大苞荆芥化学成分及其抗炎活性研究
2023-11-27赵鹏安力瓦衣丁买合苏提马国需孙照翠许旭东石磊岭高兴旺
赵鹏安,力瓦衣丁·买合苏提,马国需, ,孙照翠,许旭东,石磊岭*,高兴旺
大苞荆芥化学成分及其抗炎活性研究
赵鹏安1, 2,力瓦衣丁·买合苏提2,马国需2, 3,孙照翠3,许旭东3,石磊岭2*,高兴旺1*
1. 新疆大学,新疆 乌鲁木齐 830046 2. 新疆维吾尔自治区中药民族药研究所,新疆 乌鲁木齐 830002 3. 中国医学科学院北京协和医院药用植物研究所,北京 100193
阐明维药大苞荆芥中的活性物质并研究其抗炎活性。采用多种色谱方法对大苞荆芥化学成分进行系统的分离纯化,并利用波谱解析和理化性质对化合物的结构进行鉴定。同时采用MTT比色法,以氢化可的松作为阳性对照,对化合物进行体外抗炎活性实验。从大苞荆芥中分离鉴定了20个化合物(包括2个酚酸类化合物和18个二萜类化合物),分别为7-氧代去氢松香酸(1)、脱氢松香醇(2)、朗伯酸(3)、3β,7α-二羟基-松香-8,11,13-三烯(4)、雷酚萜L(5)、13-hydroxy-9-oxo-9,10-seco-abiet-8(14)-en-18,10α-olide(6)、8,11,13-阿松香三烯-7a,18-二醇(7)、3β,19-二羟基-松香-8,11,13-三烯(8)、7α,15,18-三羟基-松香-8,11,13-三烯(9)、松香烷-8,11,13-三烯-7β-醇(10)、6α-羟基脱氢松香酸(11)、15-羟基-松香-8,11,13-三烯-19-乙酸酯(12)、7a-羟基-松香-8,11,13-三烯-19-乙酸酯(13)、水杨酸(14)、反式-3-甲氧基-4-羟基肉桂酸(15)、15-羟基松香酸(16)、7β-羟基脱氢松香酸(17)、6-羟基脱氢松香酸(18)、角果酸G(19)、家地非诺酸K(20)。抗炎活性实验结果表明,化合物11对脂多糖(lipopolysaccharide,LPS)诱导的小鼠巨噬细胞系RAW264.7中NO释放有抑制作用,半数抑制浓度(median inhibition concentration,IC50)值为0.256 mg/mL。化合物5、6、11、13为首次从唇形科植物中分离得到;化合物1、3、4、7、8、10、12为首次从荆芥属植物中分离得到;化合物2、9、14、15为首次从大苞荆芥中分离得到;化合物11具有一定的抗炎活性,具有进一步深入研究的价值。
大苞荆芥;二萜类化合物;抗炎活性;雷酚萜L;6α-羟基脱氢松香酸;7a-羟基-松香-8,11,13-三烯-19-乙酸酯
炎症是具有血管系统的活体组织面对损伤性刺激,如感染、组织损伤等,所发生的复杂防御反应[1]。病毒和细菌是诱导炎症反应的主要介质,物理因素、化学因素以及创伤都会对这一反应产生影响[2-3]。发炎是身体对抗细菌和病毒所致感染,长时间或过度炎症在体内引发的一系列异常反应,引起各种疾病(如糖尿病、哮喘和疟疾、类风湿性关节炎),在严重的情况下甚至出现多器官功能障碍综合征,威胁人类健康[4]。因此,不断地研究安全有效的抗炎药是一项长期任务。Byun等[5]研究发现,荆芥的乙醇提取物能抑制脂多糖(lipopolysaccharide,LPS)诱导的细胞表面分子(CD80和CD86)的表达和促炎细胞因子,如肿瘤坏死因子α(tumor necrosis factor-α,TNF-α)、白细胞介素-1β (Interleukin-1β,IL-1β)、IL-6的产生。Choi等[6]研究发现,荆芥可降低特异性皮炎模型小鼠血清中的免疫球蛋白E(immunoglobulin E,IgE)、TNF-α、IL-6的含量。Zhang等[7]研究发现二萜类是荆芥属植物发挥临床抗炎作用的物质基础。Yang等[8]通过研究发现松香烷型二萜类化合物的是大苞荆芥在临床上发挥抗炎作用的物质基础,值得进一步研究。
大苞荆芥Benth.为唇形科(Labiatae)荆芥属Linn.植物,主要分布于巴基斯坦、尼泊尔、伊朗等国家[9-10],是我国一种主要依靠进口的民间习用植物药材,维吾尔名为“祖发”。据《维吾尔药志》记载,大苞荆芥全草含挥发油、黄酮类、多糖类、萜类、有机酸类等化学成分,具有镇咳平喘、清热利湿之功效,用于气管炎、咳嗽气喘、感冒发烧等症[11]。大苞荆芥常被作为神香草的替代品在民医中广泛用使用,因而对神香草的了解和研究较多,对大苞荆芥的研究较少,缺乏对大苞荆芥化学成分及其药用活性的系统研究。因此本研究选择大苞荆芥作为研究对象,应用薄层色谱、硅胶柱色谱、反相C18柱色谱以及半制备型HPLC分别对大苞荆芥95%乙醇提取液的二氯甲烷和石油醚萃取部位进行了分离纯化,针对大苞荆芥活性部位中的二萜类活性成分进行系统研究,通过核磁共振手段结合性状特征鉴定化合物结构,共鉴定20个化合物,包括18个二萜类化合物,分别为7-氧代去氢松香酸(7-oxodehydroabietic acid,1)、脱氢枞香醇(dehydroabietol,2)、朗伯酸(lambertic acid,3)、3β,7α-二羟基-松香-8,11,13-三烯(3β,7α-dihydroxy- abieta-8,11,13- triene,4)、雷酚萜L(triptobenzene L,5)、13-hydroxy-9-oxo-9,10--abiet-8(14)-en-18,10a- olide(6)、8,11,13-阿松香三烯-7a,18-二醇(8,11,13- abietatriene-7a,18-diol,7)、3β,19-二羟基-松香- 8,11,13-三烯(3β,19-dihydroxy-abieta-8,11,13-triene,8)、7α,15,18-三羟基-松香-8,11,13-三烯(7α,15, 18-trihydroxyabieta-8,11,13-triene,9)、松香烷- 8,11,13-三烯-7β-醇(abieta-8,11,13-trien-7β-ol,10)、6α-羟基脱氢松香酸(6α-hydroxycallitrisic acid,11)、15-羟基-松香-8,11,13-三烯-19-乙酸酯(19-acetoxy- 15-hydroxyabieta-8,11,13-triene,12)、7a-羟基-松香- 8,11,13-三烯-19-乙酸酯(7a-hydroxyabieta-8,11,13- trien-19-ylacetate,13)、水杨酸(salicylis acid,14)、反式-3-甲氧基-4-羟基肉桂酸(-3-hydroxy-4- methoxy-cinnamic acid,15)、15-羟基松香酸(15-hydroxyabieta-8,11,13-trien-18-oic acid,16)、7β-羟基脱氢松香酸(7β-hydroxycallitrisic acid,17)、6-羟基脱氢松香酸(6-hydroxycallitrisic acid,18)、角果酸G(angustanoic acid G,19)、家地非诺酸K(jiadifenoic acid K,20)。化合物5、6、11、13为首次从唇形科植物中分离得到;化合物1、3、4、7、8、10、12为首次从荆芥属植物中分离得到;化合物2、9、14、15为首次从大苞荆芥中分离得到。对分离得到的二萜类化合物进行了体外MTT法抗炎活性测试,其中化合物1、2、11、18对LPS诱导的RAW264.7巨噬细胞NO的释放均具有不同程度的抑制作用,其中化合物11的抑制作用相对较好,半数抑制浓度(median inhibition concentration,IC50)值为0.256 mg/mL。本研究为民间药大苞荆芥的临床应用提供质量保障和参考依据,也为大苞荆芥的二次开发和推广应用奠定科学基础。
1 仪器与材料
R-1005双回流旋转蒸发仪(方圆仪器科技有限公司);BrukerAvanceIII600型NMR(德国布鲁克公司);KH-700DB台式数控超声波清洗器(苏州安源仪器有限公司);BYLABUVIII紫外灯(诺雷信达科技有限公司);半制备型液相色谱仪(硅仪生化科技有限公司);乙醇、甲醇、石油醚、二氯甲烷(琳盛化工有限公司);柱色谱硅胶(创益化工有限公司);薄层色谱硅胶HF254(永德茂科技有限公司);Rp-C18(北京谱朋科技有限公司);纯净水(福万家纯净水有限公司);氢化可的松(批号B21001,上海源叶生物有限公司)。
药材于2020年购自于新疆维吾尔自治区麦迪森药业有限公司,经新疆维吾尔自治区药品检验研究院苏莱曼·哈力克主任药师鉴定为大苞荆芥Benth.的干燥全草,凭证药材标本(M20191025)保存于新疆中药民族药研究所药材室。
实验所用小鼠单核巨噬细胞RAW264.7购自中国医学科学院基础医学研究所基础医学中心。
2 方法
2.1 提取与分离
取6 kg大苞荆芥全草打粉,用95%乙醇加热回流提取3次,每次2 h。合并提取液,浓缩后得浸膏437 g。将浸膏用水分散后依次用石油醚和二氯甲烷萃取,萃取液浓缩后得石油醚部位134.8 g、二氯甲烷部位67.4 g。
石油醚部位经100~200目硅胶色谱分离,石油醚-醋酸乙酯(90∶1→10∶1)洗脱后得7个流分Fr. B1~B7。Fr. B6(12.0 g)依次用硅胶2次分离得4个流分Fr. B6-3a~B6-3d。Fr. B6-3a(3.0 g)用制备液相色谱(HPLC-5C18E,250 mm×10 mm,3 μm,90%甲醇,2.0 mL/min)分离得化合物1(3.6 mg)。Fr. B6-3b(2.1 g)用制备液相色谱(HPLC-5C18E,250 mm×10 mm,3 μm,85%甲醇,2.0 mL/min)分离得化合物2(3.6 mg)。Fr. B6-3d(1.2 g)用制备液相色谱(HPLC-5C18E,250 mm×10 mm,3 μm,85%甲醇,2.0 mL/min)分离得化合物3(2.5 mg)。Fr. B7(23.0 g)用硅胶柱得Fr. B7-1~B7-5共5个部位。Fr. B7-3(10.0 g)用硅胶柱洗脱,得4个流分Fr. B7-3a~B7-3d。Fr. B7-3a(2.8 g)用制备液相(HPLC-5C18E,250 mm×10 mm,3 μm,85%甲醇,2.0 mL/min)得化合物4(2.0 mg)、5(4.2 mg)。Fr. B7-3b(1.5 g)用制备液相色谱(HPLC-5C18E,250 mm×10 mm,3 μm,75%甲醇,2.0 mL/min)得到化合物6(2.0 mg)、7(2.8 mg)。Fr. B7-3c(1.6 g)用制备液相色谱分离(HPLC-5C18E,250 mm×10 mm,3 μm,80%甲醇,2.0 mL/min)得化合物8(2.5 mg)、9(3.3 mg)、10(3.7 mg)。Fr. B7-2(14.3 g)用硅胶柱得4个部位Fr. B7-2a~B7-2d。Fr. B7-2a(4.0 g)用制备液相色谱(HPLC-5C18E,250 mm×10 mm,3 μm,90%甲醇,2.0 mL/min)分离得化合物11(4.1 mg)、12(2.9 mg)、13(3.1 mg)。
二氯甲烷部位经100~200目硅胶柱色谱分离,二氯甲烷-甲醇(100∶1→2∶1)洗脱后得7个流分Fr. A1~A7,Fr. A1(15.0 g)通过硅胶柱再次分离得7个部位Fr. A1-1~A1-7。Fr. A1-2(2.1 g)用制备液相色谱(HPLC-5C18E,250 mm×10 mm,3 μm,60%甲醇,2.0 mL/min)分离,得化合物14(3.6 mg)。Fr. A1-4(3.6 g)用制备液相色谱(HPLC-5C18E,250 mm×10 mm,3 μm,40%甲醇,2.0 mL/min)分离得化合物15(3.0 mg)。Fr. A1~A3(12.0 g)用C18柱分离得4个流分(Fr. A1-3a~A1-3d)。Fr. A1-3b(1.4 g)用制备液相色谱(HPLC-5C18E,250 mm×10 mm,3 μm,75%甲醇,2.0 mL/min)分离,得化合物16(2.0 mg)、17(2.7 mg)。Fr. A1-3c(1.1 g)用制备液相色谱(HPLC-5C18E,250 mm×10 mm,3 μm,38%甲醇,2.0 mL/min)分离,得化合物18(2.6 mg)。Fr. A2(10.0 g)用硅胶柱分离得6个部分 Fr. A2-1~A2-6。Fr. A2-2(2.3 g)用制备液相色谱(HPLC-5C18E,250 mm×10 mm,3 μm,85%甲醇,2.0 mL/min)分离,得化合物19(2.3 mg)、20(3.6 mg)。
2.2 化合物抗炎活性测定
首先对巨噬细胞RAW264.7进行培养并制成单细胞悬液,将制好的单细胞悬液,以200 μL/孔接种于96孔板内,37 ℃培养,随后放入5% CO2培养箱贴壁24 h,各组加入磷酸缓冲液,使质量浓度为1 μg/mL,继续培养24 h后离心,取上清液放于96孔板中备用;氢化可的松用含10% FBS的DMEM培养液稀释,样品在96孔板中以50 μL/孔加入。之后室温下在每孔加50 μL的Griess Reagent I和II,混匀,室温避光放置10 min,用酶标仪540 nm测定吸光度()值,计算NO含量。
NO含量=1-(给药-对照)/对照
3 结果
3.1 结构鉴定
化合物1:白色无定形粉末。ESI-MS/: 337 [M+Na]+,13C-NMR、APT谱确定分子式C20H26O3。1H-NMR (600 MHz, CDCl3): 7.90 (1H, d,= 3.6 Hz, H-14), 7.43 (1H, dd,= 1.8, 2.4 Hz, H-12), 7.34 (1H, d,= 8.4 Hz, H-11), 2.30 (1H, d,= 13.2 Hz, H-5), 2.19 (1H, m, H-2b), 2.11 (1H, m, H-3b), 2.10 (1H, m, H-1b), 1.73 (1H, m, H-6b), 1.66 (1H, m, H-3a), 1.55 (1H, m, H-6a), 1.46 (1H, m, H-2a), 1.43 (1H, m, H-1a), 1.32 (3H, s, H-16), 1.32 (3H, s, H-17), 1.21 (3H, s, H-19), 1.17 (1H, d,= 7.0 Hz, H-15), 0.96 (3H, d,= 7.8 Hz, H-20);13C-NMR (150 MHz, CDCl3): 199.3 (C-7), 180.6 (C-18), 152.1 (C-9), 147.1 (C-13), 132.8 (C-14), 130.6 (C-8), 129.0 (C-12), 125.0 (C-11), 50.3 (C-5), 43.8 (C-4), 38.6 (C-1), 37.7 (C-10), 37.5 (C-3), 33.8 (C-15), 30.7 (C-6), 28.3 (C-20), 24.0 (C-17), 19.8 (C-2), 13.9 (C-19)。经数据比对[12],确定化合物1为7-氧代去脱氢松香酸。
化合物2:白色无定形粉末。ESI-MS/: 309 [M+Na]+,13C-NMR、APT谱确定分子式C20H30O。1H-NMR (600 MHz, CDCl3): 7.18 (1H, dd,= 7.8, 4.2 Hz, H-11), 7.05 (1H, d,= 7.2 Hz, H-12), 6.93 (1H, s, H-14), 3.72 (1H, d,= 11.4 Hz, H-18b), 3.49 (1H, d,= 11.4 Hz, H-18a), 2.31 (1H, m, H-1b), 2.44 (1H, m, H-5), 2.22 (1H, m, H-3b), 2.01 (1H, m, H-6b), 1.93 (1H, m, H-7b), 1.87 (1H, m, H-2b), 1.60 (1H, m, H-3a), 1.54 (1H, m, H-2a), 1.51 (1H, m, H-6a), 1.40 (1H, m, H-1a), 1.39 (6H, s, H-16, 17), 1.21 (1H, d,= 7.2 Hz, 1.19 (1H, m, H-7a), 1.19 (3H, d,= 6.8 Hz, H-20), 1.12 (3H, s, H-19);13C-NMR (150 MHz, CDCl3): 146.5 (C-9), 144.7 (C-13), 134.9 (C-8), 129.0 (C-14), 127.2 (C-12), 124.8 (C-11), 67.2 (C-18), 51.9 (C-4), 43.8 (C-5), 37.3 (C-1), 37.3 (C-10), 36.0 (C-3), 33.7 (C-15), 33.6 (C-20), 30.1 (C-7), 30.0 (C-6), 24.8 (C-17), 24.1 (C-16), 20.0 (C-2), 16.8 (C-19)。经数据比对[13],确定化合物2为脱氢松香醇。
化合物3:白色无定形粉末。ESI-MS/: 349 [M+Na]+,13C-NMR、APT谱确定分子式C20H28O3。1H-NMR (600 MHz, CDCl3): 6.83 (1H, s, H-14), 6.63 (1H, s, H-11), 3.21 (1H, m, H-15), 2.81 (1H, m, H-7b), 2.67 (1H, m, H-7a), 2.43 (1H, m, H-2b), 2.13 (1H, d,= 12.0 Hz, H-5), 2.19 (1H, m, H-1b), 2.11 (1H, m, H-3b), 1.68 (1H, m, H-6b), 1.65 (1H, m, H-2a), 1.59 (1H, m, H-3a), 1.43 (1H, m, H-6a), 1.35 (1H, m, H-1a), 1.32 (6H, s, H-16, 17), 1.19 (3H, d,= 7.2 Hz, H-18), 1.10 (3H, s, H-20);13C-NMR (150 MHz, CDCl3): 183.5 (C-19), 151.0 (C-12), 132.0 (C-13), 127.6 (C-8), 126.8 (C-14), 126.8 (C-9), 112.1 (C-11), 52.9 (C-5), 44.0 (C-4), 39.5 (C-10), 38.4 (C-1), 37.5 (C-3), 31.4 (C-7), 29.8 (C-6), 28.9 (C-15), 26.9 (C-18), 23.3 (C-20), 22.9 (C-17), 22.7 (C-16), 19.6 (C-2)。经数据比对[14],确定化合物3为朗伯酸。
化合物4:白色无定形粉末。ESI-MS/: 323 [M+Na]+,13C-NMR、APT谱确定分子C20H30O2。1H-NMR (600 MHz, CDCl3): 7.13 (1H, s, H-11), 7.12 (1H, s, H-14), 7.08 (1H, s, H-12), 4.31 (1H, m, H-7), 3.37 (1H, m, H-3), 2.89 (1H, m, H-15), 1.82 (1H, m, H-1b), 1.79 (1H, m, H-6b), 1.72 (1H, m, H-2b), 1.61 (1H, m, H-5), 1.57 (1H, m, H-1a), 1.48 (1H, m, H-6a), 1.47 (1H, m, H-2a), 1.23 (3H, d,= 7.2 Hz, H-20), 1.13 (3H, s, H-16), 1.10 (3H, s, H-17), 0.94 (6H, s, H-18, 19);13C-NMR (150 MHz, CDCl3): 150.0 (C-13), 146.9 (C-9), 136.1 (C-8), 126.9 (C-14), 126.0 (C-11), 124.7 (C-12), 78.9 (C-3), 69.7 (C-7), 44.3 (C-5), 38.6 (C-10), 37.9 (C-4), 36.8 (C-1), 33.7 (C-15), 30.9 (C-6), 28.5 (C-20), 28.2 (C-2), 24.2 (C-16), 24.0 (C-17), 15.7 (C-18), 15.7 (C-19)。经数据比对[15],确定化合4为3β,7α-二羟基-松香-8,11,13-三烯。
化合物5:白色无定形粉末。ESI-MS/: 325 [M+Na]+,13C-NMR、APT谱确定分子式C20H30O2。1H-NMR (600 MHz, CDCl3): 7.13 (1H, d,= 7.2 Hz, H-11), 7.00 (1H, d,= 7.2 Hz, H-12), 6.89 (1H, s, H-14), 4.33 (1H, d,= 11.2 Hz, H-19b), 3.53 (1H, dd,= 11.7, 4.4 Hz, H-3), 3.43 (1H, d,= 11.2 Hz, H-19a), 2.92 (1H, m, H-7b), 2.83 (1H, m, H-7a), 2.79 (1H, d,= 8.2 Hz, H-15), 2.34 (1H, ddd,= 13.3, 3.4, 3.4 Hz, H-1b), 2.01 (1H, m, H-2b), 1.97 (1H, m, H-6b), 1.91 (1H, m, H-2a), 1.66 (1H, m, H-6a), 1.54 (1H, ddd,= 13.3, 13.3, 3.5 Hz, H-1a), 1.46 (1H, dd,= 12.3, 1.2 Hz, H-5), 1.32 (3H, s, H-18), 1.22 (6H, d,= 6.9 Hz, H-16, 17), 1.15 (3H, s, H-20);13C-NMR (150 MHz, CDCl3): 146.3 (C-9), 146.0 (C-13), 134.4 (C-8), 126.8 (C-14), 124.5 (C-11), 124.1 (C-12), 80.7 (C-3), 64.2 (C-19), 50.7 (C-5), 43.0 (C-4), 37.2 (C-10), 36.8 (C-1), 33.5 (C-15), 31.1 (C-7), 28.6 (C-2), 26.0 (C-20), 24.0 (C-16), 24.0 (C-17), 22.5 (C-18), 19.0 (C-6)。经数据比对[16],确定化合物5为雷酚萜L。
化合物6:白色无定形粉末。ESI-MS/: 357 [M+Na]+,13C-NMR、APT谱确定分子式C20H30O4。1H-NMR (600 MHz, CDCl3): 6.50 (1H, d,= 0.9 Hz, H-14), 2.69 (1H, ddd,= 17.2, 10.3, 5.3 Hz, H-11b), 2.44 (1H, ddd,= 17.2, 6.4, 4.8 Hz, H-11a), 2.28 (1H, dddd,= 13.5, 10.8, 5.9, 0.9 Hz, H-7b), 2.20 (1H, dddd,= 13.5, 10.8, 5.0, 0.9 Hz, H-7a), 2.14 (1H, ddd,= 13.5, 6.4, 5.3 Hz, H-12b), 1.96 (1H, ddd,= 13.5, 10.3, 4.8 Hz, H-12a), 1.92 (1H, d,= 6.9 Hz, H-15), 1.80 (1H, t,= 6.1 Hz, H -5), 1.70 (1H, m, H-1b), 1.68 (1H, m, H-2b), 1.53 (1H, m, H-2a), 1.52 (1H, m, H-3b), 1.51 (1H, m, H-6b), 1.46 (1H, m, H-6a), 1.45 (3H, s, H-20) 1.39 (1H, m, H-3a), 1.29 (1H, m, H-1a), 1.26 (3H, s, H-19), 1.01 (3H, d,= 7.2 Hz, H-16), 0.96 (3H, d,= 7.4 Hz, H-17);13C-NMR (150 MHz, CDCl3): 199.1 (C-9), 180.4 (C-18), 28.4 (C-1), 148.1 (C-14), 139.4 (C-8), 85.0 (C-10), 72.3 (C-13), 55.2 (C-5), 48.5 (C-4), 37.0 (C-15), 34.1 (C-11), 30.8 (C-12), 29.7 (C-7), 25.4 (C-3), 23.7 (C-6), 22.2 (C-20), 19.7 (C-2), 19.7 (C-19), 17.5 (C-17), 16.4 (C-16)。经数据比对[17],确定化合物6为13-hydroxy-9-oxo-9,10--abiet-8 (14)-en-18,10a-olide。
化合物7:白色无定形粉末。ESI-MS/: 325 [M+Na]+,13C-NMR、APT谱确定分子式C20H30O2。1H-NMR (600 MHz, CDCl3): 7.22 (1H, d,= 7.2 Hz, H-11), 7.16 (1H, s, H-12), 7.14 (1H, d,= 8.2 Hz, H-14), 3.87 (1H, d,= 11.4 Hz, H-19b), 3.55 (1H, d,= 10.8 Hz, H-19a), 3.21 (1H, m, H-7), 2.88 (1H, m, H-15), 2.61 (1H, m, H-3b), 2.35 (1H, m, H-3a), 2.22 (1H, m, H-1b), 2.07 (1H, m, H-2b), 1.99 (1H, m, H-6b), 1.92 (1H, m, H-2a), 1.71 (1H, m, H-1a), 1.61 (1H, m, H-6a), 1.30 (1H, m, H-5), 1.24 (3H, s, H-18), 1.23 (3H, s, H-16), 1.13 (3H, s, H-17), 1.08 (3H, s, H-20);13C-NMR (150 MHz, CDCl3): 147.1 (C-13), 146.5 (C-9), 135.7 (C-8), 127.7 (C-14), 126.7 (C-11), 124.6 (C-12), 68.3 (C-7), 65.5 (C-19), 45.1 (C-5), 38.5 (C-4), 37.8 (C-10), 35.2 (C-1), 33.5 (C-15), 29.7 (C-3), 28.6 (C-2), 26.7 (C-20), 24.7 (C-18), 24.1 (C-16), 23.8 (C-17), 19.0 (C-6)。经数据比对[18],确定化合物7为8,11,13-阿松香三烯-7α,18-二醇。
化合物8:白色无定形粉末。ESI-MS/: 325 [M+Na]+,13C-NMR、APT谱确定分子式C20H30O2。1H-NMR (600 MHz, CDCl3): 7.19 (1H, d,= 6.2 Hz, H-11), 7.17 (1H, s, H-12), 7.09 (1H, s, H-14), 4.44 (1H, d,= 11.4 Hz, H-19b), 4.22 (1H, d,= 11.8 Hz, H-19a), 3.36 (1H, dd,= 4.2, 4.8 Hz, H-3), 2.95 (1H, m, H-7b), 2.87 (1H, m, H-15), 2.37 (1H, m, H-1b), 2.28 (1H, m, H-7a), 2.23 (1H, m, H-2b), 2.09 (1H, m, H-6b), 1.76 (1H, m, H-2a), 1.63 (1H, m, H-6a), 1.62 (1H, m, H-1a), 1.56 (3H, s, H-18), 1.49 (1H, m, H-5), 1.25 (3H, s, H-16), 1.23 (3H, s, H-17), 1.20 (3H, s, H-20);13C-NMR (150 MHz, CDCl3): 147.0 (C-9), 146.3 (C-13), 134.5 (C-14), 134.4 (C-8), 124.8 (C-11), 122.3 (C-12), 78.9 (C-3), 65.3 (C-19), 51.1 (C-5), 42.3 (C-4), 37.2 (C-10), 35.4 (C-1), 34.5 (C-15), 31.6 (C-7), 28.0 (C-2), 25.4 (C-20), 22.5 (C-16), 22.5 (C-17), 19.6 (C-6), 19.5 (C-18)。经数据比对[16],确定化合物8为3β,19-二羟基-松香-8,11,13-三烯。
化合物9:白色无定形粉末。ESI-MS/: 341 [M+Na]+,13C-NMR、APT谱确定分子式C20H30O3。1H-NMR (600 MHz, CDCl3): 7.32 (1H, s, H-11), 6.89 (1H, s, H-12), 6.78 (1H, s, H-14), 4.43 (1H, m, H-19b), 4.18 (1H, m, H-19a), 3.23 (1H, m, H-7), 2.32 (1H, m, H-1b), 2.28 (1H, m, H-3b), 2.17 (1H, m, H-6b), 1.99 (1H, m, H-2b), 1.92 (1H, m, H-3a), 1.82 (1H, m, H-2a), 1.81 (1H, m, H-6a), 1.70 (1H, m, H-5),1.59 (1H, m, H-1a), 1.53 (3H, s, H-18), 1.36 (3H, s, H-16), 1.28 (3H, s, H-17), 1.06 (3H, s, H-20);13C-NMR (150 MHz, CDCl3): 146.8 (C-9), 144.8 (C-13), 135.7 (C-8), 127.3 (C-14), 126.1 (C-11), 125.6 (C-12), 68.7 (C-7), 66.3 (C-19), 58.3 (C-15), 45.7 (C-5), 43.6 (C-4), 38.9 (C-1), 37.5 (C-3), 37.5 (C-10), 28.6 (C-2), 28.6 (C-20), 24.2 (C-16), 24.0 (C-17), 20.0 (C-6), 16.1 (C-18)。经数据比对[19],确定化合物9为7α,15,18-三羟基-松香-8,11,13-三烯。
化合物10:白色无定形粉末。ESI-MS/: 309 [M+Na]+,13C-NMR、APT谱确定分子式C20H30O。1H-NMR (600 MHz, CDCl3): 7.44 (1H, s, H-11), 7.14 (1H, d,= 7.2 Hz, H-12), 7.08 (1H, d,= 6.8 Hz, H-14), 2.87 (1H, s, H-15), 1.61 (1H, m, H-1a), 2.62 (1H, m, H-3b), 2.57 (1H, m, H-7), 2.56 (1H, m, H-1b), 2.36 (1H, m, H-6b), 2.33 (1H, m, H-3a), 2.03 (1H, m, H-2b), 1.99 (1H, m, H-5), 1.97 (1H, m, H-2a), 1.48 (1H, m, H-6a), 1.32 (3H, s, H-18), 1.24 (3H, s, H-16), 1.23 (3H, s, H-17), 1.21 (3H, s, H-20), 1.16 (3H, s, H-19);13C-NMR (150 MHz, CDCl3): 146.7 (C-9), 145.3 (C-13), 138.3 (C-8), 126.0 (C-14), 125.4 (C-11), 124.6 (C-12), 71.7 (C-7), 50.2 (C-5), 43.6 (C-4), 39.1 (C-10), 37.4 (C-1), 33.9 (C-15), 32.1 (C-3), 29.9 (C-2), 28.8 (C-20), 24.2 (C-16), 24.0 (C-17), 23.3 (C-18), 19.9 (C-6), 14.3 (C-19)。经数据比对[20],确定化合物10为松香烷-8,11,13-三烯-7β-醇。
化合物11:白色无定形粉末。ESI-MS/: 339 [M+Na]+,13C-NMR、APT谱确定分子式C20H28O3。1H-NMR (600 MHz, CDCl3): 7.18 (1H, d,= 7.4 Hz, H-11), 7.00 (1H, d,= 7.8 Hz, H-12), 6.91 (1H, s, H-14), 1.54 (1H, m, H-1a), 2.81 (1H, m, H-15), 2.76 (1H, m, H-3b), 2.61 (1H, dd,= 9.0, 10.2 Hz, H-6), 2.36 (1H, m, H-7b), 2.33 (1H, m, H-3a), 2.19 (1H, m, H-5), 2.19 (1H, m, H-1b), 2.03 (1H, m, H-2b), 1.99 (1H, m, H-2a), 1.67 (1H, m, H-7a), 1.37 (3H, s, H-18), 1.23 (3H, s, H-17), 1.22 (3H, s, H-16), 1.10 (3H, s, H-19);13C-NMR (150 MHz, CDCl3): 182.6 (C-20), 146.2 (C-9), 144.6 (C-13), 134.8 (C-8), 127.1 (C-14), 125.3 (C-11), 124.4 (C-12), 65.0 (C-6), 52.2 (C-5), 44.9 (C-4), 39.6 (C-10), 33.6 (C-15), 32.0 (C-3), 31.9 (C-1), 29.7 (C-2), 24.3 (C-16), 24.1 (C-17), 24.1 (C-18), 20.7 (C-7), 14.3 (C-19)。经数据比对[19],确定化合物11为6α-羟基脱氢松香酸。
化合物12:白色无定形粉末。ESI-MS/: 367 [M+Na]+,13C-NMR、APT谱确定分子式C22H32O3。1H-NMR (600 MHz, CDCl3): 7.39 (1H, s, H-12), 7.19 (1H, s, H-11), 7.16 (1H, s, H-14), 4.33 (1H, d,= 11.2 Hz, H-20b), 4.01 (1H, d,= 11.4 Hz, H-20a), 2.90 (1H, m, H-7b), 2.34 (1H, m, H-7a), 2.07 (3H, s, H-22), 1.82 (1H, m, H-1b), 1.72 (1H, m, H-6b), 1.57 (1H, m, H-1a), 1.56 (1H, m, H-3b), 1.55 (1H, m, H-5), 1.52 (6H, s, H-16/17), 1.43 (1H, m, H-2b), 1.43 (1H, m, H-6a), 1.21 (1H, m, H-3a), 1.21 (3H, s, H-18), 1.19 (1H, m, H-2a), 1.04 (3H, s, H-19);13C-NMR (150 MHz, CDCl3): 171.5 (C-21), 148.0 (C-9), 146.0 (C-13), 134.7 (C-8), 124.9 (C-14), 124.6 (C-11), 122.1 (C-12), 73.5 (C-20), 67.0 (C-15), 51.2 (C-5), 38.7 (C-4), 37.2 (C-10), 36.0 (C-1), 31.6 (C-16), 31.6 (C-17), 31.2 (C-3), 30.4 (C-7), 29.4 (C-2), 27.4 (C-18), 25.7 (C-19), 21.1 (C-22), 19.3 (C-6)。经数据比对[21],确定化合物12为15-羟基-松香-8,11,13-三烯-19-乙酸酯。
化合物13:白色无定形粉末。ESI-MS/: 367 [M+Na]+,13C-NMR、APT谱确定分子式C22H32O3。1H-NMR (600 MHz, CDCl3): 7.26 (1H, s, H-12), 7.20 (1H, d,= 7.4 Hz, H-11), 7.13 (1H, d,= 2.4 Hz, H-14), 4.28 (1H, d,= 8.6 Hz, H-20b), 3.98 (1H, d,= 12.2 Hz, H-20a), 2.93 (1H, m, H-7), 2.86 (1H, m, H-15), 1.99 (3H, s, H-22), 1.82 (1H, m, H-1b), 1.72 (1H, m, H-6b), 1.67 (1H, m, H-2b), 1.60 (1H, m, H-3b), 1.54 (1H, m, H-1a), 1.54 (1H, m, H-5), 1.41 (1H, m, H-6a), 1.29 (1H, m, H-2a), 1.25 (3H, s, H-16), 1.24 (3H, s, H-17), 1.15 (1H, m, H-3a), 1.15 (3H, s, H-18), 1.07 (3H, s, H-19);13C-NMR (150 MHz, CDCl3): 171.6 (C-21), 147.1 (C-9), 146.89 (C-13), 135.9 (C-8), 36.2 (C-1), 128.0 (C-14), 126.9 (C-11), 124.9 (C-12), 79.2 (C-7), 69.9 (C-20), 45.3 (C-5), 38.7 (C-10), 38.7 (C-4), 33.8 (C-15), 32.7 (C-3), 28.7 (C-2), 27.3 (C-16), 24.9 (C-17), 24.2 (C-18), 24.1 (C-19), 21.2 (C-22), 19.1 (C-6)。经数据比对[22],确定化合物13为7a-羟基-松香-8,11,13-三烯-19-乙酸酯。
化合物14:淡黄色油状物。ESI-MS/: 161 [M+Na]+,13C-NMR、APT谱确定分子式C7H6O3。1H-NMR (600 MHz, CDCl3): 7.93 (1H, dd,= 8.0, 1.4 Hz, H-6), 7.52 (1H, dd,= 7.8, 1.6 Hz, H-3), 7.02 (1H, brd,= 8.0 Hz, H-4), 6.94 (1H, t,= 7.4 Hz, H-5), 10.40 (1H, s, OH);13C-NMR (150 MHz, CDCl3): 175.0 (1-COOH), 162.3 (C-2), 137.0 (C-4), 131.1 (C-6), 119.7 (C-5), 111.8 (C-1), 118.0 (C-3)。经数据比对[23],确定化合物14为水杨酸。
化合物15:淡黄色固体。ESI-MS/: 217 [M+Na]+,13C-NMR、APT谱确定分子式C10H10O4。1H-NMR (600 MHz, CDCl3): 7.69 (1H, d,= 16.2 Hz, H-7), 7.11 (1H, d,= 1.8 Hz, H-6), 6.99 (1H, dd,= 7.4, 1.2 Hz, H-2), 6.94 (1H, d,= 8.4 Hz, H-3), 6.31 (1H, d,= 15.6 Hz, H-8), 3.94 (3H, s, 5-OCH3);13C-NMR (150 MHz, CDCl3): 172.0 (C-9), 143.1 (C-7), 139.9 (C-5), 138.6 (C-4), 126.8 (C-1), 126.3 (C-2), 114.1 (C-3), 114.0 (C-6), 109.5 (C-8), 56.1 (5-OCH3)。经数据比对[24],确定化合物15为反式-3-甲氧基-4-羟基肉桂酸。
化合物16:白色无定形粉末。ESI-MS/: 339 [M+Na]+,13C-NMR、APT谱确定分子式C20H28O3。1H-NMR (600 MHz, CDCl3): 7.22 (2H, s, H-11, 12), 7.16 (1H, s, H-14), 2.91 (1H, dd,= 16.0, 5.6 Hz, H-7b), 2.56 (1H, m, H-7a), 2.38 (1H, m, H-3b), 2.21 (1H, m, H-6b), 2.12 (1H, m, H-2b), 2.11 (1H, m, H-6a), 2.07 (1H, m, H-1b), 1.59 (1H, d,= 10.2 Hz, H-5), 1.58 (1H, m, H-2a), 1.56 (6H, s, H-16, 17), 1.33 (3H, s, H-18), 1.24 (1H, m, H-1a), 1.23 (1H, m, H-3a), 1.12 (3H, s, H-20);13C-NMR (150 MHz, CDCl3): 183.2 (C-19), 146.6 (C-9), 146.1 (C-13), 135.3 (C-8), 125.7 (C-11), 125.0 (C-14), 122.3 (C-12), 72.5 (C-15), 52.9 (C-5), 43.9 (C-4), 38.8 (C-1), 38.5 (C-10), 34.8 (C-3), 32.3 (C-7), 31.79 (C-16), 31.67 (C-17), 28.9 (C-18), 23.3 (C-20), 21.1 (C-6), 19.9 (C-2)。经数据比对[20],确定化合物16为15-羟基松香酸。
化合物17:白色无定形粉末。ESI-MS/: 339 [M+Na]+,13C-NMR、APT谱确定分子式C20H28O3。1H-NMR (600 MHz, CDCl3): 7.19 (1H, d,= 7.2 Hz, H-11), 7.15 (1H, d,= 1.8 Hz, H-12), 7.13 (1H, d,= 1.8 Hz, H-14), 4.79 (1H, d,= 4.8 Hz, H-7), 2.33 (1H, m, H-1b), 2.29 (1H, d,= 7.2 Hz, H-5), 2.09 (1H, m, H-2b), 2.09 (1H, m, H-6b), 2.00 (1H, m, H-3b), 1.47 (1H, m, H-3a), 1.44 (1H, m, H-2a), 1.43 (1H, m, H-1a), 1.36 (1H, m, H-6a), 1.35 (6H, s, H-16, 17), 1.23 (1H, d,= 7.2 Hz, H-15), 1.10 (3H, s, H-19), 1.04 (3H, s, H-20);13C-NMR (150 MHz, CDCl3): 183.3 (C-18), 146.9 (C-9), 145.8 (C-13), 136.0 (C-8), 128.0 (C-14), 127.0 (C-12), 125.7 (C-11), 68.7 (C-7), 45.8 (C-5), 43.7 (C-4), 38.6 (C-1), 37.5 (C-3), 37.5 (C-10), 33.7 (C-15), 29.8 (C-6), 28.7 (C-20), 24.2 (C-17), 24.0 (C-16), 22.2 (C-19), 20.0 (C-2)。经数据比对[25-26],确定化合物17为7β-羟基脱氢松香酸。
化合物18:白色无定形粉末。ESI-MS/: 339 [M+Na]+,13C-NMR、APT谱确定分子式C20H28O3。1H-NMR (600 MHz, CDCl3): 7.44 (1H, d,= 5.2 Hz, H-14), 7.17 (1H, d,= 7.8 Hz, H-11), 7.10 (1H, d,= 1.8 Hz, H-12), 4.75 (1H, dd,= 6.6, 6.0 Hz, H-6), 2.54 (1H, d,= 6.6 Hz, H-5), 2.33 (1H, m, H-1b), 2.18 (1H, m, H-7b), 2.03 (1H, m, H-2b), 1.98 (1H, m, H-3b), 1.96 (1H, m, H-2a), 1.59 (1H, d,= 12.2 Hz, H-3a), 1.59 (1H, d,= 12.6 Hz, H-7a), 1.33 (6H, s, H-16, 17), 1.25 (1H, m, H-1a), 1.22 (1H, m, H-15), 1.16 (3H, s, H-19), 0.60 (3H, s, H-20)。13C-NMR (150 MHz, CDCl3): 182.8 (C-18), 146.7 (C-9), 145.2 (C-13), 138.2 (C-8), 126.0 (C-14), 125.4 (C-12), 124.6 (C-11), 71.7 (C-6), 50.2 (C-5), 43.6 (C-4), 39.1 (C-10), 39.0 (C-1), 37.1 (C-3), 33.8 (C-15), 32.1 (C-7), 24.2 (C-17), 24.1 (C-16), 23.3 (C-20), 20.0 (C-2), 14.6 (C-19)。经数据比对[25-26],确定化合物18为6-羟基脱氢松香酸。
化合物19:白色无定形粉末。ESI-MS/: 323 [M+Na]+,13C-NMR、APT谱确定分子式C19H24O3。1H-NMR (600 MHz, CDCl3): 7.65 (1H, d,= 8.2 Hz, H-12), 7.65 (1H, s, H-14), 7.24 (1H, d,= 5.4 Hz, H-11), 2.75 (1H, m, H-6b), 2.63 (1H, m, H-7b), 2.57 (3H, s, H-17), 2.47 (1H, m, H-7a), 2.44 (1H, m, H-1b), 2.18 (1H, m, H-2b), 2.07 (1H, m, H-3b), 1.98 (1H, m, H-6a), 1.54 (1H, d,= 11.6 Hz, H-5), 1.40 (1H, m, H-2a), 1.37 (3H, s, H-18), 1.37 (1H, m, H-1a), 1.17 (1H, m, H-3a), 1.01 (3H, s, H-20);13C-NMR (150 MHz, CDCl3): 198.4 (C-15), 183.0 (C-19), 153.8 (C-9), 136.0 (C-13), 134.6 (C-8), 129.0 (C-14), 126.1 (C-11), 126.0 (C-12), 52.6 (C-5), 44.0 (C-4), 39.3 (C-10), 38.5 (C-1), 36.9 (C-3), 32.1 (C-7), 29.9 (C-18), 24.2 (C-17), 22.1 (C-20), 20.8 (C-6), 20.3 (C-2)。经数据比对[12],确定化合物19为角果酸G。
化合物20:白色无定形粉末。ESI-MS/: 339 [M+Na]+,13C-NMR、APT谱确定分子式C20H28O3。1H-NMR (600 MHz, CDCl3): 6.92 (1H, s, H-14), 6.91 (1H, d,= 7.6 Hz, H-12), 6.70 (1H, d,= 6.4 Hz, H-11), 2.88 (1H, m, H-7b), 2.82 (1H, m, H-15), 2.01 (1H, m, H-1b), 1.81 (1H, m, H-3b), 1.76 (1H, m, H-6b), 1.74 (1H, m, H-2b), 1.73 (1H, m, H-7a), 1.50 (3H, s, H-20), 1.49 (1H, m, H-3a), 1.44 (1H, m, H-2a), 1.43 (1H, m, H-1a), 1.34 (1H, m, H-6a), 1.26 (3H, s, H-19), 1.19 (6H, d,= 7.2 Hz, H-16, 17);13C-NMR (150 MHz, CDCl3): 180.8 (C-18), 151.5 (C-9), 141.5 (C-13), 128.1 (C-8), 128.1 (C-14), 125.1 (C-12), 115.2 (C-11), 85.3 (C-10), 55.4 (C-5), 48.8 (C-4), 36.8 (C-1), 36.6 (C-3), 33.5 (C-15), 29.2 (C-7), 26.5 (C-6), 24.4 (C-16, 17), 22.4 (C-20), 20.0 (C-2), 18.4 (C-19)。经数据比对[7],确定化合物20为家地非诺酸 K。
3.2 化合物抗炎活性评价
由表1可知,部分二萜类化合物对RAW 264.7巨噬细胞具有抑制作用。以氢化可地松为阳性对照,化合物1、2、11和18具有微弱的抗炎活性,其中化合物11的抗炎活性相对较好,IC50值为0.256 mg/mL。具有抗炎活性的化合物1、2、11、18均为松香烷二萜结构,且六元环上的取代基都有1个羟基或1个羧基、或二者皆有,根据IC50值比较,这4个化合物的活性由强到弱依次为化合物11、18、2、1;根据结构进行比较,化合物11和18六元环上的取代基都有1个羟基和1个羧基,化合物2六元环上的取代基有1个羟基,化合物1六元环的取代基是1个羧基。由此推断,此类化合物六元环上的羧基或羟基可能是使化合物具有抗炎活性关键基团,并且羟基和羧基同时存在化合物的抗炎活性更好,取代基为羟基的化合物抗炎活性要优于取代基为羧基的化合物。
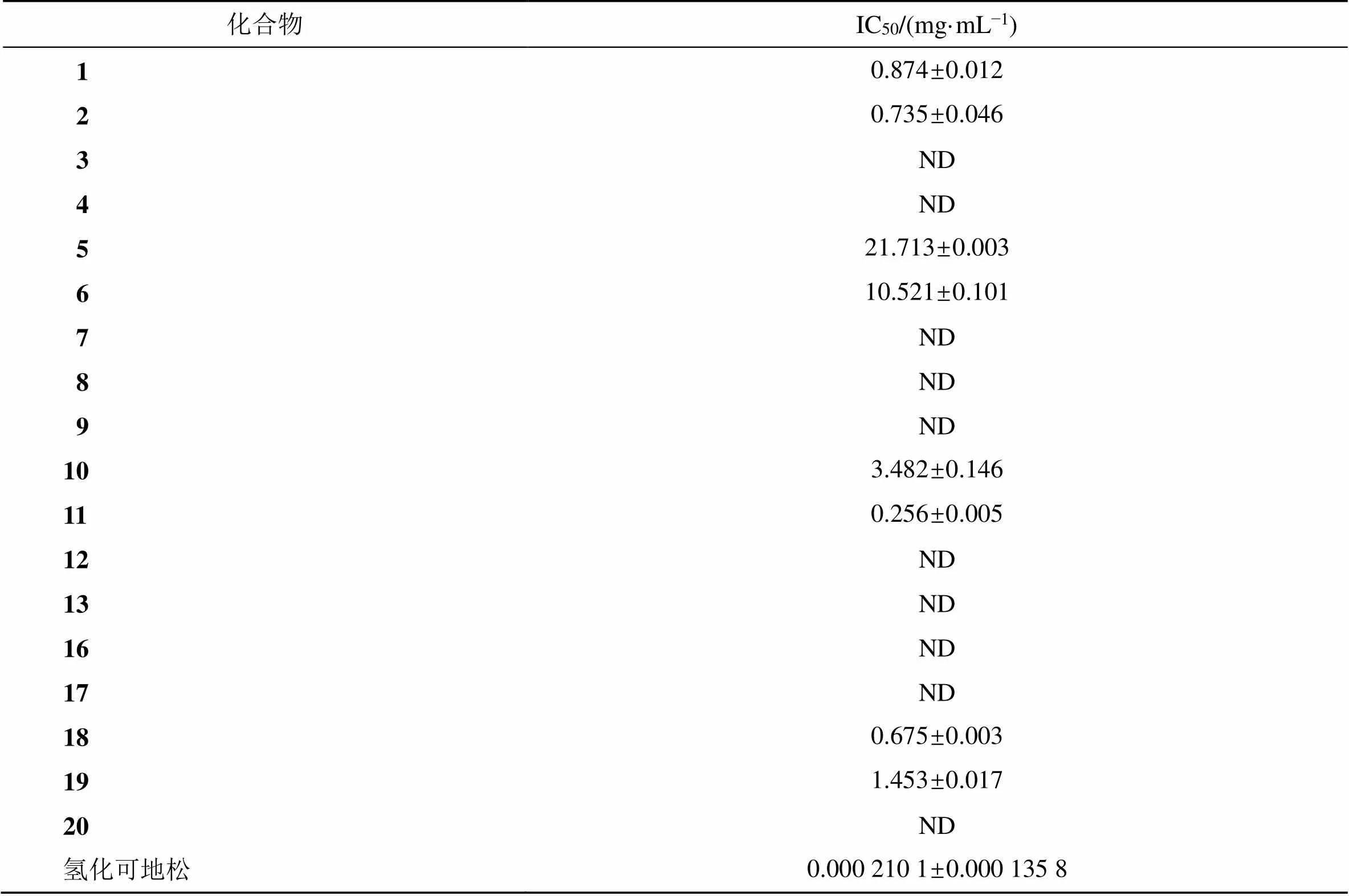
表1 化合物对RAW 264.7细胞的抗炎作用
4 讨论
大苞荆芥是维吾尔族民医习用药材,具有镇咳平喘、清热利湿之功效,用于治疗气管炎、咳嗽气喘、感冒发烧等症。大苞荆芥常被作为神香草的替代品在民医中广泛用使用,因而对大苞荆芥的研究较少,缺乏对大苞荆芥化学成分及其药用活性的系统研究。因此,本研究以大苞荆芥为研究对象,从其95%乙醇提取物石油醚和二氯甲烷萃取部位共分离纯化了20种化合物,经波谱解析和理化性质鉴定,其中有18个化合物为二萜类化合物。NO作为炎症组织损伤的重要致病因子,在急、慢性炎症反应的发生发展过程中发挥重要作用。当免疫细胞遭受微生物内毒素、炎症介质等刺激时,会生成大量的诱导型一氧化氮合成酶,产生NO进行免疫应答[27]。因此,本研究通过各化合物对LPS诱导的小鼠RAW264.7巨噬细胞产生NO的影响来评价各化合物的抗炎活性。结果表明化合物1、2、11、18对LPS诱导的RAW264.7巨噬细胞NO的释放均具有不同程度的抑制作用,其中化合物11的抑制作用相对较好,其IC50值为0.256 mg/mL。本研究阐明了大苞荆芥抗炎作用的药效物质基础,为大苞荆芥的进一步开发利用提供了科学依据。
利益冲突 所有作者均声明不存在利益冲突
[1] 刘英男, 牛凤菊, 辛义周, 等. 荆芥的化学成分、药理作用及临床应用研究进展 [J]. 中国药房, 2020, 31(11): 1397-1402.
[2] Zippi M, Corrado C, Pica R,. Extraintestinal manifestations in a large series of Italian inflammatory bowel disease patients [J]., 2014, 20(46): 17463-17467.
[3] Wlodarska M, Kostic A D, Xavier R J. An integrative view of microbiome-host interactions in inflammatory bowel diseases [J]., 2015, 17(5): 577-591.
[4] Heo S J, Yoon W J, Kim K N,. Evaluation of anti-inflammatory effect of fucoxanthin isolated from brown algae in lipopolysaccharide-stimulated RAW264.7 macrophages [J]., 2010, 48(8/9): 2045-2051.
[5] Byun M W.ethanol extract exerts anti-inflammatory activity through the inhibition of TLR4 signaling in lipopolysaccharide-stimulated macrophage cells [J]., 2014, 17(3): 350-356.
[6] Choi Y Y, Kim M H, Kim J H,.inhibits the development of atopic dermatitis in mice [J]., 2013, 27(8): 1131-1135.
[7] Zhang M L, Chen M Y, Hou Y,. Inflammatory and cytotoxic activities of abietane terpenoids fromBenth [J]., 2021, 26(18): 5603.
[8] Yang E L, Hou Y, Ma G X,. Abietane-type diterpenoids fromBenth. and their anti-inflammatory activity [J]., 2022, 10: 944972.
[9] Latif A, Mahmood Z, Siddiqui N,. Physiochemical standardization of market sample of Gul-e-Zofa (Benth.) [J]., 2013, 4: 76-86.
[10] Siddiqui N, Rauf A, Latif A,. Spectrophotometric determination of the total phenolic content, spectral and fluorescence study of the herbal Unani drug Gul-e-Zoofa (Benth) [J]., 2017, 12(4): 360-363.
[11] 刘勇民, 沙吾提·伊克木. 维吾尔药志(上册) [M]. 乌鲁木齐: 新疆人民出版社, 1999: 423-429.
[12] Yang X W, Feng L, Li S M,. Isolation, structure, and bioactivities of abiesadines A-Y, 25 new diterpenes fromOrr [J]., 2010, 18(2): 744-754.
[13] Fieser L F, Campbell W P. Hydroxyl and amino derivatives of dehydroabietic acid and dehydroabietinol [J]., 1939, 61(9): 2528-2534.
[14] Zhang G J, Li Y H, Jiang J D,. Anti-coxsackie virus B diterpenes from the roots of[J]., 2013, 69(3): 1017-1023.
[15] Chiu L C, Wang J Y, Lin C H,. Diterpenoid compounds isolated fromSolms exert anti-inflammatory effects by inhibiting the IKK/NF-κB pathway [J]., 2021, 26(21): 6540.
[16] Duan H Q, Takaishi Y, Momota H,. Immunosuppressive diterpenoids from[J]., 1999, 62(11): 1522-1525.
[17] Tanaka R, Wada S I, Kinouchi Y,. A new-abietane-type diterpene from the stem bark of[J]., 2004, 70(9): 877-880.
[18] Wu W M, Liu Y, Chen X,. Diterpenoids from the branch and leaf of[J]., 2016, 110: 123-128.
[19] Georges P, Legault J, Lavoie S,. Diterpenoids from the buds ofLamb [J]., 2012, 17(8): 9716-9727.
[20] Zhang C L, Bie P Y, Peng X J,. Total synthesis of (±)-abieta-8, 11, 13-trien-7β-ol [J]., 2003, 50(3A): 429-432.
[21] Guo K, Liu Y C, Liu Y,. Diversified abietane family diterpenoids from the leaves ofand their cytotoxic activity [J]., 2019, 157: 43-52.
[22] Chang C I, Cheng M J, Wang S Y,. Two new dimeric abietane-type diterpenoids from the bark ofand their enzyme inhibitory activity [J]., 2019, 33: 84-89.
[23] Carvalho M J, Carvalho L M, Ferreira A M,. A new xanthone from[J]., 2003, 17(6): 445-449.
[24] 董玉, 石任兵, 刘斌. 石菖蒲非挥发性部位化学成分研究 [J]. 中国药业, 2008, 17(20): 18-20.
[25] Andrew Cheung H T, Miyase1 T, Lenguyen M P,. Further acidic constituents and neutral components ofResin [J]., 1993, 49(36): 7903-7915.
[26] Gouiric S C, Feresin G E, Tapia A A,. 1β, 7β-Dihydroxydehydroabietic acid, a new biotransformation product of dehydroabietic acid by[J]., 2004, 20(3): 281-284.
[27] 杨智. 华美牛肝菌和猪屎豆的化学成分研究[D]. 昆明: 昆明理工大学, 2018.
Chemical constituents ofand its anti-inflammatory activities
ZHAO Peng-an1, 2, LIVARIDING Mahesuti2, MA Guo-xu2, 3, SUN Zhao-cui3, XU Xu-dong3, SHI Lei-ling2, GAO Xing-wang1
1. Xinjiang University, Urumqi 830046, China 2. Xinjiang Institute of Traditional Chinese Medicine and Ethnic Medicine, Urumqi 830002, China 3. Institute of Medicinal Plant Development, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing 100193, China
To elucidate the active substances inand their anti-inflammatory activities.The chemical constituents ofwere systematically isolated and purified by various chromatographic methods, and the structures of the compounds were identified by spectral analysis and physicochemical properties. Meanwhile, MTT colorimetry was used to test the anti-inflammatory activities of the compoundswith hydrocortisone as the positive control.Twenty compounds (two phenolic acids and 18 diterpenoids) were isolated and identified fromand identified as 7-oxodehydroabietic acid (1), dehydroabietol (2), lambertic acid (3), 3β,7α-dihydroxy-abieta-8,11,13-triene (4), triptobenzene L (5), 13-hydroxy-9-oxo-9,10-seco-abiet-8(14)-en-18,10a-olide (6), 8,11,13-abietatriene-7a,18-diol (7), 3β,19-dihydroxy-abieta-8,11,13- triene (8), 7α,15,18-trihydroxy-abieta-8,11,13-triene (9), abieta-8,11,13-trien-7β-ol (10), 6α-hydroxycallitrisic acid (11), 19-acetoxy-15-hydroxy-abieta-8,11,13-triene (12), 7a-hydroxy-abieta-8,11,13-trien-19-ylacetate (13), salicylis acid (14),-3-hydroxy-4-methoxy-cinnamic acid (15), 15-hydroxycallitrisic acid (16), 7β-hydroxycallitrisic acid (17), 6-hydroxycallitrisic acid (18), angustanoic acid G (19) and Jiadifenoic acid K (20). The results of anti-inflammatory activity showed that compound 11 inhibited the release of NO in LPS induced mouse macrophages RAW264.7, with IC50value of 0.256 mg/mL.Compounds 5, 6, 11 and 13 were isolated from Lamiaceae for the first time. Compounds 1, 3, 4, 7, 8, 10, and 12 are isolated fromgenus for the first time. Compounds 2, 9, 14 and 15 were isolated fromfor the first time. Compounds 11 had certain anti-inflammatory activity, which had the value for further in-depth research.
Benth.; diterpenoid; anti-inflammatory activity; triptobenzene L; 6α-hydroxycallitrisic acid; 7a-hydroxy- abieta-8,11,13-trien-19-ylacetate
R284.1
A
0253 - 2670(2023)22 - 7342 - 09
10.7501/j.issn.0253-2670.2023.22.011
2023-04-12
新疆维吾尔自治区公益性科研院所基本科研业务经费资助项(Ky2023zmy005);中国医学科学院医学与健康科技创新工程(2022-I2M-1-017)
赵鹏安(1998—),男,硕士研究生,研究方向为中药学。E-mail: 1049867556@qq.com
通信作者:石磊岭(1977—),男,硕士,研究员,研究方向为中药学研究。E-mail: shileiling@sina.com
高兴旺(1983—),男,博士,主要从事天然产物化学研究。E-mail: gxw@xju.edu.cn
[责任编辑 王文倩]
