Human bocavirus 1 and 2 genotype-specific antibodies for rapid antigen testing in pediatric patients with acute respiratory infections
2023-12-02RiDeYanPengXuFangWangYuTongZhouPanDengShiRuNanZhuYuSunLiYingLiuLiPingJiaHuiJinDongHuiZhaoChengFengQinLinQingZhao
Ri De · Yan-Peng Xu · Fang Wang · Yu-Tong Zhou · Pan-Deng Shi · Ru-Nan Zhu · Yu Sun · Li-Ying Liu ·Li-Ping Jia · Hui-Jin Dong · Hui Zhao · Cheng-Feng Qin · Lin-Qing Zhao
Abstract Background Previous serological studies of human bocavirus (HBoV) 1 could not exclude cross-reactivity with the other three HBoVs,particularly HBoV2.Methods To search for genotype-specific antibodies against HBoV1 and HBoV2,the divergent regions (DRs) located on the major capsid protein VP3 were defined through viral amino acid alignment and structure prediction.DR-deduced peptides were used as antigens to harvest corresponding anti-DR rabbit sera.To determine their genotype specificities for HBoV1 and HBoV2,these sera samples were used as antibodies against the antigens VP3 of HBoV1 and HBoV2 (expressed in Escherichia coli) in western blotting (WB),enzyme-linked immunosorbent assay (ELISA),and bio-layer interferometry (BLI)assays.Subsequently,the antibodies were evaluated with clinical specimens from pediatric patients with acute respiratory tract infection by indirect immunofluorescence assay (IFA).Results There were four DRs (DR1–4) located on VP3 with different secondary and tertiary structures between HBoV1 and HBoV2.Regarding the reactivity with VP3 of HBoV1 or HBoV2 in WB and ELISA,high intra-genotype cross-reactivity of anti-HBoV1 or HBoV2 DR1,DR3,and DR4,but not anti-DR2,was observed.Genotype-specific binding capacity of anti-DR2 sera was confirmed by BLI and IFA,in which only anti-HBoV1 DR2 antibody reacted with HBoV1-positive respiratory specimens.Conclusion Antibodies against DR2,located on VP3 of HBoV1 or HBoV2,were genotype specific for HBoV1 and HBoV2,respectively.
Keywords Divergent regions · Genotype-specific antibody · Human bocavirus 1 and 2 · Major capsid protein VP3
Introduction
Human bocavirus (HBoV) 1 was first identified in nasopharyngeal aspirates (NPAs) from children with respiratory tract infection in 2005 by Allander et al.through new nucleic acid-based techniques,metagenomic detection systems,for active virus hunting,which paved the way for virus discoveries [1].In close succession,three HBoVs (HBoV2,3,and 4)were similarly detected in 2008–2010 in human stool samples when scientists searched for new viruses responsible for gastroenteritis [2,3].In addition to stool samples and NPAs,these viruses have been detected in serum,cerebrospinal fluid,sewage,and river water [4,5].The causative role of HBoVs in respiratory and enteric pathology is under investigation,which is a long journey full of potential pitfalls [6,7].In fact,the tissue tropisms of HBoV1 and HBoV2 were different,as evidenced by their different detection frequencies in different clinical samples and patients,resulting in the identification of HBoV1 as the human respiratory bocavirus and HBoV2 as the human enteric bocavirus [4,8,9].
All HBoVs are members of the familyParvoviridae,subfamilyParvovirinae,and genusBocaparvovirus,with linear single-stranded DNA genomes encoding non-structural proteins NS1–4 and NP1 and capsid structural viral proteins VP1–3 [10–12].The three capsid proteins VP1–3 share a common C terminus,with an expression ratio of 1:1:10,to form a non-enveloped icosahedral capsid,which was structured as depressions at the twofold axis,protrusions surrounding the threefold axis,and a canyon surrounding a channel at the fivefold axis of symmetry.
The HBoV capsid carries host and tissue tropism determinants and domains for pathogenicity,genome packaging,assembly,and antigenicity required for viral infection [13].In HBoV1,the major capsid protein VP3 can assemble virus-like particles (VLPs) containing neutralizing epitopes and receptor binding sites [14].The variable surface region (VR) III (202 LDGNAAGG 209) of HBoV1 VP3 has been proposed as a relevant factor of tissue tropism.These regions in enteric HBoV2 VRIII (202 MEDA 205) and HBoV3–4 VRIII (202 MEDS 205) are structurally similar but different from those of respiratory HBoV1 [15,16].These observations suggest that this region might be an important determinant of the genotype specificity of HBoV1 and HBoV2.
Serological studies have used recombinant HBoV1 capsid proteins as antigens to establish causal relationships between acute respiratory tract infection (ARTI) and HBoV1 based on seroconversion or an increase in immunoglobulin G (IgG) level ≥ fourfold,which could not exclude crossreactivity with the capsid proteins of the other three HBoVs,particularly HBoV2,resulting in the original antigenic sin(OAS) phenomenon due to the absence of genotype-specific antibodies [17,18].Although monoclonal antibodies against HBoV1 and HBoV2 have been previously reported,they have not been tested in clinical specimens [13].To search for genotype-specific antibodies,the divergent regions(DRs) on VP3 were aligned,and antibodies targeting DRs of HBoV1 or HBoV2 VP3 were harvested and evaluated by western blotting (WB),enzyme-linked immunosorbent assay (ELISA) and bio-layer interference (BLI),followed by indirect immunofluorescence assay (IFA) with clinical samples.
Methods
Searching for DRs between HBoV1 and HBoV2 by amino acid sequence alignment and structure prediction
Genomic sequences of HBoV1 and HBoV2 from countries around the world were downloaded from GenBank and numbered with their GenBank number,country and year[19,20].Phylogenetic analysis was conducted using the neighbor-joining method and maximum composite likelihood model by MEGA7.0 and Clustal X to align VP3 amino acid sequences.Based on DR data of theVP3gene,the residues exposed on the surface of VP3 from ABK32030.1 and AFW98869.1 reported by our laboratory in Beijing,belonging to HBoV1 or HBoV2,respectively,were predicted by ProtScale (https:// web.expasy.org/ prots cale/).The helix,turn,random coil,and extend stand were analyzed using Jpred4 (http:// www.compb io.dundee.ac.uk/jpred/ index.html) to obtain this VP3 secondary structural information.The antigenic index of the proteins was predicted in the Jameson-Wolf method by DNAStar Protean software.The tertiary structure model of VP3 was predicted by Swiss-Model (https:// swiss model.expasy.org) homology modeling according to the homologous template,named HBoV1–7L0W and HBoV2–7L0U in the Protein Data Bank (http:// www.wwpdb.org).By PyMOL (https:// pymol.org/2/),the VP3 tertiary structure models were visualized and searched for regions with a significant difference at the amino acid level of VP3 between HBoV1 and HBoV2.
Synthesis of peptides according to the DRs of VP3 from HBoV1 and HBoV2 for specific antibody preparation
Peptides deduced from the DRs of VP3 from HBoV1 and 2 were synthesized by GL Biochem (Shanghai,China),with expecting purity >98%,and kept at − 80 °C.These peptides were then dissolved in 8 M urea–phosphate-buffered saline(PBS) buffer for immunization of 6-to 8-week-old clean Japanese white rabbits from Hubei province of China (n=2 per group) with Freund's adjuvant using the following doses of 0.7,0.35,0.35,0.35,and 0.25 μg on weeks 0,2,4,6 and 8,respectively,for specific antibody preparation,while PBS was used in the control group (n=2 per group).Blood samples were then collected from the marginal auricular vein of rabbits at weeks 0 (before primary immunization),2,4,6,and 8.Finally,all rabbits were euthanized at week 8 for serum collection.
Expression and purification of VP3
Recombinant VP3 of HBoV1 and HBoV2 was expressed inEscherichia coli(E.coli) as previously described [21].In brief,the coding regions of the HBoV1VP3gene (nt 3443–5071 of ABK32030.1) and HBoV2VP3gene (nt 3426–5042 of AFW98869.1) were amplified and cloned into the expression vector PET30b and transformed intoE.coliDH5α for the selection of positive clones.Then,the recombinant plasmids PET30b-HBoV1 VP3 or PET30b-HBoV2 VP3 were transformed intoE.coliBL21 (DE3)(Novagen,USA) and induced by 1 mmol/L isopropyl-β-D-thiogalactopyranoside for 19 hours.VP3 was harvested mostly in inclusion bodies with an N-terminal 6×His tag downstream of the T7 promoter,yielding a protein with a molecular weight of 68 kDa.After ultrasonication,the expressed VP3 was subjected to partial affinity purification with nickel-nitrilotriacetic acid agarose (Qiagen,USA).
Western blotting
Purified VP3 proteins were separated by 8%–20% SDSPAGE and then transferred to nitrocellulose membranes.After blocking with 5% non-fat dry milk solution in TBST(Tris–HCl,0.5% Tween-20) at room temperature for 2 hours,the membranes were incubated with antibodies against peptides of VP3 prepared in the study at 4 °C overnight.On the next day,the membranes were washed three times in TBST and then incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG antibodies (Zhongshan,China)at room temperature for 1 hour.The color was developed by addition of the Luminata Forte Western HRP Substrate,and the intensity of the immunoblot bands was quantified using a System GelDoc XR+ImageLab.10–8.
Enzyme-linked immunosorbent assay
The concentration of purified VP3 was determined by the Bradford Protein Quantitative Kit (Sangon Biotech No.C503031) according to the standard curve of bovine serum albumin with an absorption wavelength of A595.Subsequently,50 μL per well of purified VP3 (2 μg/mL,diluted in PBS) was coated onto 96-well microplates for incubation overnight at 4 °C.After blocking with 5% non-fat dry milk solution in TBST at 37 °C for 1 hour,50 μL per well of antibodies against peptides (5 μg/mL,by double dilution) were added to each well and incubated at 37 °C for 1 hour,followed by incubation at 37 °C for 1 hour with 50 μL per well of HRPconjugated goat anti-rabbit immunoglobulin G,(1:5000 dilution in TBST).Then,3,3′,5,5′-tetramethylbenzidine substrates were dispensed into wells and incubated at room temperature for 15 minutes,which was stopped by adding 100 μL per well of H2SO4.The absorbance was read using a spectrophotometer with a 450 nm filter.The cut-off value was determined as 2.1 times the value of the blank control.
Bio-layer interferometry assay
As a tool to assess the binding potency,a BLI assay [22]was chosen to detect the interaction of antibodies with HBoV1 VP3 (1.43×103nM) and HBoV2 VP3 (3.71× 102nM).In brief,antibodies against HBoV1 DRs and HBoV2 DRs that showed no intra-genotype cross-reaction in WB and ELISA were double diluted.The purified VP3,anti-His-probe,and antibodies were added to the test tube according to the protocol.The kinetics module was run with the probes.The size of antibody affinity can be expressed by the affinity constant Kd {L/mass molar concentration,Kd=Koff÷Kon=[Ab×H]∕{[Ab][H]},andR2≥ 0.95 means effective detection.
Immunofluorescence assay
Anti-DR antibodies with no intra-genotype cross-reactivity in WB and ELISA were used in IFA.The specificity of anti-DR antibodies was tested using control slides of respiratory syncytial virus (RSV),influenza virus A and B(FluA and B),adenovirus (ADV),parainfluenza virus I,II,and III (PIV 1–3) from the D 3 Ultra™ DFA Respiratory Virus Screening &ID Kit (Diagnostic Hybrids Inc.,Athens,OH,USA) as antigens.
Clinical specimens (NPAs) were collected from pediatric patients hospitalized at the Capital Institute of Pediatrics,which were HBoV positive identified by viral screening using a capillary electrophoresis-based multiplex PCR (CEMP)assay (Ningbo HEALTH Gene Technologies Ltd.,Ningbo,China) [23] and genotyped as HBoV1 by PCR [4].These specimens were centrifuged at 500gfor 10 minutes to have the cell pellets resuspended and spotted onto slides.These slides were then acetone fixed and kept at − 20 °C for IFA.
For IFA,anti-DR antibodies diluted to 1:20,1:40,and 1:80 were added to control or clinical specimen slides and then incubated at 37 °C for 30 minutes in a humidified box.The slides were washed three times in phosphate buffered saline with Tween-20 (PBST) and then incubated with fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit IgG antibodies (1:1000 dilution;Zhongshan,China) at 37 °C for 30 minutes.After three washes in PBST,the slides were observed under a fluorescence microscope (Nikon Eclipse 80i,Nikon Corporation,Japan).
Statistical analysis
All ELISA measurements were performed in triplicate for calculation of the mean values±standard deviations using GraphPad Prism 9.0.Statistical analysis was conducted by one-way analysis of variance (ANOVA).Pvalues <0.05 were considered statistically significant.
Results
DRs on the amino acid sequences and structures of VP3 from HBoV1 and HBoV2
Based on the alignment of 31 reference amino acid sequences of HBoV1 VP3 (542 aa) and HBoV2 VP3 (538 aa),four regions with differences >8 amino acids between HBoV1 and HBoV2 were named DR 1–4 (Supplementary Fig.1a).The amino acids of DR2 were exposed more often at the surface than those of DR1,3,and 4 (Supplementary Fig.1b).Comparison of the tertiary structural alignment and VP3 main chain region showed that DR1 of HBoV2 DR1,with a divergence of 12 amino acid mutations from HBoV1 DR1,formed a β-sheet independently,while HBoV1 DR4,with seven amino acid mutations,formed an extra α-helix.More importantly,HBoV2 DR2 (located on a fivefold region) formed an α-helix in addition to a β-turn and random coil with the deletion of four conserved amino acids (204 GNAA 207,located on a random coil between two α-helices of HBoV1 DR2),resulting in an incomplete loop that was exposed on the surface (Supplementary Fig.1c).Using a value >1 as a marker of high antigenic ability,DR1-DR4 all showed high antigenic indices and could be used as antigens for the development of specific antibodies(Table 1).
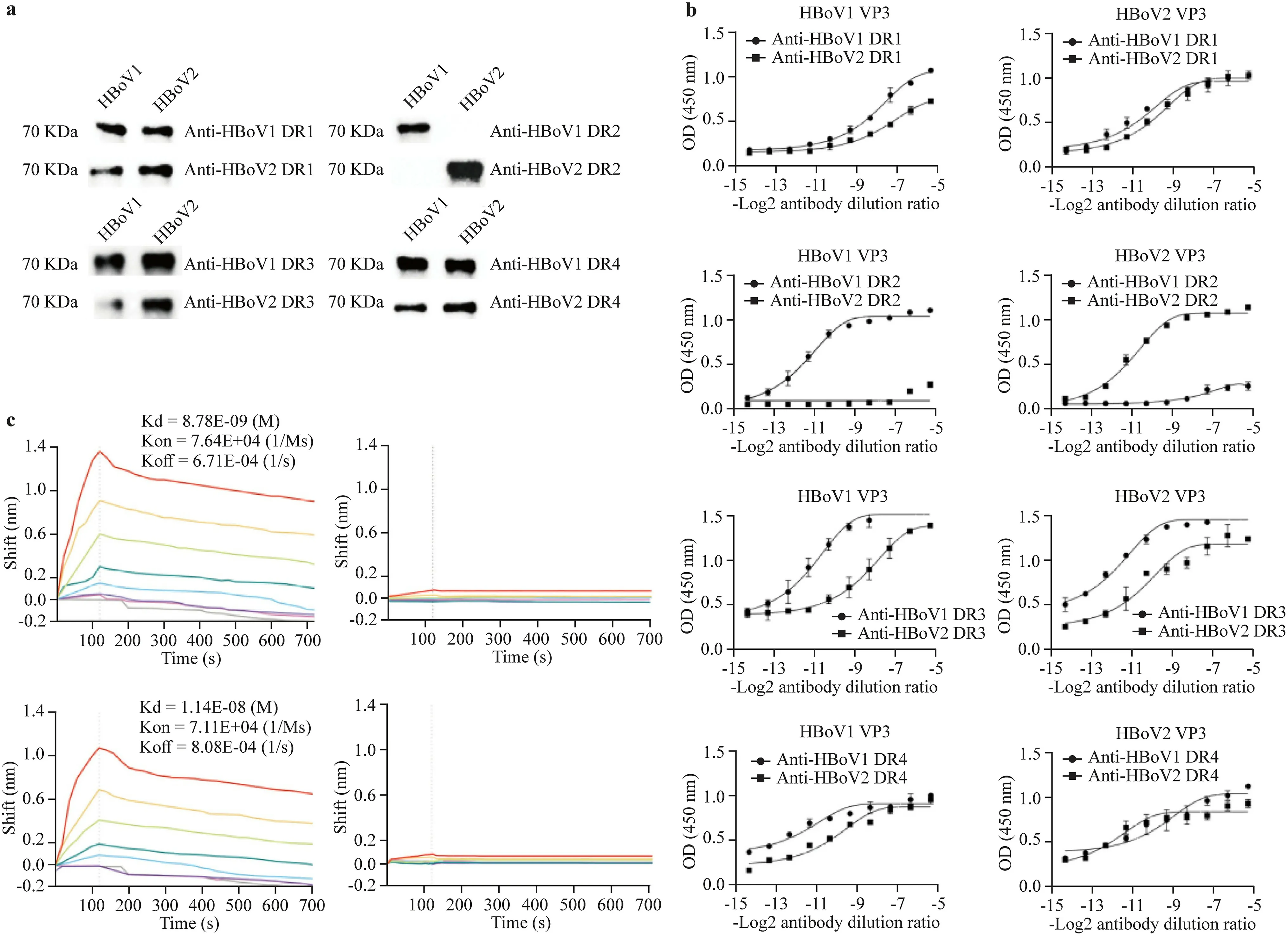
Fig.1 Evaluating the genotypic specificity of anti-DRs in reacting with VP3 of HBoV1 or HBoV2 expressed in Escherichia coli BL21.a In western blotting,VP3 proteins of HBoV1 (lane HBoV1)or HBoV2 (lane HBoV2) expressed in E.coli BL21 was used as an antigen to react with antibodies labeled with anti-HBoV1 or HBoV2 DR1,DR2,DR3,and DR4.The immunoblot bands with a weight of about 68 kDa were shown when anti-DR1,3,and 4 of HBoV1 and HBoV2 reacted with VP3 of HBoV1 or HBoV2,respectively,which were missed when anti-HBoV1 DR2 reacted with VP3 of HBoV2,as well as anti-HBoV2 DR2 reacted with VP3 of HBoV1;b in ELISA,VP3 (100 ng) of HBoV1 or HBoV2 expressed in E.coli BL21 were used as antigens to react with antibodies labeled with anti-HBoV1 or HBoV2 DR1,DR2 in,DR3,and DR4,respectively.The average OD450 values of anti-DR1,3,and 4 to HBoV1 or HBoV2 VP3 showed no significant difference,while that of anti-DR2 to HBoV1 or HBoV2 VP3 showed a significant difference in these cross-reaction assays;c the binding curve graph of HBoV1 VP3 with anti-HBoV1 and anti-HBoV2 DR2,as well as HBoV2 VP3 with anti-HBoV2 and anti-HBoV1 DR2,calculated by Gator software.The highest concentration of polyclonal antibody was 250 nM in the red line.The other five colored lines indicate the ability of the twofold diluted antibody to bind to the VP3 antigen.The gray line indicates the blank control.The affinity constant Kd (unit is liter/mass molar concentration) was used to evaluate the size of antibody affinity{Kd=K of/f K on=[Ab×H]/([Ab][H])}.K of f dissociation rate constant,K on the speed of intermolecular association,Ab antibody,H antigen,DR divergent region,VP capsid structural viral protein,HBoV human bocavirus,ELISA enzyme-linked immunosorbent assay
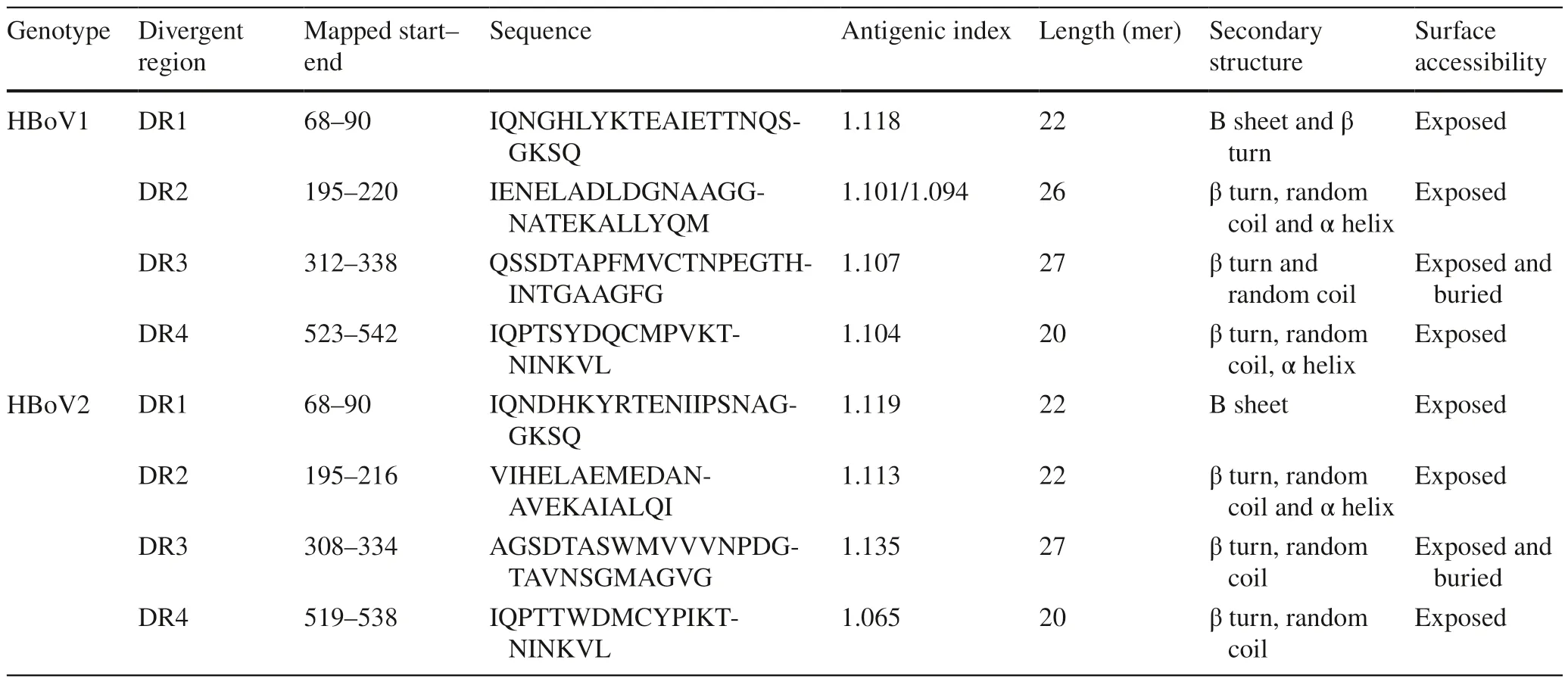
Table 1 Conformational information antigenic index of divergent region DR1–4 located on the major capsid protein VP3 of HBoV1 or HBoV2
Intra-genotype cross-reactivity of antibodies specific to peptides deduced from DR1–4 of HBoV1 and HBoV2 in WB and ELISA
In WB,all immunoblot bands with a molecular weight of approximately 68 kDa were present when antibodies against DR1,3,and 4 of HBoV1 and HBoV2 reacted with VP3 of HBoV1 or HBoV2,respectively.However,the bands were not detected when the anti-HBoV1 DR2 antibody was incubated with VP3 of HBoV2,and the anti-HBoV2 DR2 antibody was incubated with VP3 of HBoV1 (Fig.1 a).
In ELISA,the half effective concentrations (EC50)of anti-HBoV1 or anti-HBoV2 DR2 antibodies against HBoV1 VP3 (7.271e−05 and 2.192e−03) and HBoV2 VP3 (8.168e−04 and 9.209e−05) were different.The average OD450 values of the anti-HBoV1 DR1 antibody to HBoV1 and HBoV2 VP3 were 0.486 and 0.336(t=1.743,P=0.089),respectively,and those of the anti-HBoV2 DR1 antibody to HBoV1 and HBoV2 VP3 were 0.646 and 0.600,(t=0.454,P=0.653),respectively,with no significant difference in these cross-reactivity assays.Similar results were observed in cross-reactivity assays of anti-DR3 and anti-DR4 antibodies.However,the average OD450 values of the anti-HBoV1 DR2 antibody were significantly higher in the reaction with HBoV1 VP3 (0.721)than in the reaction with HBoV2 VP3 (0.099) (t=7.382,P=0.000).The average OD450 values of the anti-HBoV2 DR2 antibody were significantly higher in the reaction with HBoV2 VP3 (0.701) than in the reaction with HBoV1 VP3(0.121) (t=6.401,P=0.000) (Fig.1 b).
The binding ability of anti-HBoV1 or HBoV2 DR2 antibodies to VP3 in BLI assay
According to the BLI assay,as shown in Fig.1 c,the Kd,Kon,andKoffvalues of the anti-HBoV1 DR2 antibody to HBoV1VP3 were 8.78×10–9(M),7.64×104(1/Ms),and6.71×10–4(1/s),while the Kd,Kon,andKoffvalues of the anti-HBoV2 DR2 antibody to HBoV1 VP3 could not be calculated by Gator,which showed no cross-reactivity.Similarly,theKd,Kon, andKoffvaluesoftheanti-HBoV2 DR2 antibody toHBoV2VP3were1.14 ×10–8(M),7.11× 104(1/Ms),and 8.08×10–4(1/s),respectively.Additionally,the Kd,Kon,andKoffvalues of the anti-HBoV1 DR2 antibody to HBoV2 VP3 could not be calculated.
Specific reaction of anti-HBoV1 DR2,not anti-HBoV2 DR2,antibodies to HBoV1-positive clinical specimens in IFA
Anti-HBoV1 and anti-HBoV2 DR2 antibodies showed negative results in reaction with control slides of RSV,Flu A and B,ADV,and PIV 1–3 in IFA.Three clinical specimens were tested in IFA.One specimen (D9466),collected on August 17,2021,from a 14-month-old boy who was diagnosed with ARTI with the underlying disease of acute lymphoblastic leukemia,was positive for HBoV (6.11×104copies/mL),RSV,and PIV determined by CEMP and then genotyped to HBoV1 by PCR,with PIV3 positivity and RSV negativity in IFA using individual FITC-labeled monoclonal antibodies against PIV3 or RSV,respectively,in the D 3 Ultra™ DFA Respiratory Virus Screening &ID Kit.Another specimen(D10398),collected on November 23,2021,from a 2-yearold girl who was diagnosed with pneumonia,was only positive for HBoV (6.13×104copies/mL) determined by CEMP and then genotyped to HBoV1 by PCR.The third specimen(D9174),collected on July 19,2021,from a 1-year 9-monthold girl who was diagnosed with pneumonia,was positive only for HBoV (5.85×104copies/mL) determined by CEMP and then genotyped to HBoV1 by PCR.
In the reaction with NPAs D9466,more positive cells with specific green fluorescence were observed at a 1:20 dilution of anti-HBoV1 DR2 antibody than at a 1:80 dilution.Similarly,in the reaction with NPAs D10398,more positive cells were observed at the 1:20 dilution of anti-HBoV1 DR2 antibody than at a 1:40 dilution.However,no positive cells were observed in these clinical specimens in the reaction with the anti-HBoV2 DR2 antibody (Fig.2).For D9174,no positive cells were detected in the reaction with either anti-HBoV1 or anti-HBoV2 DR2 antibodies.
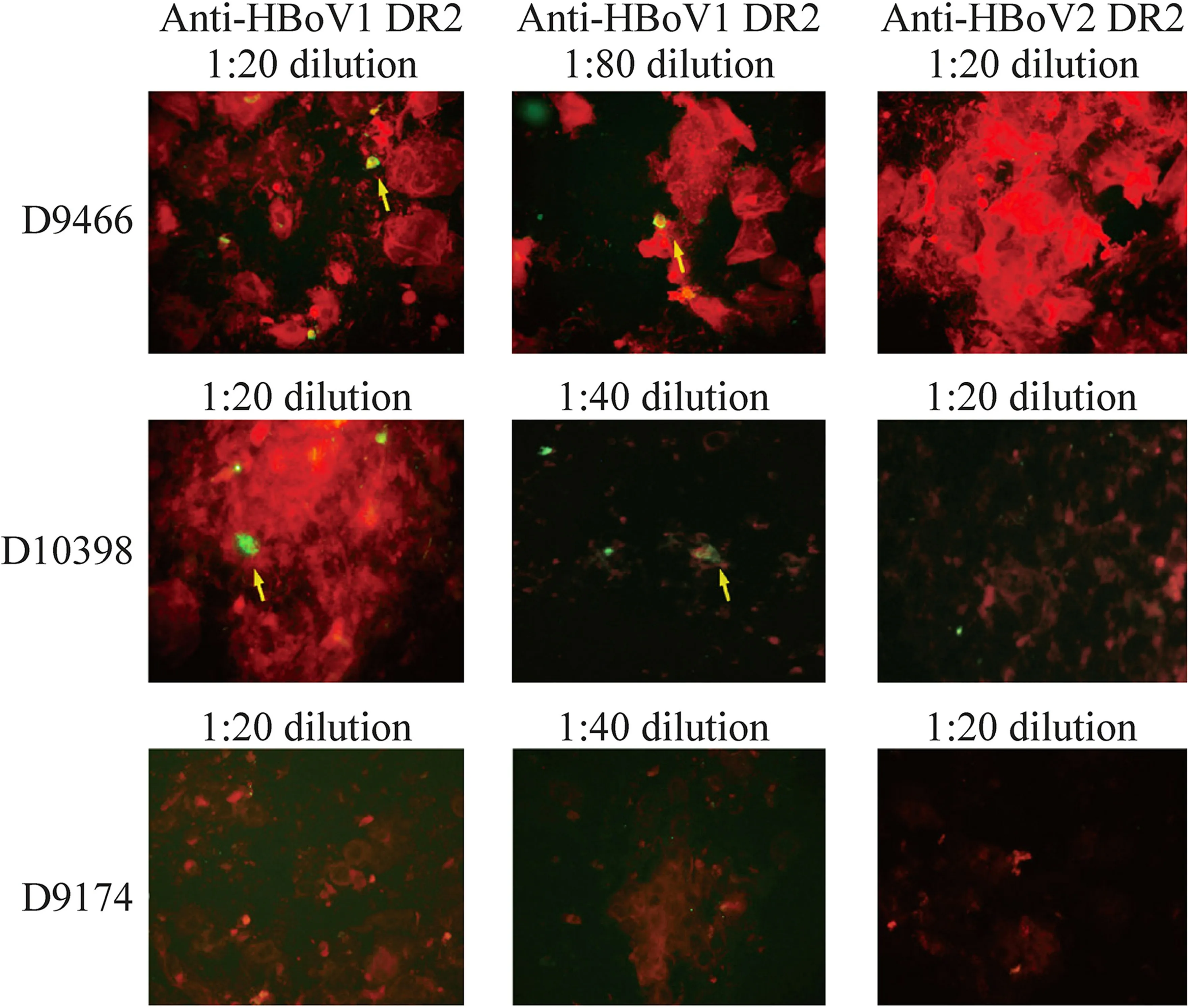
Fig.2 The genotypic specificity of anti-HBoV1 DR2 was confirmed in the indirect immunofluorescence assay.Positive cells with specific green fluorescence (shown by arrows) were observed in clinical specimens (D9466 and D10398) reacting with anti-HBoV1 DR2 but not with anti-HBoV2 DR2.Only negative cells with no specific green fluorescence were shown in a clinical specimen (D9174) reacting with anti-HBoV1 and HBoV2 DR2.DR divergent region,HBoV human bocavirus
Discussion
HBoVs possess three main structural proteins,VP1,VP2,and VP3,with an expression ratio of 1:1:10 of the virus capsid.Among these proteins,VP3,containing neutralizing epitopes and receptor binding sites,could be expressed as VLPs,which had good immunogenicity and induced highly specific antibodies in rabbits and mice [24].However,the lack of genotype specificity of antigens and antibodies in serological studies has led to cross-reactivity and the OAS phenomenon [17,18].This study searched the major viral capsid protein VP3 for its DRs between HBoV1 and HBoV2.Then,genotype-specific antibodies were harvested from rabbits immunized with peptides deduced from these DRs.
By comparison of 31 reference sequences,four DRs(DR1–4) in HBoV1 and HBoV2 were located on VP3,not only at the amino acid level with >8 mutations but also at the secondary and tertiary structure levels.Notably,four conserved amino acids (204 GNAA 207) located at the apex of a half-loop structure in the form of a random coil between two a-helices were detected in HBoV1-DR2 (195 IENELADLDGNAAGGNATEKALLYQM 220) but not in HBoV2-DR2 (195 VIHELAEMEDANAVEKAIALQI 216),which is similar to those of HBoV1-VRIII (198 ELADLDGNAAGGNATEKALL 217) and HBoV2-VRIII (196 IHELAEMED(A/S)NAVEKAI 212) [15].
To enhance the antigenic index,three amino acids(195 IEN 197) were added to HBoV1-DR2.The loss of the halfloop in the tertiary structure of HBoV2,which results from the deletion mutations of four amino acids in VP3,was also reported in HBoV3 and HBoV4 compared to that of HBoV1 at the apex of the loop by cryo-electron microscopy [15,16].Therefore,genotype specificity between HBoV1 and HBoV2 might be determined by DR2.
To harvest genotype-specific antibodies,peptides were synthesized according to the amino acid sequences of DR1–4,which were used as antigens to immunize rabbits for collection of anti-HBoV1 or anti-HBoV2 DR1–4 antibodies.Then,the genotype specificities of anti-HBoV1 or anti-HBoV2 DR1–4 antibodies in reaction with VP3 were tested by WB and ELISA.WB results showed high intra-genotype cross-reactivity of anti-HBoV1 or anti-HBoV2 DR1,3,and 4 antibodies.However,anti-HBoV1 DR2 and anti-HBoV2 DR2 antibodies clearly showed no intra-genotype crossreaction to VP3 of HBoV2 and HBoV1,respectively,which supported the hypothesis that the DR2 region might contain some genotype-specific features.
This hypothesis was further confirmed by ELISA.While the EC50values of anti-HBoV1 and anti-HBoV2 DR1,3,and 4 antibodies showed no significant difference in reaction with VP3 of HBoV1 and HBoV2,the EC50of anti-HBoV1 and anti-HBoV2 DR2 antibodies showed a significant difference in reaction with VP3 of HBoV1 and HBoV2.The BLI assay was used as an efficient tool to determine the binding ability between antigens and antibodies.BLI results corroborated the intra-genotype specificity of anti-HBoV1 and anti-HBoV2 DR2 antibodies,which was further validated with the presence of positive cells in the reaction of HBoV1-positive NPAs from ARTI pediatric patients with anti-HBoV1 DR2 antibody but not anti-HBoV2 DR2 antibody.
The specimen D9174,which was positive for HBoV1 DNA,showed a negative result in the antigen test by IFA.Since PCR is more sensitive than immunofluorescence detection and shows positive results even in the incubation period and recovery periods,different detection methods should be selected at different disease stages.More complete collection of clinical history and laboratory data will improve the accuracy of the interpretation of the results.Our results also suggested that more large-scale antigen tests among ARTI pediatric patients can be carried out using these genotype-specific antibodies to build the correlation between HBoV1 and the disease.
In the specific reaction of anti-HBoV1 DR2 antibody to HBoV1-positive clinical specimens in IFA,only three samples with medium HBoV1 viral load were tested,which was a limitation of our study.Testing more clinical specimens,particularly those with or without concurrent detection of other pathogens and those with low,medium,or high viral loads,will provide a wider spectrum of results,which will empower a better understanding of HBoV1 infection and potential applications of the antibodies obtained in this work.
In conclusion,the genotype specificities of anti-HBoV1 and anti-HBoV2 DR2 antibodies were examined in WB and ELISA,which was strengthened by the absence of affinity of anti-HBoV1 and anti-HBoV2 DR2 antibodies to VP3 of HBoV2 and HBoV1,respectively,in BLI assay.Furthermore,these results were validated in HBoV1-positive clinical specimens,in which positive cells were detected only in reaction with anti-HBoV1 DR2 antibody,not with anti-HBoV2 DR2 antibody.Therefore,anti-HBoV1 and anti-HBoV2 DR2 antibodies can be used for rapid antigen tests in children’s clinical samples and genotype-specific antibody detection in serological research,particularly in ARTI patients.
Supplementary InformationThe online version contains supplementary material available at https:// doi.org/ 10.1007/ s12519-023-00697-8.
AcknowledgementsWe would like to thank the Affiliated Children's Hospital and Capital Institute of Pediatrics stafffor collecting the clinical samples,and the children and their parents who supported our research work.We would like to thank Editage (http:// www.edita ge.cn) for language editing.
Author contributionsDR contributed to methodology,formal analysis,and writing of the original draft.XYP contributed to validation,reviewing and editing.WF and ZYT contributed to data curation and methodology.SPD contributed to software.ZRN contributed to investigation.SY,LLY,JLP,and DHJ contributed to resources.ZH contributed to visualization.QCF contributed to supervision,project administration,reviewing and editing.ZLQ contributed to conceptualization,supervision,reviewing and editing,and funding acquisition.All the authors approved the final version of the manuscript.
FundingThis work was supported by the Beijing Natural Science Foundation (7192029),the National Natural Science Foundation of China (82172277),and the Beijing Municipal Commission of Health(2060399 PXM2017_026268_00005_00254486).
Data availabilityData will be made available on reasonable request.
Declarations
Ethical approvalThe research was approved by the Ethics Committee of the Capital Institute of Pediatrics (approval number: SHERLLM2019013) as a retrospective study.
Conflict of interestNo financial or non-financial benefits have been received or will be received from any party related directly or indirectly to the subject of this article.The authors have no conflict of interest to declare.
杂志排行
World Journal of Pediatrics的其它文章
- Instructions for Authors
- Editors
- Information for Readers
- Efficacy of inactivated SARS-CoV-2 vaccination in pediatric hematology/oncology patients: a real-world study
- Real-world performance analysis of a novel computational method in the precision oncology of pediatric tumors
- Determinants of infant behavior and growth in breastfed late preterm and early term infants: a secondary data analysis
