Protective effect of lycopene on Parkinson's disease cell model based on endoplasmic reticulum stress
2023-12-02BAOBoCHAIXingxingDENGZiliangLIULuluZHUShaopingLILili
BAO Bo, CHAI Xing‑xing, DENG Zi‑liang, LIU Lu‑lu, ZHU Shao‑ping, LI Li‑li,4✉
1.Department of Pathology and Pathophysiology, Guangdong Medical University, Zhanjiang 524023, China
2.Laboratory Animal Center, Guangdong Medical University, Dongguan 523808, China
3.Department of Histology and Embryology, Guangdong Medical University, Dongguan 523808, China
4.Dongguan Key Laboratory for Development and Application of Experimental Animal Resources in Biomedical Industry, Dongguan 523808, China
Keywords:
ABSTRACT Objective: To evaluate the effect of lycopene on Parkinson's disease cell model and its possible mechanism.Methods: The SH‑SY5Y cells were treated with 0.5 μmol/L rotenone for 24 h to establish Parkinson's disease cell model.The experiments were randomly divided into the control group, the lycopene group, the rotenone group, the pretreatment groups of different concentrations lycopene (low, medium, high concentration).Cell viability was detected by CCK‑8 assay, the morphological changes of cells were observed under an inverted microscope,Hoechst staining was used to observe cell apoptosis, the expression and distribution of endoplasmic reticulum stress marker proteins GRP78 and CHOP in each group were detected by Western blot and cell immunofluorescence.Results: The study found that compared with the control group, the cell viability in the rotenone group was significantly decreased with obvious apoptosis; compared with the rotenone group, the cell viability of the lycopene pretreatment group was improved, and the difference was statistically significant(P<0.05); The apoptosis in the lycopene pretreatment group was decreased.The expression of GRP78 and CHOP in the rotenone group was significantly higher than that in the control group (P<0.01),while the expression of both in the high concentration lycopene pretreatment group was lower than that in the rotenone group (P<0.05).Conclusion: Lycopene pretreatment had a significant protective effect on rotenone‑induced SH‑SY5Y cells, which may be related to the fact that lycopene pretreatment can effectively alleviate endoplasmic reticulum stress in SH‑SY5Y cells damaged by rotenone.
1.Introduction
Parkinson’s disease(PD) is the second most common neurological degenerative disease in the middle‑aged and elderly , following Alzheimer[1].It affects over 6 million people worldwide[2].Studies have shown that PD has become one of the fastest‑growing numbers of cases among neurological disorders diseases[3].Current research has demonstrated that a combination of factors[4], including aging, genetic factors, and environmental factors, contribute to the development of PD.Recent reports have highlighted the significant role of endoplasmic reticulum stress (ERS) in the pathogenesis of PD[5-9].The study of Ioanna C.Stefani and his colleagues[10]discovered that ERS initially aims to restore cell homeostasis in the early stage; however, excessive stress can trigger pro‑apoptotic signals and promote neuron cell death.At present, the treatment of PD primarily focuses on symptom control[11] and does not address the underlying pathological progression of the disease[12].Over time, the efficacy of treatment diminishes, and side effects may arise[13].With an improved understanding of the pathogenesis of PD, the hypothesis of potential neuroprotective strategies has been proposed.Early adoption of neuroprotective or disease‑modifying therapies may alter the disease progression[14].In conclusion,the authors concur that early intervention targeting endoplasmic reticulum stress holds great significance in the prevention and treatment of PD.
Lycopene is a pharmacologically active carotenoid known for its anti‑cancer, antioxidant, cardiac protection and antihypertensive effects[15, 16].In recent years, numerous studies have reported the neuroprotective effects of lycopene[17-19].Previous studies have shown that the neuroprotective mechanism of lycopene involves antioxidant, inhibition of inflammation, inhibition of apoptosis and restoration of intracellular Ca2+homeostasis[20].However, the relationship between the neuroprotective effect of lycopene and ERS remains largely unexplored.Therefore, this study aims to investigate the preventive effect of lycopene on Parkinson’s disease cell models based on endoplasmic reticulum stress.The findings of this study could provide valuable insights for the prevention and treatment of PD.
2.Materials and methods
2.1 Materials
DMEM high glucose medium, fetal bovine serum(FBS), trypsin,Penicillin‑Streptomycin (Gibco, USA); Dimethyl sulfoxide,rotenone (Lot No.R8875, 95% purity)(Sigma Aldrich (Shanghai)Trading Co., LTD, China).Lycopene (98% purity, Xi’an Fedders Biotechnology Co., LTD, China); CCK‑8 assay kit (Dojindo,Japan); RIPA lysis buffer, BCA Protein Assay Kit, DAPI, super Sensitive ECL Chemiluminescence Kit (Beyotime, China).β‑Actin antibodies, CHOP antibodies (Lot No.3700, 2895, CST, USA);GRP78 antibodies (Lot No.ARG54026, Arigo, China); Horseradish peroxidase‑labeled goat against rabbit secondary antibody IgG(H+L), Horseradish peroxidase‑labeled goat against mouse secondary antibody IgG(H+L) (Lot No.ZB‑5301, ZB‑5305, ZSGB‑BIO, China).Human neuroblastoma cells were provided by our research group.Rotenone and lycopene were dissolved in DMSO to form mother liquor, which was diluted to working concentration using serum‑free DMEM medium.
2.2 Methods
2.2.1 Cell culture
Frozen SH‑SY5Y cells were resuscitated and cultured in a 5%CO2, 37 ℃ incubator with DMEM medium with 10% FBS and 1%penicillin‑streptomycin.The culture medium was timely adjusted;the cells were digested and passaged with 0.25% trypsin when the cell confluence reached 80%‑90%.Experiments were carried out with cells in logarithmic growth phase.
2.2.2 Cell grouping and treatment methods
Control Group: Cells were treated with normal culture medium.Lycopene Group: Cells were treated with 20 μmol/L lycopene for 2 h and then incubated with normal medium for 24 h.Rotenone Group:Cells were treated with 0.5 μmol/L rotenone for 24 h.Lycopene Pretreated Group: Cells were pretreated with 5 μmol/L, 10 μmol/L,or 20 μmol/L lycopene for 2 h and then incubated with 0.5 μmol/L rotenone for 24 h.
2.2.3 The assay of cell viability
SH‑SY5Y cells were seeded in 96‑well plates at a density of 5.0×104cells per well.Each well was inoculated with 100 μL cell suspension and three multiple wells.After fully attached, drug treatment was performed.Afterwards, CCK‑8 assay kit was used to detect cell viability.
2.2.4 Hoechst staining
SH‑SY5Y cells were seeded in 24‑well plates at a density of 1.0×105cells per well.Each well contained 500 μL cell suspensions.After the cells fully attached, they were treated with the respective drugs according to the experimental groups.Following the drug treatment, Hoechst 33258 was performed to observe cell apoptosis.
2.2.5 Western blot analysis
After drug treatment, the culture medium was discarded and the cells were washed twice with pre‑cooled PBS.The cells were then lysed in RIPA buffer containing 1 mM PMSF for 0.5 h on ice.The lysates were centrifuged at 14 000 r/min and 4 ℃ for 15 min, and the supernatant were collected.The protein concentration in the supernatant was determined using the BCA Protein Assay Kit.Whole cell lysates were separated by SDS‑PAGE and transferred to PVDF membrane.At room temperature, the membranes were blocked with 5% nonfat milk for 2 h and incubated overnight at 4°C with primary antibodies β‑actin (1:1 000), GRP78 (1:1 000),CHOP (1:1 000).The signals were detected with ECL reagents after incubation with secondary antibodies (1:1 000).The gray value of the target bands was analyzed using Image J software.
2.2.6 Cell immunofluorescence
SH‑SY5Y cells were seeded in laser confocal dishes at a density of 5.0×104cells per dish.Each dish was inoculated with 1 mL cell suspension.After the cells were treated with drugs, the culture medium was discarded and the cells were washed twice with pre‑cooled PBS.A mixture of acetone and methanol in equal volumes was added into each dish to fix and permeabilize at room temperature for 20 min.The mixture was then aspirated, and the cells were washed three times with PBS for 5 minutes each.Appropriate 5%BSA was added to each dish and blocked at room temperature for 30 min.The dishes were incubated overnight at 4 ℃ with primary antibodies (GRP78 at 1:100, CHOP at 1:70).After removing the primary antibodies and the cells were washed with PBS for 3 times,5 min per time.Fluorescently labeled secondary antibodies (1:300)were to the dishes and incubated at room temperature for 1 hour.The cells were then washed with PBS and stained with DAPI.Finally,the cells were observed and photographed at the laser confocal workstation as early as possible.
2.2.7 Statistical processing
Statistical analysis of the data was performed by GraphPad Prism5.0 software.The data were represented by mean±standard error.The comparison between two groups was conducted by t test, and the comparison among multiple groups of samples was conducted by analysis of variance.P<0.05 were considered statistically significant.
3.Results
3.1 Effect of lycopene on SH‑SY5Y cells
In order to further investigate the role of lycopene in rotenone‑damaged SH‑SY5Y cells, we first examined the effect of lycopene on SH‑SY5Y viability.The results are presented in Figure 1.Compared to the control group, the viability of SH‑SY5Y cells did not show significant changes after 24 h treatment with different concentrations of lycopene.This suggests that within the tested concentration range, lycopene did not have a significant impact on the viability of SH‑SY5Y cells.Therefore, lycopene can be considered safe for further research.

Fig 1 Effects of different concentrations of lycopene on cell viability(n=3,±s)
3.2 Effect of lycopene pretreatment on rotenone-damaged SH-SY5Y cells
Next, we investigated the effect of lycopene pretreatment on rotenone‑damaged SH‑SY5Y cells.The results are shown in Figure 2, in comparison to the control group, the cell viability of rotenone group decreased significantly (P<0.01).However, pretreatment with different concentrations of lycopene improved cell viability to varying degrees, with cell viabilities of [(74.74±0.47)%, (81.70±1.90)% and (83.17±1.37)%].The differences between the lycopene pretreatment group and the rotenone group were statistically significant (P<0.05,P<0.01).Microscopic examination of cell morphology revealed that the cells in the lycopene pretreatment group exhibited similar growth and good shape as those in the control group.Conversely, cell growth was inhibited in rotenone group, characterized by a significant reduction in cell number,blurred cell boundaries, and the presence of shrunken and rounded cells.No significant difference in cell morphology between lycopene pretreatment group and rotenone group, indicating that lycopene pretreatment had no obvious effect on improving cell morphology.
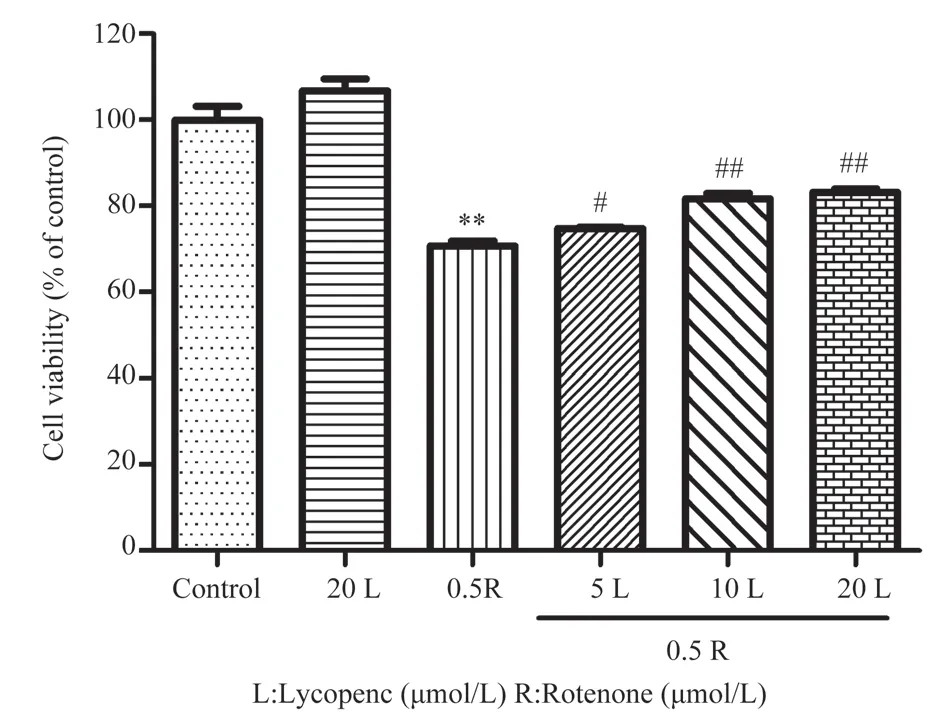
Fig 2 Effects of lycopene pretreatment on rotenone‑induced SH‑SY5Y cell viability(n=3, ±s)

Fig 3 Effects of lycopene pretreatment on rotenone‑induced SH‑SY5Y cell morphology (100×)
3.3 Effect of lycopene pretreatment on apoptosis of rotenone‑damaged SH‑SY5Y cells
To further investigate the effect of lycopene pretreatment on SH‑SY5Y cells, Hoechst staining was utilized to observe cell apoptosis under a fluorescence microscope.The results, as depicted in Figure 4, reveal that the cells in the control group and the lycopene treatment group displayed normal staining patterns with uniform fluorescence.However, in the rotenone group, cells exhibited denser staining, intensified fluorescence, and a whitish appearance,indicating a higher degree of chromatin condensation and marginalization.These characteristics suggest that the intracellular chromatin was highly condensed and on the verge of decomposing into fragmented apoptotic bodies.Compared with rotenone group,the cells in lycopene pretreated group showed less dense staining,indicating a reduction in apoptotic features.
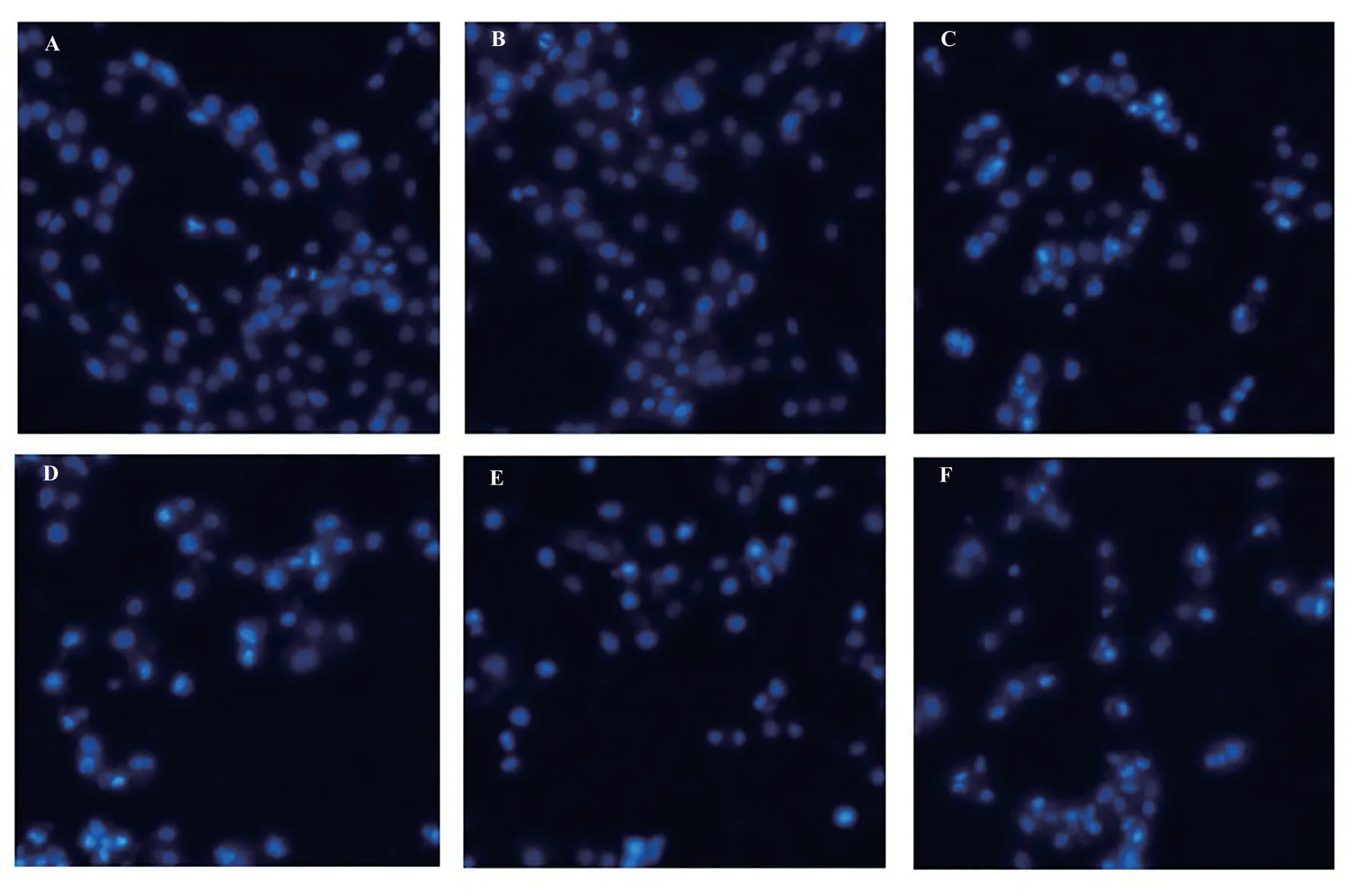
Fig 4 Cell apoptosis was detected by Hoechst staining (200×)
3.4 Effect of lycopene pretreatment on endoplasmic reticulum stress induced by rotenone in SH-SY5Y cells
In Figure 5A and B, the expression of GRP78 in the rotenone group(1.13±0.16) was significantly increased compared to the control group (0.50±0.17) (P<0.01).In the lycopene pretreatment groups at different concentrations, the expression level of GRP78 were as follows: 5 μmol/L (1.25±0.23), 10 μmol/L (1.02±0.23), and 20 μmol/L (0.58±0.15).The expression level of GRP78 in the 20 μmol/L lycopene pretreatment group was significantly decreased compared to the rotenone group, and this difference was statistically significant (P<0.05).The results of cellular immunofluorescence in Figure 5E were consistent with the Western blot results, showing consistent expression patterns of GRP78 in each experimental group.Regarding CHOP expression, as shown in Figure 5C and D the rotenone group (1.33±0.04) exhibited significantly higher expression levels of CHOP compared to the control group (0.53±0.03)(P<0.01).In the lycopene pretreatment groups at different concentrations, the expression levels of CHOP were as follows: low concentration (1.10±0.02), medium concentration (0.98±0.08), and high concentration (0.86±0.03), all of which were lower than that in rotenone group(P<0.01).The results of cellular immunofluorescence in Figure 5F revealed that CHOP was mainly located in the nucleus.Rotenone treatment increased fluorescence of CHOP, while lycopene pretreatment attenuated the fluorescence intensity of CHOP.

Fig 5 Effects of lycopene pretreatment on rotenone‑induced endoplasmic reticulum stress in SH‑SY5Y cells
4.Discussion
Lycopene has attracted attention due to its strong antioxidant capacity and its role in studying antioxidant stress in various diseases.Liu Chongbin et al.found that lycopene can improve the antioxidant stress ability of rotenone‑induced PD model mice and alleviate the cognitive and motor dysfunction[21].Additionally,lycopene has been found to protect SH‑SY5Y cells from autophagy‑induced cell death by inhibiting oxidative stress‑activated AMPK/mTOR pathways[22].These findings indicate the neuroprotective effects of lycopene, although the underlying mechanisms are not fully understood.The endoplasmic reticulum is a crucial site for protein synthesis, processing and transport in eukaryotes.ERS has been detected in samples from PD patients[23], and rotenone has been shown to induce ERS[24-26].Chen Yuanyuan et al.reported that ERS may play a more significant role than reactive oxygen species in the early death of SK‑N‑MC cells induced by rotenone[24].Based on the involvement of ERS in PD, this study aimed to investigate the effect of lycopene pretreatment on rotenone‑induced PD cell model and its underlying mechanism.The results demonstrated that low concentration of lycopene pretreatment improve cell viability,significantly compared to the rotenone group (P<0.05).Medium and high concentrations of lycopene pretreatment significantly enhanced cell viability (P<0.01).Furthermore, lycopene pretreatment reduced the apoptosis of SH‑SY5Y cells, indicating its protective effect against rotenone‑induced injury.These findings align with the results of previous studies by Li Tan et al.[22] and Feng Chunsheng et al.[27],further confirming the protective effect of lycopene on damaged SH‑SY5Y cells.
To explore the potential mechanism underlying the protective role of lycopene pretreatment in rotenone‑damaged SH‑SY5Y cells, we investigated ERS in cells.GRP78, a protein located in endoplasmic reticulum whose upregulation indicates ERS, was examined[28].The expression of GRP78 was significantly upregulated in rotenone‑induced SH‑SY5Y cells (P<0.01), indicating ERS induction.In the low‑concentration lycopene pretreatment group, the expression of GRP78 increased, but there was no statistical significance compared to the rotenone group (P>0.05).The medium‑concentration lycopene pretreatment group exhibited decreased expression of GRP78, , although it was not statistically significant compared to the rotenone group (P>0.05).However, the high‑concentration lycopene pretreatment group showed a significant decrease in GRP78 expression compared to the rotenone group (P<0.05).Considering that early ERS may have a protective effect on nerve cells, the study aimed to evaluate the degree of rotenone‑induced ER stress by examining CHOP (also known as GADD153).CHOP is a proapoptotic molecule induced by ERS[29].The results indicated a significant increase in CHOP expression (P<0.01), suggesting that rotenone induced excessive ERS in SH‑SY5Y cells, leading to ERS‑induced apoptosis.In contrast, CHOP expression was downregulated in the lycopene pretreatment group, suggesting that lycopene pretreatment alleviated ERS and prevented the occurrence of apoptosis.
In conclusion, lycopene pretreatment can significantly enhance cell viability and reduce rotenone‑induced apoptosis, indicating a role of lycopene pretreatment to protect cells.The Lycopene pretreatment down‑regulated the expression of ERS indicator protein GRP78 and inhibited the expression of ERS apoptotic protein CHOP, indicating that lycopene could alleviate ER stress in PD cell models, avoiding cell apoptosis derived from excessive ERS.However, the detailed mechanism of its effect requires further study.
Author’s contribution
The participants of the experiment design were Li Li‑li, Bao Bo,Chai Xing‑xing; The experimenters were Bao Bo, Chai Xing‑xing,Deng Zi‑liang, Liu Lu‑lu, Zhu Shao‑ping, Li Li‑li; The article was written by Bao Bo, Deng Zi‑liang and Li Li‑li.
All authors declare no conflict of interest.
杂志排行
Journal of Hainan Medical College的其它文章
- Research progress on key genes of vitamin D signaling pathway
- Research progress on the influence of local hemodynamics on carotid atherosclerosis
- Copy number variation sequencing for diagnosis of cytomegalovirus infection based low‑depth whole‑genome sequencing technology in fetus: Three cases and literature review
- Exploration of the molecular mechanism of Qishen decoction in regulating miR-495/FTO pathway mediated macrophage polarization to improve insulin resistance therapy of type 2 diabetes
- Intervention of Xuduan Zhongzi Formula on spermatogenesis epididymal morphological changes in a mice model of oligospermia
- Expression of miR-9-5p and RHOA in aluminum-induced rat cognitive dysfunction
