Establishment and Application of A Human Primary Keratinocyte Inflammation Model for Cosmetic Raw Material Screening
2023-11-29SunFanghuiSongXiaojieHuogang
Sun Fanghui,Song Xiaojie,Huo gang
R&D Center,Osmun Biological Co.,Ltd.,China;
Abstract Using inducers to induce cells to produce inflammatory response is a common in vitro experimental method to study inflammation.However,there are relatively few inflammatory models developed for the cosmetic industry,and there are also great differences in the control of model induction,the selection of inflammatory indicators,and the concentration of inducers.Therefore,in this paper,by systematically studying the effects of Lipopolysaccharide (LPS) on the cell viability,the levels of IL-1α,IL-8 and ROS of human primary keratinocytes,a skin inflammation model based human primary keratinocyte was developed.The results showed that 0.01~100 µg/mL LPS had no significant effect on the cell viability of human primary keratinocytes,while 100 µg/mL LPS could simultaneously induce human primary keratinocytes to produce large amounts of IL-1α and IL-8,and 0.01 µg/mL LPS could induce plentiful ROS.Therefore,a skin inflammation model for differential induction of different inflammatory indicators was established,and the sample OSM2021041301 was tested with this model,it was found that sample OSM2021041301 could significantly inhibit LPS-induced elevated IL-1α and IL-8 levels,the inhibitory effect on LPS-induced elevated ROS level was weak.The results indicated that OSM2021041301 has certain anti-inflammatory effect on inflammation caused by the increase of IL-1α,IL-8 and ROS induced by LPS and its analogues.
Key words LPS;human primary keratinocytes;inflammation model;IL-1α;IL-8;ROS
Skin inflammation refers to a state of high response caused by external stimulation of the skin,mainly manifested as paroxysmal burning,redness,stinging,itching,tightness and other symptoms.With the increasing complexity of the living environment,increasing numbers of people are facing the trouble of skin inflammation.Therefore,screening of active substances with anti-inflammatory effects is a research hotspot in the cosmetics industry at present[1,2].The construction of inflammation model in vitro by inducer stimulation is a commonly used experimental method for screening anti-inflammatory substances.If the tested substance can inhibit the inflammatory response caused by inducers,it indicates that the substance has certain anti-inflammatory activity.But most inflammation research has focused on medicine and life sciences,relatively few inflammation models have been developed specifically for the cosmetic industry.For instance,most studies have used immune cells such as RAW264.7 and THP-1 to carry out anti-inflammatory experiments[3-6].However,skin inflammation is a combination of immune cells and keratinocytes.In particular,keratinocytes,as the outermost layer of skin cells,have been underestimated in the role of skin inflammation.In addition,there are no a unified standard in terms of skin cell type,detection indicator,and the selection of inducer`s type and concentration in the existing models of skin inflammation[7,8],which provides relatively limited reference for cosmetics enterprises to carry out research on inflammation.
Damaged keratinocytes can secrete lots of inflammatory factors and activate more advanced immune cells (macrophages,lymphocytes,mast cells,T cells and granulocytes) into the immune state,thus causing a cascade amplification effect of inflammatory response[9].Compared with epidermal cell lines such as HaCAT,which are commonly used in current inflammation models,human primary keratinocytes are directly extracted from human skin tissues without long-term in vitro culture,thus preserving the characteristics of human keratinocytes to the greatest extent.The study of human primary keratinocytes on skin inflammation can more accurately simulate the process of skin inflammation caused by external factors.
The inducers for the construction of inflammatory models include LPS,capsaicin,and tumor necrosis factor,among which LPS is the most widely used[6,10,11].It stimulates host cells to produce inflammatory response through toll-like receptors and promotes the expressions of transcription factors (NF-kB),inflammatory factors (IL-1,IL-6,IL-8,IL-10,TNF-a,etc.) and inflammatory enzymes (iNOS,COX2),and a large amount of ROS is produced[12].The up-regulation of IL-1α can recruit other immune cells,causing fever symptoms and chronic inflammation.The up-regulation of IL-8 can cause the aggregation of local neutrophils,leading to changes in vascular permeability and itching and tingling symptoms.ROS increases the synthesis of prostaglandin E2 by up-regulating the level of COX2,thus amplifying erythema and inflammatory reactions[13].It can be seen that these three inflammatory indicators are most closely related to the symptoms of skin inflammation such as redness,swelling,heat and pain.
In this paper,the effects of LPS at different concentrations on the expression of IL-1α,IL-8,and ROS in human primary keratinocytes were systematically studied.An attempt was made to establish a skin inflammation model based on human primary keratinocytes,in order to construct an inflammation model for more scientific screening of anti-inflammatory active materials from the perspective of keratinocytes.
1 Experimental part
1.1 Reagents and instruments
Human primary keratinocyte (102-05n) (Cell Application);LPS (E.coli 0111:B4) (Sigma);MTT(Biosharp Life Science);DMSO (Sinopharm);OSM2021041301 (Yunchu Biology);Penicillin(Biological Industries);Streptomycin (Biological Industries);Human IL-1α ELISA kit (Abcam);Human IL-8 ELISA kit(Abcam);Keratinocyte-SFM(1×) (Gibco);DCFH-DA (Sigma);Microplate reader(PerkinElmer).
1.2 Experimental method
1.2.1 Cell culture
Human primary keratinocytes were cultured in KSFM complete medium and incubated in a incubator at 37 ℃ and 5% CO2,the medium was changed every 1 day.
1.2.2 Cell viability assay
Human primary keratinocytes were seeded into 96-well plates at a density of 3×103cells/well.When the confluency reached 90%,the cells were treated with 0.01~100 µg/mL LPS.After incubation for 24 h,0.5 mg/mL MTT was added,and the cells were incubated for 4 h.Formazan was extracted with DMSO,and the absorbance at 560 nm was detected with Microplate reader,and the relative cell viability was calculated.
1.2.3 IL-1α and IL-8 content assay
Human primary keratinocytes were seeded in 24-well plates at a density of 5×103cells/well.When the confluency reached 90%,LPS (0.01~100 µg/mL)or a mixture of LPS and OSM2021041301 was administered.After incubation for 24 h,the medium was collected and centrifuged.IL-1α and IL-8 concentrations in the medium were determined by Enzyme-related immunosorbent assay (ELISA).At the same time,the cell number per well was measured by MTT,the ratio of the final cytokine concentration to the number of cells per well is the concentration of cytokines (IL-1α or IL-8) per unit cell.
1.2.4 ROS content assay
Human primary keratinocytes were seeded at a density of 3×103cells/well in 96-well plates.When the confluency reached 90%,the cells were treated with LPS of different concentrations for 1h,then was incubated with 10μmol/L DCFH-DA for 30 min,and the fluorescence intensity at 535nm (Ex:485 nm/Em:535 nm) was detected by microplate reader with fluorescence detection function[14].At the same time,the number of cells per well was measured by MTT,the final fluorescence intensity per well was divided by the number of cells to obtain the fluorescence intensity per unit cell.When testing samples,cells were treated with OSM2021041301 for 24 h prior to LPS treatment,other procedures as previously described.
1.2.5 Data Analysis
There were three multiple wells for each experimental condition.The data results were expressed as mean ± standard deviation (SD),and the significance analysis of the data was conducted by t test.“*” indicatesp<0.05;“**” indicatesp<0.01;“***” indicatesp<0.001.
2 Results and discussion
2.1 Effects of LPS on the viability of human primary keratinocytes
Human primary keratinocytes were treated with LPS in the 0.01~100 μg/mL concentration range,and there was no significant difference in cell viability between the NT group and the 0.01~1 μg/mL LPS treated group,meanwhile although there was statistical difference when LPS concentration was 10~100 μg/mL compared with NT group,the cell viability was greater than 80% (Figure 1).These results indicated that LPS had no significant effect on the viability of human primary keratinocytes in the concentration range of 0.01~100 μg/mL.
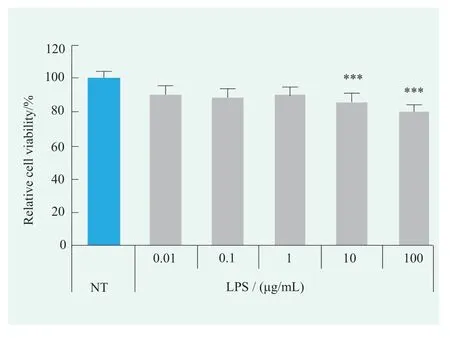
Figure 1. Effects of LPS on cell viability of human primary keratinocytes (Statistical analysis with NT)
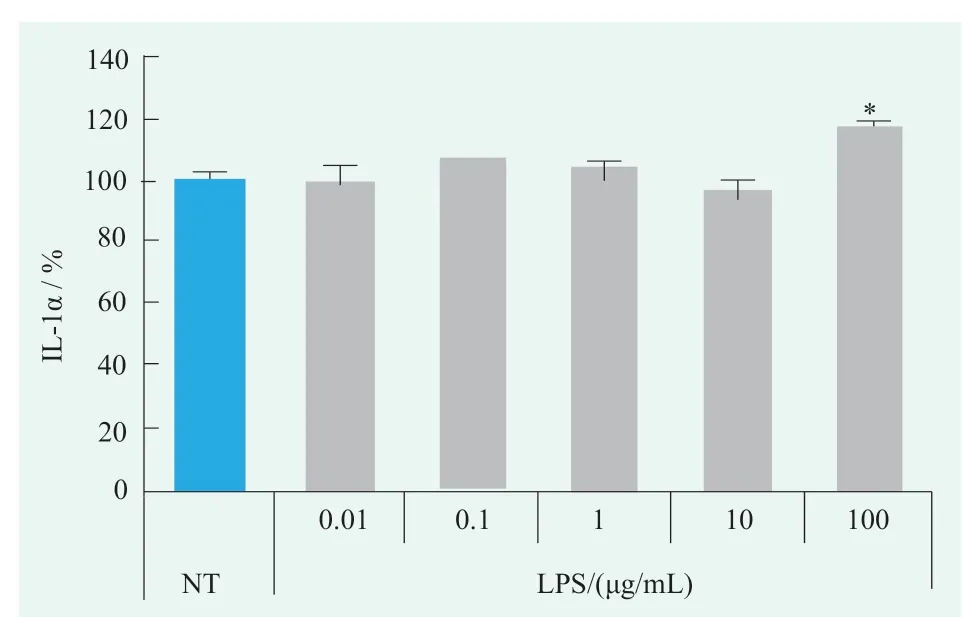
Figure.2 Effects of LPS on IL-1α levels in human primary keratinocytes (Statistical analysis with NT)
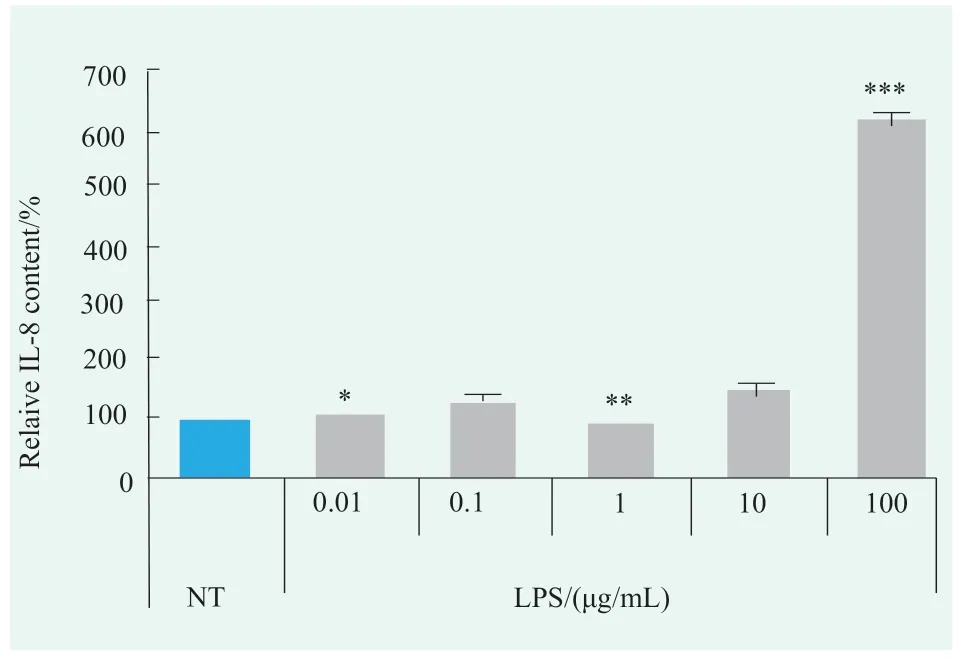
Figure.3 Effects of LPS on IL-8 levels in human primary keratinocytes (Statistical analysis with NT)
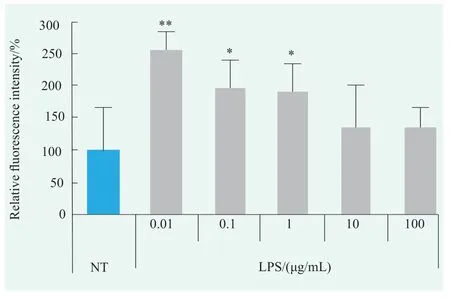
Figure.4 Effects of LPS on ROS levels in human primary keratinocytes (Statistical analysis with NT)

Figure.5 Effects of OSM2021041301 on LPS-induced IL-1α,IL-8 and ROS levels
2.2 Effects of LPS on levels of inflammatory cytokines in human primary keratinocytes
2.2.1 Effect of LPS on IL-1α level
contentThe effect of LPS on IL-1α levels in human primary keratinocytes was examined using the concentration range identified above,as shown in Figure 2.There was no significant change in IL-1α level after 0.01~10 μg/mL LPS treatment,but was significantly increased by 17% after 100 µg/mL LPS treatment compared with NT group,indicating that 100 μg/mL LPS can induce significant changes in the IL-1α level of human primary keratinocytes.
2.2.2 Effects of LPS on IL-8 level
Detection results showed that the IL-8 level in the human primary keratinocytes fluctuated slightly between 0.01~1 μg/mL LPS treatment groups,while increased 46% and 514% when treated with 10 μg/mL LPS and 100 μg/mL LPS compared with NT group,respectively(Figure 3).These results indicated that both 10 µg/mL and 100 µg/mL LPS could induce large amounts of IL-8 production in human primary keratinocytes,and the effect of 100 µg/mL LPS was more obvious.
2.3 Effects of LPS on ROS levels in human primary keratinocytes
ROS production in human primary keratinocytes treated with LPS was detected using DCFHDA (Figure 4).Compared with NT group,ROS levels were increased in 0.01~100 µg/mL LPS treatment groups,and the effect was more obvious at 0.01~1 µg/mL.ROS levels were most significantly increased at 0.01 µg/mL,which were 2.55 times higher than NT group.
2.4 Application of human primary keratinocyte inflammation model induced by LPS
Based on the above results,a differentiallyinduced inflammation model was established by selecting 100 μg/mL LPS to induce IL-1α,IL-8 and 0.01 μg/mL LPS to induce ROS in human primary keratinocytes.This inflammatory model has been attempt to test the efficacy of OSM2021041301.
Preliminary cytotoxicity assay data showed that 0.01%~0.5% OSM2021041301 had no cytotoxicity to human primary keratinocytes (Data not shown).Therefore,0.01%~0.5% OSM2021041301 were chosen to co-treat human primary keratinocytes with LPS of appropriate concentration,then the levels of IL-1α,IL-8 and ROS were measured.The results showed that the levels of IL-1α and IL-8 in the group co-treated with OSM2021041301 and LPS were lower than those treated with LPS alone.This indicated that OSM2021041301 could effectively inhibit the expresstion of IL-1α and IL-8 induced by LPS,and the inhibition rates both on IL-1α and IL-8 were about 50% (Figure 5a and Figure 5b).In addition,LPSinduced ROS content in human primary keratinocytes decreased after OSM2021041301 treatment,but there was no statistically significant difference compared with LPS alone treatment group.This suggests that OSM2021041301 at experimental concentrations may have an inhibitory effect on the ROS increase in human primary keratinocytes induced by LPS,but the effect is not significant (Figure 5c).
3 Conclusion
According to the experimental results of this paper,it’s more appropriate to use 100 µg/mL LPS to induce the expression of IL-1a and IL-8,and 0.01 µg/mL LPS to induce the expression of ROS when constructing the human primary keratinocyte inflammation model.The situation which the ability of low-dose LPS to up-regulate ROS levels in human primary keratinocyte is greater may be explained by the greater sensitivity of ROS to the earliest stages of inflammatory response.Attempts were made to screen the anti-inflammatory effects of OSM2021041301 using this inflammatory model,and the experimental results showed that 0.01%~0.5% OSM2021041301 has a better transient effect on the relief of inflammatory symptoms such as fever and tingling by inhibiting IL-1α and IL-8 levels,meanwhile,the reduction of IL-1α and IL-8 levels could attenuate the cascade amplification effect of the immune response.However,ROS levels in KC could not be adequately suppressed by 0.01%~0.5% OSM2021041301.
In conclusion,a model of skin inflammation based on human primary keratinocytes has been successfully established,and this model can successfully screen out cosmetic ingredients with anti-inflammatory effects on the LPS-induced inflammatory response.LPS induction with different concentrations for distinct inflammatory indicators and the introduction of human primary keratinocytes are two highlights of this model,which makes this model more suitable for skin inflammation research in the cosmetic industry compared with other traditional models.In addition,the characteristic indicators closely related to various inflammatory symptoms such as burning,redness,tingling and swelling are detected by enzyme linked immunosorbent assay,which makes the method simple to operate,low cost and relatively comprehensive anti-inflammatory targets.It can be used as a tool for rapid preliminary screen and filter out effective anti-inflammatory raw materials for cosmetic enterprises.
