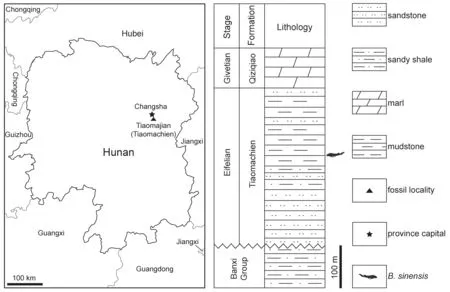
Fig.1 Map of the fossil site and the fish-bearing horizon in this study Based on: GS(2019)3266
2 Material and methods
To explore the phylogeny ofBothriolepis,we choseGrossilepistuberculata(Lukševičs,1996) andDianolepisliui(Chang,1965;Pan et al.,2018),two bothriolepidoid species as the out-groups.We compiled a matrix containing 75Bothriolepisspecies,2 outgroups,and 69 characters (see “Bothriolepisdataset” matrix in Supplementary Information) using Mesquite v.3.61 (Maddison,2019) and performed the phylogenetic analysis using TNT v.1.5(Goloboff and Catalano,2016).In the phylogenetic analysis,we excluded 11Bothriolepisspecies that have a rate of character coding completeness of less than 10% (see Supplementary Information) to get a more resolved result.The characters and codings are mainly based on Long (1983),Lukševičs (2001,2021),Young (2010),and Dupret et al.(2023).All characters are set as unordered.
Institutional abbreviationsIVPP,Institute of Vertebrate Paleontology and Paleoanthropology,Chinese Academy of Sciences,Beijing,China;GMC,Geological Museum of China,Beijing,China.
Anatomical abbreviationsADL,anterior dorsolateral plate;AMD,anterior median dorsal plate;alr,postlevator thickening of anterior median dorsal plate;a.PrL,attachment area for prelateral plate;a1SM,anterior attachment area for submarginal plate;a2SM,posterior attachment area for submarginal plate;CD1-3,dorsal central plate 1-3;cf.ADL,area overlapping anterior dorsolateral plate;cf.AMD,area overlapping anterior median dorsal plate;cf.MxL,area overlapping mixilateral plate;cr.pm,paramarginal crista of head shield;cr.pto,postorbital crista of head shield;cr.tp,posterior internal transverse crista;cr.tv,transverse nuchal crista of head shield;fm,insertion fossa on head shield for levator muscles;f.retr,levator fossa of anterior median dorsal plate;g,pit on postpineal plate;grm,ventral median groove on dorsal wall of trunk shield;L,lateral plate;ML2-3,lateral marginal plate 2-3;MM2-3,mesial marginal plate 2-3;MxL,mixilateral plate;mvr,ventral median ridge of anterior median dorsal plate;Nu,nuchal plate;nprl,prelateral notch of head shield;oa.AMD,area overlapped by anterior median dorsal plate;oa.AVL,area overlapped by anterior ventrolateral plate;oa.PMD,area overlapped by posterior median dorsal plate;oa.PVL,area overlapped by posterior ventrolateral plate;ood,otic-occipital depression of head shield;PM,postmarginal plate;PMD,posterior median dorsal plate;PNu,paranuchal plate;PP,postpineal plate;PrM,premedian plate;PVL,posterior ventrolateral plate;p,lateral pit of head shield;pr.dm,median dorsal process of mixilateral plate;prh,preorbital recess of head shield;pro,obstantic process of trunk shield;proc,preobstantic corner of head shield;pr.po,depression on head shield for dorsal face of the endocranial postorbital process;prv1,anterior ventral process of anterior median dorsal plate;pt1,anterior ventral pit of dorsal wall of anterior median dorsal plate;sot,supraotic thickening of head shield;tlg,transverse lateral groove of head shield paleoanthropology.
3 Systematic paleontology
Class Placodermi McCoy,1848
Order Antiarcha Cope,1885
Suborder Euantiarcha Janvier &Pan,1982
Infraorder Bothriolepidoidei Gross,1965
Family Bothriolepidae Miles,1968
Genus Bothriolepis Eichwald,1840
Bothriolepis sinensis Chi,1940
(Figs.2-5)
BothriolepissinensisChi,1940,p.57-73,pl.I,figs.1-7;text-fig.3
BothriolepissinensisPan,1959,p.27-28,pl.IV,figs.1,2
BothriolepissinensisLu,1988,p.69-77,pl.I,fig.1;pl.II,figs.1-4;text-figs.1-4
HolotypeIVPP V467a,internal mould of an AMD (Chi,1940:pl.I-1).
Referred specimensIVPP V467b,internal mould of an ADL (Chi,1940:pl.I-7);V467c,internal mould of an incomplete AMD (Chi,1940:pl.I-3);V467d,internal mould of a MxL (Chi,1940:pl.I-5);GMV1961,internal mould of a head shield,articulating with part of the trunk shield (Lu,1988:fig.1,pl.I-1);GMV1972,a complete proximal segment of pectoral fin (Pan,1959:pl.I-2);GMV1974a,an incomplete proximal segment of pectoral fin (Lu,1988:pl.III-1);GMV1974b,external mould of an incomplete distal segment of pectoral fin;GMV1975,a PrM.
Diagnosis(emended)BothriolepissinensisresemblesB.shaokuanensisin the broad orbital fenestra (greater than 1/3 of the head shield width) and slender pectoral fin (length/width ratio greater than 7).Bothriolepissinensiscan be characterized by its enlarged supraotic thickening of the head shield (longer than half of the nuchal plate);length/width ratio of the head shield 1.4-1.6;fan-shaped preorbital recess;similar length and width of the PrM;high transverse nuchal crista;length/width ratio of AMD about 1.5;ventral median ridge reaching the anterior margin of AMD;high anterior ventral process;AMD overlapped by MxL.
4 Description
The head shield itself was largely eroded except the PrM (Fig.2B).The PrM is broad,with its width and length nearly equal,and its anterior margin 1.5 times its posterior margin.The radiation center of ornamentation is visible on the anterior margin of the preorbital recess(prh,Fig.2B).A round-shaped poriferous area on the visceral side of the PrM is located at the anterior one-fourth of the plate and engages the anterior margin of the preorbital recess,resembling some AustralianBothriolepisspecies such asB.askinaeandB.karawaka(Young,1988).A similar feature can be also found in some other antiarch genera likeLivnolepis(Moloshnikov,2004,2008),however,the poriferous area in the latter is a narrow lozenge that does not reach the anterior margin of the preorbital recess.
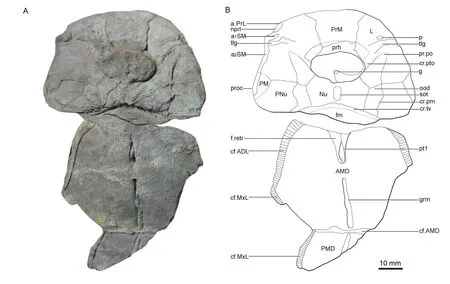
Fig.2 Photo (A) and illustrative drawing (B) of the referred specimen of Bothriolepis sinensis (GMV1961)
Thanks to the complete internal mould of the head shield,visceral details of the head shield are preserved (Fig.2).An enlarged lateral pit on the lateral plate (p,Fig.2B) is visible anteriorly of the transverse lateral groove of head shield (tlg,Fig.2B).Young (1988) discussed the large lateral pit in some of the “Victorian species” without clear identification criteria.Here we regard lateral pits that are larger than the minimum width of the transverse lateral groove as large lateral pits,as inB.sinensis.But the lateral pit ofB.sinensisis still much smaller than that ofB.barretti(Young,1988).The transverse lateral groove runs between the anterior and the posterior attachment areas for submarginal plate (a1SM,a2SM,Fig.2B).The anterior attachment area is placed anterolaterally to the transverse lateral groove (Fig.2B),and the posterior attachment area extends posteriorly behind the preobstantic corner (proc,Fig.2B),similar toB.macphersoni(Young,1988).The lateral and posterolateral margins of the head shield form a 145° angle at the preobstantic corner,as inB.shaokuanensis(Chang,1965) andB.niushoushanensis(Pan et al.,1980,1987).A pit (g,Fig.2B) is present on the postpineal plate.The attachment area for prelateral plate (a.PrL,Fig.2B) lies at the anterolateral margin of the PM (Fig.2B).The preorbital recess is fan-shaped,which Lu (1988) considered as a transitional form between “trilobate” type(e.g.,B.nitida,Leidy,1856;Young,1988;Thomson and Thomas,2001) and “simple”type (e.g.,B.askinae,Young,1988).The oticoccipital depression (ood,Fig.2B) shows that the dorsal face of the endocranial postorbital process (pr.po,Fig.2B) reaches the level of the anterior margin of the orbital fenestra.The transverse nuchal crista (cr.tv,Fig.2B) is high and protrudes anteriorly.The unpaired insertion fossa on the head shield for levator muscles (fm,Fig.2B) is relatively large,and reaches half the length of the PNu (Fig.2B).The supraotic thickening (sot,Fig.2B) is present on the visceral side of the Nu (Fig.2B),and its size is much larger than that in otherBothriolepisspecies (Long and Werdelin,1986;Young,1988;Johanson;1997;Moloshnikov,2009;Downs et al.,2016).
The holotype IVPP V467a shows a visceral aspect of AMD (Fig.3A-C).The length/width ratio is about 1.5.The anterior ventral process and pit (prv1,pt1,Fig.3C) are located at the anterior two-thirds of the midline.The index between the width of the anterior margin and the maximum width of AMD is about 1.6,and the index between the anterior and posterior divisions of the AMD is about 1.5.The area overlapping ADL (cf.ADL,Fig.3C) is on the anterolateral margin of the AMD.The levator fossa and postlevator thickening of AMD (f.retr,alr,Fig.3C) are situated on the anterior portion of the plate.The MxL overlaps the AMD completely in the holotype and IVPP V467c.However,in GMV1961 the AMD bears an area overlapping the MxL (cf.MxL,Fig.2B) that occupies one-third of the posterolateral margin of AMD,representing the individual variation.The ventral median groove of the AMD (grm,Fig.3C) runs behind the ventral median ridge (mvr,Fig.3C) and extends into the PMD.The PMD is incomplete,and its length/width ratio remains unknown.
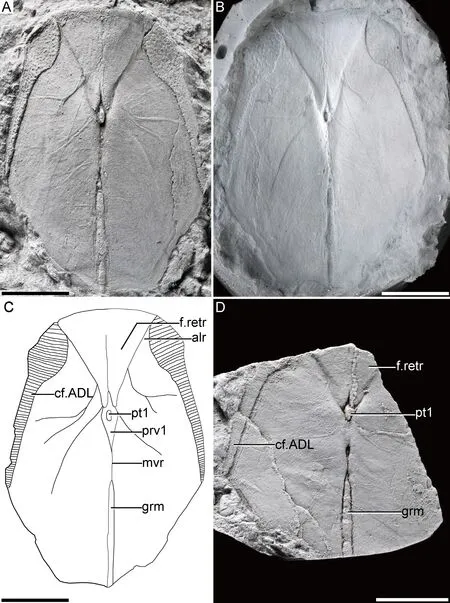
Fig.3 Anterior median dorsal plate of Bothriolepis sinensis
The ADL is preserved as an incomplete internal mould (Fig.4A),which was previously referred to as an indeterminable dermal bone (Chi,1940:pl.I-7).The dorsal lamina is 42 mm long by 14 mm wide,and the lateral lamina is 21 mm wide.The angle between the dorsal and lateral laminae is about 120°.The area overlapped by the AMD (oa.AMD,Fig.4A) is on the dorsal margin,and the fragmented area overlapped by the AVL (oa.AVL,Fig.4A) is on its ventral margin.A well-developed but short obstantic process (pro,Fig.4A) on the anterior margin of the ADL only extends one-fifth of the total length of the plate,similar toB.fergusoni(Long and Werdelin,1986).
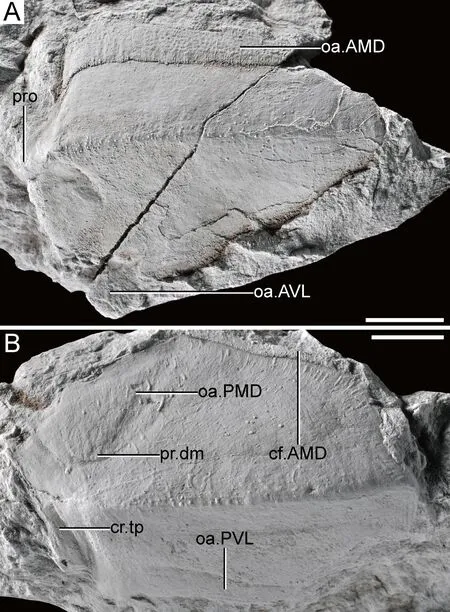
Fig.4 Other referred specimens of B.sinensis collected by Chi in the 1930s
The MxL (Fig.4B) is well preserved as an internal mould,which was previously referred to as an ADL (Chi,1940:pl.I-5).The dorsal lamina is 42 mm long by 18 mm wide,and the lateral lamina is 14 mm wide.The angle between the dorsal and lateral laminae is about 135°.A strong posterior internal transverse crista (cr.tp,Fig.4B) is placed posteriorly.The area overlapping AMD (cf.AMD,Fig.4B) is on the anterior dorsal margin,with depressions that mark the areas overlapped by PMD (oa.PMD,Fig.4B) and PVL (oa.PVL,Fig.4B).The median dorsal process of MxL is low and lacks an obtrusive corner,suggesting that the PMD bears a rounded lateral corner.
The pectoral fin specimens are well preserved to show the proximal and distal segments.The proximal segment is slender,with the length/width ratio greater than 7.In GMV1974a,the proximal segment is 78 mm long by 11 mm wide.The distal segment of the pectoral fin is preserved as an incomplete external mould in GMV1974b.The contact relationships of distal segment plates remain unknown.Spines are present on the lateral margins of lateral marginal plate 2 (ML2,Fig.5) and distal segment.
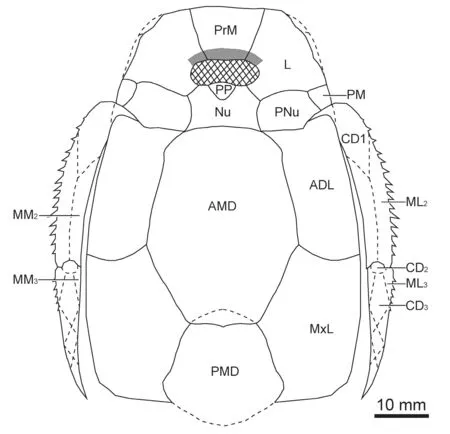
Fig.5 Reconstruction of the trunk armour of Bothriolepis sinensis
5 Discussion
5.1 Interrelationships of Bothriolepis
The phylogeny ofBothriolepishas received attention for decades.A few studies (Long,1983;Lukševičs,2001;Young,2010) that discussed the interrelationships ofBothriolepisonly used synapomorphies to show their phylogeny without any parsimony analysis.Dupret et al.(2023) compiled a character matrix based on previous studies,which contains a majority of theBothriolepisspecies,but the outgroup he used for the analysis (B.askinae) was not suitable for resolving the interrelationships ofBothriolepis.Following Long (1983),Lukševičs(2001),Young (2010),and Dupret et al.(2023),we established a more comprehensive matrix to discuss the interrelationships ofBothriolepis.Our phylogenetic analysis yielded 264 most parsimonious trees of length 324,with a consistency index (CI) of 0.235,and a retention index(RI) of 0.507.The low CI represents extensive convergences and reversals (Qiao and Zhu,2015).Though the strict consensus tree is poorly resolved,reflecting the inherent difficulties in resolving the interrelationships ofBothriolepis,we can still extract phylogenetic signals for the paleobiogeographic discussion.
Our study disagrees with Young (1988),Lukševičs (2001),and Dupret et al.(2023)in thatB.askinaeis not placed in the most basal position of the genus.The clade (Node 1,Fig.6) contains allBothriolepisspecies and is supported by two synapomorphies: anterior and posterior edges of orbitonasal fenestra straight and parallel (Character 7,state 0);semicircular groove on lateral plate developed (Character 60,state 1).We agree Lukševičs (2001)in thatB.primaandB.obrutschewiform a clade (Node 2,Fig.6),which is characterized by two synapomorphies: the posterior margin of orbit anterior to the junction formed by lateral,postmarginal and paranuchal plates (Character 11,state 0),and the junction of rostral infraorbital sensory line in midline on PrM straight or with a weak posterior indentation(Character 59,state 0).A majority of the Chinese species (B.niushoushanensis,B.lochangensis,B.tungseni,B.kwangtungensis,andB.sinensis) in our research are placed in the same clade (Node 3,Fig.6) by a synapomorphy that the pectoral pit-line on the ventral central plate 1 extending to the ventral central plate 2 (Character 66,state 1).Besides,B.sinensisandB.kwangtungensisform a monophyletic group (Node 4,Fig.6) by their slender proximal segment of the pectoral fin (length/width ratio greater than 7,Character 50,state 1).These four clades (Nodes 1-4,Fig.6) are both manifested by a suboptimal tree that is retained in Bremer supports analysis (retain trees suboptimal by 100),which represents considerable stability.
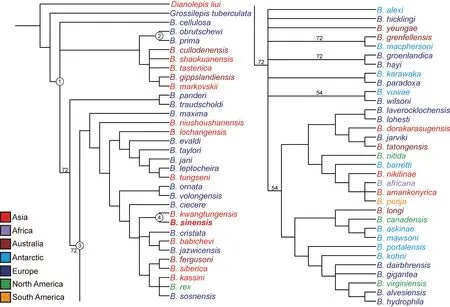
Fig.6 The 50% majority rule consensus tree of 264 most parsimonious trees,shows the phylogenetic position of B.sinensis within Bothriolepis
5.2 Paleobiogeography of Bothriolepis
The cosmopolitan antiarchBothriolepisis one of the most diverse genera in placoderms(Fig.7A).Moreover,Bothriolepisexhibits some features that reveal endemic signals,e.g.,the trilobate preorbital recess only appears in the Antarctic species (Young,1988);the trifid preorbital recess appears in the species from Greenland (Stensiö,1948),Europe (Miles,1968),and Australia (Long and Werdelin,1986);and the fan-shaped preorbital recess only appears in the Chinese species (Chi,1940;Pan,1959;Pan et al.,1980;Lu,1988).

Fig.7 Geographical distribution of Bothriolepis
Here we used the data from DeepBone (Pan and Zhu,2019) to perform the data visualization.We converted the latitude and longitude coordinates of these fossil sites into pixel coordinates of raster tile maps using the Web Mercator algorithm (Fig.7A).To reveal the paleobiogeography ofBothriolepis,we plotted these fossil sites on each paleo map by the paleogeographic coordinate calculations (PointTrack version 7.0) and reconstructions (Scotese,2002;Ke et al.,2016;Pan et al.,2022) (Fig.7B).The general trend of radiation inBothriolepisis from South China and East Gondwana to the coasts of the Rheic Ocean (Fig.7B).The specimens we counted in the Deepbone database included 106 taxa ofBothriolepis(75 species and their specimens with solid identification,location,and horizon information;31 specimens were considered indeterminate,but their localities can still be used in the calculation of geographical distribution).
TwoBothriolepisspecies,B.verrucose(Young and Gorter,1981) andB.yunnanensis(Liu,1962;Zhao and Zhu,2010) that showed the most early-diverging forms ofBothriolepis,appeared in South China and East Gondwana (Australia) of Eifelian (Fig.7B),which represented the initial rise ofBothriolepis.Bothriolepisgradually became more diverse in Givetian,and multiple species (16 taxa) widely spread from the Paleo-Tethyes Ocean to the East of Australia,and Antarctica (Fig.7B).The migration ofBothriolepiswas kept westwards in Frasnian.35 species ofBothriolepisfrom Australia,the Paleo-Tethyes Ocean,Baltica,and Laurentia were described for this stage (Fig.7B).In Famennian,22 species ofBothriolepiswere described on the coasts of the Rheic Ocean,which were South America,Africa,Baltica,and Laurentia.Moreover,sporadic species appeared in Australia and Antarctica (Fig.7B).
6 Conclusion
We described several additional specimens ofB.sinensis,which included some head shields (GMV1961,GMV1975),trunk plates (GMV1961,IVPP V467),and pectoral fins(GMV1972,GMV1974).According to additional specimens,B.sinensiscan be identified by the following characteristics: enlarged supraotic thickening,length/width ratio of head shield 1.4-1.6,broad orbital fenestra (greater than 1/3 of the head shield width),and fanshaped preorbital recess.We also compiled a relatively comprehensive matrix containing 75Bothriolepisspecies,2 outgroups,and 69 characters to reveal the interrelationships ofBothriolepis.The phylogenetic analysis showed thatB.sinensisandB.kwangtungensisform a monophyletic group by their slender proximal segment of pectoral fin.In addition,most of the Chinese species in our phylogenetic analysis (B.niushoushanensis,B.lochangensis,B.tungseni,B.kwangtungensisandB.sinensis) are placed in the same clade by their pectoral pitlines on the ventral central plate 1 extending to ventral central plate 2.According to the data from the DeepBone platform,we summarized the trend of dispersal ofBothriolepisfrom South China and East Gondwana to the coasts of the Rheic Ocean.
AcknowledgementsWe thank Jia Lian-Tao and Cui Xin-Dong for their assistance during this work.This project was supported by the National Natural Science Foundation of China(42002015,41872023,42130209,42272028),Youth Innovation Promotion Association CAS(2021070),and State Key Laboratory of Palaeobiology and Stratigraphy (Nanjing Institute of Geology and Palaeontology,CAS) (193121).
Supplementary material can be found on the website of Vertebrate PalAsiatica (http://www.vertpala.ac.cn/CN/2096-9899/home.shtml).
杂志排行
古脊椎动物学报(中英文)的其它文章
- An egg clutch of the Stalicoolithidae discovered in Wuning,Jiangxi,China
- Micromammal fossils from the basal part of the Jiaozigou Formation in Yagou area,Linxia Basin,Gansu Province
- A giant bamboo rat from the latest Miocene of Yunnan
- New findings of Xiyuichthys (Xiushuiaspidae,Galeaspida)from the Silurian of Jiangxi Province and Tarim Basin
