Update of latest data for combined therapy for esophageal cancer using radiotherapy and immunotherapy: A focus on efficacy,safety,and biomarkers
2023-11-20ShupingChengButuoLiJinmingYuLinlinWang
Shuping Cheng ,Butuo Li ,Jinming Yu ,Linlin Wang
1Department of Radiation Oncology,Shandong Cancer Hospital Affiliated to Shandong First Medical University,Jinan 250117,China;2Department of Radiation Oncology and Shandong Provincial Key Laboratory of Radiation Oncology,Shandong Cancer Hospital and Institute,Shandong First Medical University and Shandong Academy of Medical Sciences,Jinan 250117,China;3 Research Unit of Radiation Oncology,Chinese Academy of Medical Sciences,Jinan 250117,China
Abstract Esophageal cancer usually has a poor prognosis.Given the significant breakthrough with tumor immunotherapy,an increasing number of clinical studies have demonstrated that the combination of radiotherapy and immune checkpoint inhibitors (ICIs) may have a synergistic effect and good outcome in esophageal cancer.Clinical studies of immunoradiotherapy (iRT) for esophageal cancer have proliferated enormously from 2021 to the present.However,a summary of the efficacy and toxicity of combined therapy to guide esophageal cancer treatment in clinical practice is lacking.For this review,we integrate the latest data to analyze and assess the efficacy and safety of iRT for esophageal cancer.In addition,we discuss better predictive biomarkers,therapeutic options for specific populations,and other challenges to identify directions for future research design.
Keywords: Esophageal cancer;immunoradiotherapy;combination therapies
Introduction
Esophageal cancer,regarded as the seventh most fatal cancer in the world,has a poor prognosis.Eastern Asia has one of the highest reported rates (1,2).Both radiotherapy(RT) and immunotherapy are important modalities for esophageal carcinoma.
Many preclinical studies have demonstrated that the combination of RT and immune checkpoint inhibitors(ICIs) may have a survival benefit in various cancers (3-6).Research on immunoradiotherapy (iRT) for esophageal cancer is still in its infancy.Surprisingly,several recent clinical studies have demonstrated that a combination of RT and ICIs has a synergistic effect and good outcome in esophageal cancer (7).A considerable amount of updated data on iRT for esophageal cancer (EC-iRT) has emerged over the last two years (Table 1).For this review,we integrate the latest data to analyze and assess the efficacy and safety of EC-iRT,potentially serving as the guideline and justification for analyzing therapeutic protocols for esophageal cancer to guide clinical practice.Furthermore,we explore biomarkers to better identify patients who may benefit from EC-iRT.
Potential synergy between RT &immunotherapy

iRT stems from the rationale that RT can initiate immunotherapy,achieving synergistic antitumor response and producing synergistic antitumor immunity for durable disease control when these two treatment modalities are combined (27,28).Previously,it was shown that RT induces immunogenic cell death and cellular stress,leading to the release of inflammatory cytokines,damageassociated molecular patterns and tumor-associated antigens (27,29-33).Subsequently,the combination with immunotherapy might influence the tumor microenvironment by increasing the antigen-presenting capacity,enhancing tumor immune infiltration and tumor cell killing and decreasing myeloid-derived suppressor cells (30,34-39).A preclinical model of esophageal squamous cell carcinoma(ESCC) showed that the addition of radiation to immunotherapy increases tumor-infiltrating CD8+T cells and reduces decreased T cell exhaustion (40,41).The conclusion was that combined agents’ therapeutic effect is superior to that of single-agent immunotherapy (40).The mechanism probably operates in local tumors as well as control of secondary metastases,the so-called abscopal effect (42,43). The notion that cancer immunotherapy may promote radiosensitivity via the normalization of tumor vasculature and hypoxia is also important (Figure 1) (44-46).
As for particle therapy,previous study has shown that proton beam radiotherapy (PBT) could induce intra-tumor immune cell infiltration and be associated with activated transcriptomic antitumor immune response pathways (47).Although there is no data on the combination of proton therapy with immunotherapy for esophageal cancer,an animal study has shown that the combination could increase the infiltration of CD8-positive and CD11bpositive cells,especially in the abscopal tumor,by exploring the effects of high-energy carbon ion therapy in combination with immunotherapy in a murine model of osteosarcoma (48).
In addition,some have suggested that metabolic therapy may help RT to better overcome immunoresistance (49).Oxidative mitochondrial metabolism inhibition as part of a metabolic therapy regimen can address radiation-induced immunosuppression.Recently,it has been reported that the combination of iRT and OXPHOS inhibition is a promising approach to promote abscopal responses in programmed cell death protein 1 (PD-1) resistant disease(49),though it has not yet been validated for EC-iRT.
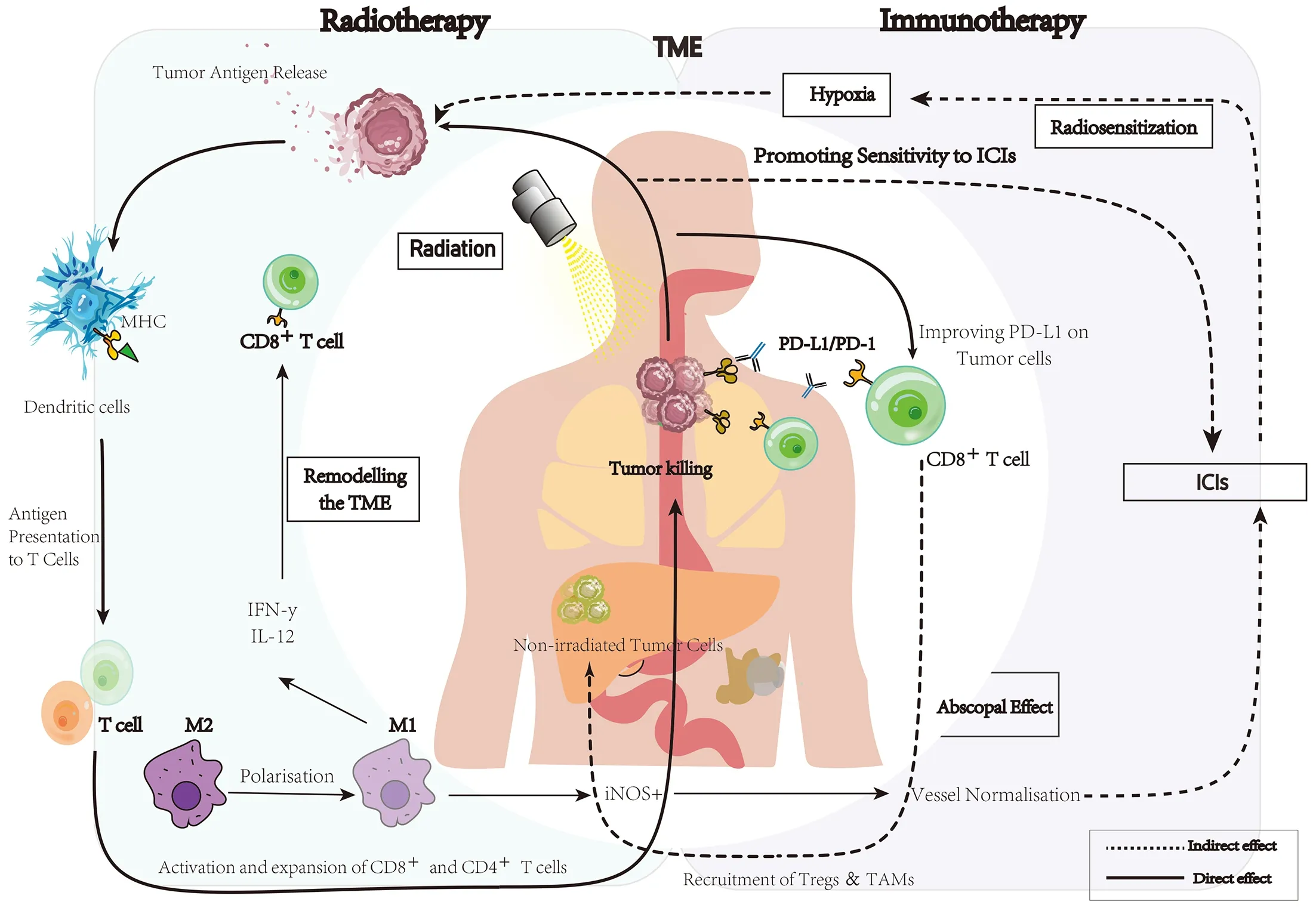
Figure 1 A basic schematic of interactions between radiotherapy and immunotherapy against tumor cells and abscopal effects.TME,tumor microenvironment;ICI,immune checkpoint inhibitor;MHC,major histocompatibility complex;PD-1,programmed cell death protein 1;PD-L1,programmed cell death ligand 1;IFN,interferon;IL-12,interleukin-12;M2,M2 macrophage;M1,M1 macrophage;iNOS,inducible nitric oxide synthase;TAM,tumor-associated macrophage.
Clinical efficacy of EC-iRT
Neoadjuvant treatment in resectable locally advanced esophageal cancer
Neoadjuvant chemoradiotherapy (CRT) plus immunotherapy (iCRT)
Neoadjuvant CRT followed by surgical resection is considered a standard treatment for patients with resectable locally advanced esophageal cancer (50).However,the 10-year follow-up for CROSS was disappointing,suggesting that distant metastasis is the primary mode of failure and that the incidence of distant metastasis is not reduced after neoadjuvant CRT.Especially in the modern era of immunotherapy,it is crucial to determine whether addition of immunotherapy enhances the efficacy and reduces the incidence of distant metastasis.
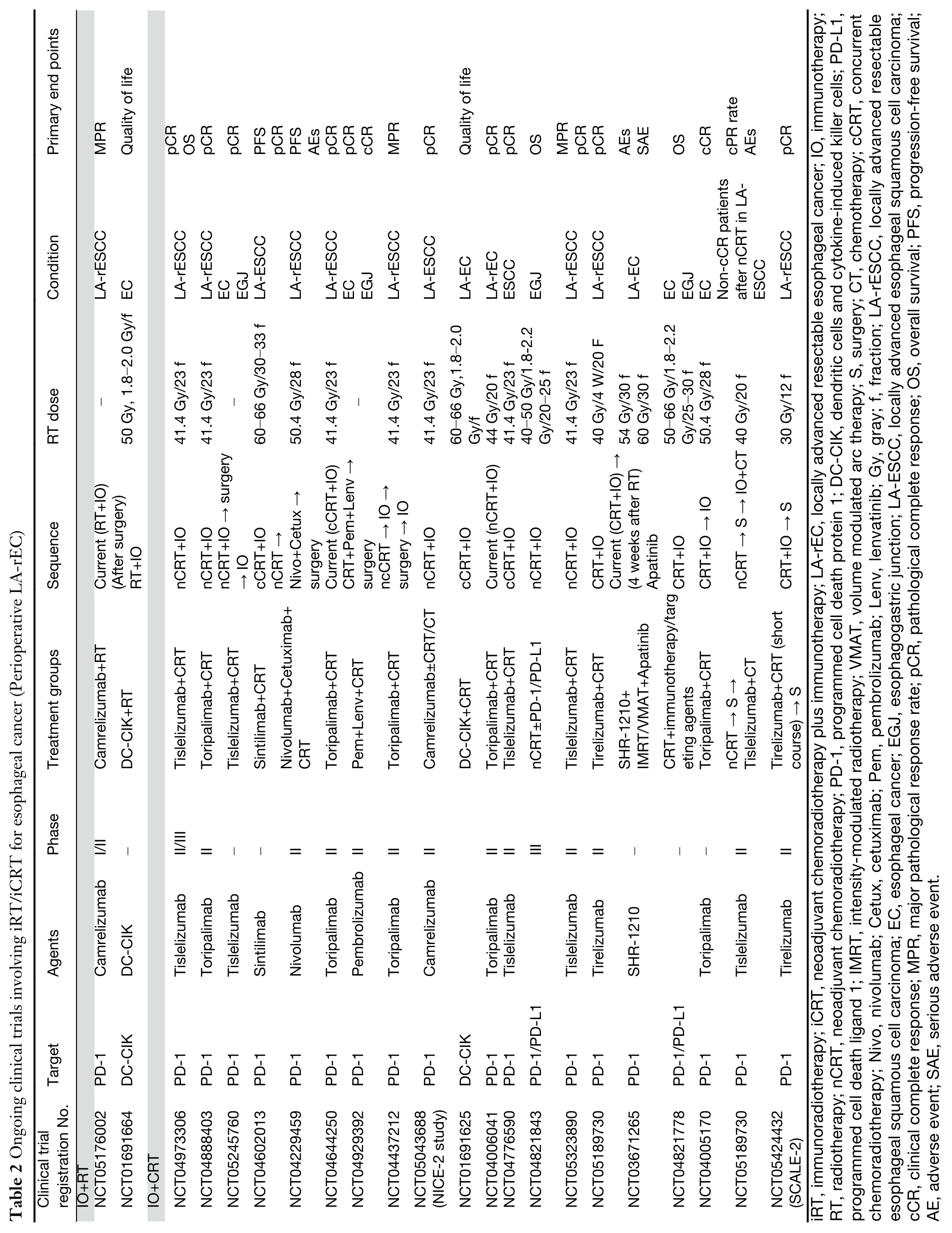
In terms of neoadjuvant iCRT of the two main histological types,the PALACE-1 study enrolled 20 patients with locally advanced resectable-ESCC (LAESCC) (15),and the combination of pembrolizumab plus CRT achieved a high pathological complete response(pCR),at 55.6%.PALACE-2 therefore focused on pCR,and results are eagerly awaited.Another aspect of concern is that no significant difference in pCR was found between neoadjuvant CRT concurrent and sequential tislelizumab(47.1%vs.46.7%) for LA-ESCC (20,21).However,patients treated with sequential tislelizumab had a higher major pathological response (MPR) (70.6%vs.86.7%)(20,21).In addition to pCR and MPR,the trial of NCT03671265 (17) and NCT02844075 (18) showed benefits from neoadjuvant iCRT with regard to overall survival (OS) and progression-free survival (PFS).In esophageal adenocarcinoma (EAC),the pCR rate in the PERFECT trial was higher than that of the CROSS trial(14).However,these preliminary clinical studies had small sample sizes.
At present,an increasing number of multicenter phase III studies of esophageal cancer are comparing neoadjuvant iCRT versus neoadjuvant CRT alone.NICE-2 is a headto-head trial comparing not only neoadjuvant iCRT and neoadjuvant CRT but also neoadjuvant immunochemotherapy.The results will provide helpful information for better comparing multimodal neoadjuvant therapeutic combinations with improved clinical efficacy.Moreover,several ongoing trials are exploring anti-epidermal growth factor receptor (EGFR) or EGFR tyrosine kinase inhibitors(cetuximab or lenvatinib) combined with iCRT for patients with resectable LA-ESCC.
Neoadjuvant RT plus immunotherapy
Although concurrent CRT (cCRT) has been the standard care for locally advanced esophageal cancer,some patients refuse or cannot tolerate CRT.A single-arm,phase Ib study (NCT03222440) enrolled 20 patients with ESCC who accepted neoadjuvant higher dose RT (total dose=60 Gy) combined with immunotherapy (9).Despite the short follow-up period,neoadjuvant iRT still showed favorable prospects.In another phase Ib clinical trial that used lower doses of RT (total dose=41.4 Gy) combined with immunotherapy,the pCR and MPR reached 57.1% and 64.3%,respectively (8).Nevertheless,there is insufficient evidence to suggest which kind of RT is better than another.The number of studies related to neoadjuvant iRT for patients with locally advanced esophageal carcinoma is still relatively small,and there has been limited exploration of combined modality therapy.
Adjuvant treatment in resectable locally advanced esophageal cancer
Postoperative adjuvant immunotherapy after neoadjuvant CRT
It is reported that 70%-75% of patients do not achieve pCR after neoadjuvant CRT and surgery (51).A previous study suggested dendritic cell vaccine combined with RT could activate the specific immune response and may lead to better survival in patients undergone surgery with LAESCC (10).Therefore,immunotherapy is currently always considered to be a paramount complement to postoperative radiochemotherapy.For patients who achieve complete resection (ypCR) of ESCC after neoadjuvant cCRT,adjuvant durvalumab was accepted in NCT02520453 (24).However,no significant difference was found between groups with regard to OS and disease-free survival (DFS).It is possible that patients with ypCR achieved better prognosis and were less likely to benefit from adjuvant durvalumab.It was suggested that caution should be exercised when using adjuvant immunotherapy in patients with ypCR in the future.In the checkmate577,a 33%reduction in risk of relapse or death was observed in the adjuvant nivolumab group,and distant metastasis-free survival approximately doubled compared with the control group (52).These data indicate that postoperative adjuvant immunotherapy can reduce the risk of local recurrence and distant metastasis for patients who do not achieve a pCR.
Postoperative adjuvant immunotherapy after total neoadjuvant therapy (TNT)
Several ongoing studies mostly tend to decide on postoperative adjuvant therapy in light of clinicopathological factors,such as pCR,ypTNM staging,peripheral nerve injuries and lymphovascular invasion,with patients divided into high-and low-risk groups (52).In the NCT05189730 study (53),patients with clinical partial response (cPR) and clinically stable disease (cSD) after neoadjuvant CRT received neoadjuvant immunochemotherapy,and postoperative tislelizumab was applied to those patients who did not achieve pCR after neoadjuvant CRT.Hence,high-risk patients may obtain better clinical outcomes with prolonged survival from the TNT model(Figure 2).
Postoperative adjuvant immunotherapy after neoadjuvant iCRT
Compared with postoperative adjuvant immunotherapy after neoadjuvant CRT,multiple small sample studies,such as NCT02844075 (54) and NCT02639065 (55),have documented that preoperative iCRT combined with postoperative immunotherapy can also achieve good perioperative survival outcomes.In this treatment regimen,the 1-year OS varied from 80% to 96%,and pCR varied from 43% to 47%.The ongoing study NCT01691664,in which the primary endpoint is quality of life,will provide comprehensive information on different clinical endpoints(Table 2).
Unresectable locally advanced esophageal cancer
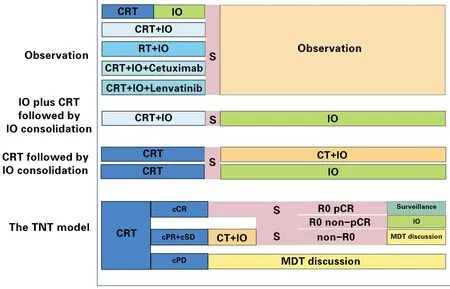
Figure 2 Perioperative iRT/iCRT treatment strategies in resectable locally advanced esophageal cancer.CRT,neoadjuvant chemoradiotherapy;IO,immunotherapy;RT,radiotherapy;S,surgery;CT,chemotherapy;TNT,total neoadjuvant therapy;cCR,clinical complete response;cPR,clinical partial response;cSD,clinical stable disease;cPD,clinical progressive disease;R0,the radical resection was performed and no residual tumor;pCR,pathologic complete response;non-pCR,did not achieved pathologic complete response;MDT,multidisciplinary team.
The RTOG8501 study sought to determine radical cCRT for locally advanced esophageal cancer,but the 5-year survival rate was only approximately 20% (56).A possible direction for exploration is cCRT combined with sequential immunotherapy. In 2022,data from the TENERGY trial were released by European Society for Medical Oncology (ESMO),in which atezolizumab maintenance therapy following radical CRT was used(54,55),with a clinical complete response (cCR) of approximately 40% and the median OS of 31 months.It is suggested that atezolizumab monotherapy following definitive CRT (dCRT) may result in a promising cCR rate and promising long-term survival.However,the median PFS was less than satisfactory at only 3.2 months.Further studies might be necessary to explain the observations.In addition to ICI monotherapy therapy,durvalumab and tremelimumab with dCRT were administered for inoperable LA-ESCC in NCT03377400 (19).Patients who received combination ICIs showed higher 2-year OS[hazard ratio (HR),0.49;95% confidence interval (95%CI),0.25-0.98;P=0.043] and 2-year PFS (HR,0.52;95%CI,0.28-0.97;P=0.040) than the dCRT group.All of the above findings show the feasibility of combining radical cCRT and immunotherapy for locally advanced esophageal cancer.
Immunochemotherapy can also play a role in the inductive setting before CRT in unresectable LA-ESCC.ImpactCRT,a single-arm,phase II trial,included 46 patients and used induction chemotherapy plus camrelizumab followed by cCRT.With a median followup of 19.1 months,8 (17.4%) patients died.The 1-year PFS and 1-year OS were 74.2% and 87.6%,respectively(25).It indicated that this combination therapy strategy had promising efficacy as a first-line treatment for unresectable LA-ESCC,however,it should be validated in a larger trial in the future.
Many head-to-head phase III clinical trials of the combination of CRT and immunotherapy for unresectable locally advanced esophageal cancer are currently underway,such as Keynote975,Rationale311,and KUNLUN.The NCT0343720 study provided a new idea for comparing consolidation therapy with single-agent and double-agent immunotherapy (nivolumab and ipilimumab) after using dCRT and nivolumab.Overall,the optimal time to implement immunotherapy and how to choose the RT dose remain hot topics in clinical research on unresectable locally advanced esophageal cancer.Further validation is required (Table 3).
Metastatic/recurrent esophageal cancer
Immunochemotherapy regimens are the standard first-line therapies for advanced esophageal cancers and commonly used.RT is considered one of the major treatments for advanced esophageal cancer,and in combination with immunotherapy may be important to maximize the efficacy of esophageal cancer treatment.There are some ongoing studies regarding the duration of combination therapy and RT doses in patients with advanced esophageal cancer.Only oligometastatic patients with ESCC were included in the NCT05183958 and NCT04821765 studies involving immunotherapy combined with stereotactic body radiotherapy (SBRT) and conventional RT.The results of the present study may suggest which iRT combination therapeutic strategy will have better efficacy.Patients with multiple metastases of ESCC were enrolled in the study NCT04512417,which used SBRT for distant metastases,and a strategy of sequential immunotherapy was applied.We look forward to the follow-up work (Table 4).
Clinical efficacy of novel immunotherapy agents for ECiRT
In addition to traditional PD-1/programmed cell death ligand 1 (PD-L1) inhibitors,various immunotherapy agents have been combined with RT to explore more efficacious regimens for patients with esophageal cancer who are unsuitable for standard treatments.One of the choices is the combination therapy involving a multi-peptide vaccine with CRT for patients with unresectable or advanced ESCC.It has been demonstrated in a phase I study of 11 patients enrolled,six cases of complete response (CR) and 5 cases of progressive disease (PD) occurred (26).Oncolytic viruses are another method of immunotherapy (57).Telomelysin (OBP-301) in combination with radiation therapy was applied in the study of NCT03213054 study for those who could not undergo surgery/chemotherapy(11).The cCR rate reached 83.3% in stage I and 60.0% in stage II/III.This finding suggested that multiple courses of endoscopic intratumoral OBP-301 injection with RT might be a feasible treatment strategy and provide clinical benefits to patients with esophageal cancer for whom standard treatments are unsuitable.In addition to primary lesions,another aspect that needs to be considered for advanced esophageal cancer is the control of distant metastasis.Several studies have demonstrated that thymalfasin combined with RT has synergistic enhancement effects and controls distant metastasis.In a study that used SBRT combined with thymalfasin for patients with metastatic ESCC (mESCC) (12),PR occurred in 3 (9.7%) patients,and the metastatic lesion control rate was 45.2%.This combination showed an initial promising abscopal effect on patients with advanced ESCC.However,because the sample size of most studies for advanced esophageal carcinoma was small and the follow-up time was short,further observation and verification are needed.

Table 4 Ongoing clinical trials involving iRT/iCRT for EC (Advanced EC/oligometastatic EC/rEC)
Optimization of RT for esophageal cancer in era of immunotherapy
Whether to choose elective nodal irradiation or involved field irradiation has been the subject of debate for patients with esophageal cancer in the era of immunotherapy.At present,researchers in China and abroad advocate for adopting involved field irradiation when RT is combined with immunotherapy or immunochemotherapy for esophageal cancer.The number of lymphocytes decreases with the increasing planning target volume during RT,and a decreased lymphocyte count is associated with worse survival.This suggests that in the era of immunotherapy,the target area for RT should not be too large.
The NEOCRTEC5010 study showed that PFS and OS between high-dose RT and standard-dose RT were not statistically distinct from one another,with a significantly higher incidence of grade 3 pneumonitis in patients undergoing the former (58).Currently,patients are treated with neoadjuvant iRT/iCRT up to total doses in the range of 40-45 Gy;the radical iRT/iCRT dose can reach 50-60 Gy.Regardless of iCRT or iRT,the clinical treatment strategy for patients with esophageal cancer usually involves irradiation with a fractionation dose of 2 Gy every time rather than disposable high-dose treatment.Overall,this daily conventionally fractionated long-course RT is safe.Relatively,short-course RT (2.5 Gy × 12 f) combined with neoadjuvant immunochemotherapy in the SCALE study also showed the feasibility of this protocol.A preclinical study reported that high-dose hypofractionated RT has a stronger immunostimulatory effect on a certain range (59).This idea seems hardly feasible,however,for the esophagus,which is one of the serial organs.Nonetheless,iRT could be considered for some distant metastases.Based on this assumption,two ongoing phase II clinical trials are adopting SBRT (8 Gy/f,3-5 f) for metastatic lesions of esophageal cancer and conventional fractionated RT (≥40 Gy) for primary lesions combined with immunotherapy.Furthermore,it has been reported in other cancers that low-dose irradiation combined with immunotherapy confers a survival benefit,which provides a new idea for iRT in esophageal cancer (60-63).In 2020,a report from ESMO indicated that compared with anti-PD-1 drugs after RT,administration of anti-PD-1 drugs before or during RT for thoracic tumors increases the incidence rate of radiation pneumonitis (64,65).Overall,the optimal treatment plan remains under discussion and needs further exploration.
Clinical safety of EC-iRT
Perioperative clinical safety
Both of immunotherapy and RT have their significant side effects or toxicity (66).In the process of exploring the combination of iRT for esophageal cancer,safety evaluation has been the subject of much recent attention.A meta-analysis suggested that approximately 51.2% of patients with neoadjuvant iCRT have preoperative grade 3-4 treatment-related adverse events (TRAEs),higher than the group with neoadjuvant immunochemotherapy (19.4%)(67). However,there was no statistically significant difference in the incidence of R0 resection and surgery between the two groups of patients.Adverse events (AEs)of neoadjuvant iCRT mainly occurred during neoadjuvant iCRT combined with ICIs.Approximately 40%-65% of patients experienced TRAEs of grade 3 or higher during the neoadjuvant treatment period,with hematological toxicity being the most common. Compared with NEOCRTEC5010,which used nCRT,no obvious difference was observed in hematologic toxicity,which was 54.3% (58).Elevation of liver enzymes was the most frequent nonhematologic toxicity of grade 3 or higher.It is noteworthy that in the PALACE-1 study,which used iCRT (15),grade 3 and higher AEs were observed in 13(65%) patients;in fact,one of the patients who experienced significant esophageal hemorrhage died while awaiting surgery. Most TRAEs with neoadjuvant CRT and concurrent and sequential tislelizumab were grade 1-2(20,21).Grade 3 postoperative complications occurred in 20.0% of patients with sequential iCRT,including anastomotic fistula (20.0%,3/15),injury of the recurrent laryngeal nerve (6.7%,1/15) and pleural effusion (6.7%,1/15).More focus on grade 3 TRAEs,such as hematologic toxicity and liver damage,of concurrent therapy is needed.In particular,one patient with concurrent iCRT died due to pneumonia before surgery (20).Overall,neoadjuvant immunotherapy combined with chemotherapy was found to be safer than neoadjuvant iCRT.Additionally,Sihaget al.compared the thirty-day mortality and readmission rates of patients who were treated with neoadjuvant iCRT or CRT (16),and the results revealed no significant differences between the control and treatment groups.However,due to the small sample size of this study,work with larger sample sizes is required. Most of the preoperative neoadjuvant iCRT for esophageal cancer was grade 3 and below AEs.Based on improving pCR,improving the safety profile will be of great help to increase clinical efficacy.
According to the results of a prospective phase Ib trial of patients treated with iRT (8),15 (68.2%) patients experienced TRAEs,and only 1 (4.5%) patient developed grade 3 AEs.The rate of grade 3 and higher TRAEs in this study was significantly lower than that of neoadjuvant iCRT.However,the limitation of the study was the small sample size;and thus,more evidence is needed to support the conclusion.
When patients received neoadjuvant CRT combined with postoperative adjuvant immunotherapy,the incidence of grade 3/4 AE was 25%-35%.However,compared with those who did not accept postoperative adjuvant immunotherapy,no visible difference was detected.The initial results of Checkmate577 and NCT02639065 indicate that neoadjuvant CRT combined with postoperative adjuvant immunotherapy is safe and feasible and does not adversely impact health-related quality of life.Most AEs of RT combined with postoperative adjuvant immunotherapy were grade 1-2 (22,23).
Clinical safety of unresectable locally advanced esophageal cancer
Adding immunotherapy to cCRT may increase toxicity while increasing efficacy.The good safety profile was highlighted in a 2022 ESMO study that used camrelizumab monotherapy followed by cCRT.The most frequent grade 1-2 AEs were reactive skin capillary hyperplasia (66.7%,10/15) and pneumonia (40.0%,6/15),and no grade 3 or 4 AEs occurred (68).Reactive skin capillary hyperplasia might occur with camrelizumab.In the TENERGY study,approximately 10% of patients experienced grade 3-5 TRAEs of pneumonia,which was lower than camrelizumab.These results suggest that cCRT followed by ICI monotherapy leads to a durable therapeutic response and has a tolerable safety profile for locally advanced esophageal cancer. However,the possible development of treatment-related pneumonia should be monitored.
In the trial of ImpactCRT which was applied for induction chemotherapy plus camrelizumab followed by cCRT,the most common grade ≥3 TRAE were hematological toxicities and liver injury (25).Of concern,one treatment-related death was observed.Further studies are still needed to evaluate safety as well as longer-term efficacy in the future.
Moreover,safety data for iRT in the NCT03222440 trials demonstrated that compared with RT alone,iRT did not increase radiation-related toxic side effects and immune-related adverse events (irAEs) (9).And iRT has less severe AEs than cCRT (56,69,70).Overall,RT/CRT combined with immunotherapy for unresectable locally advanced esophageal cancer has a good safety profile.Further research is currently underway in many large studies,such as keynote 975 and rationale 311.
Clinical safety of metastatic/recurrent esophageal cancer
Safety data for advanced esophageal cancer have mainly been extracted from studies of RT combined with a new class of immunotherapy agents.In a study of RT and chemotherapy combined with multiple-peptide vaccine(26),leucopenia (54.5%) was the most frequent grade 3 toxicity,and no subjects had serious AEs.At the same time,the combination of SBRT and Tα1 yielded acceptable toxicity.No grade 4 AEs were observed during treatment(12).Although safety data of advanced esophageal cancer were derived from small studies,iRT regimens displayed a good safety profile in a clinical trial (13).
Exploration of biomarkers in clinical trials
Cytokines and proteins
In the NCT02639065 trial (23),patients with locally advanced esophageal adenocarcinoma (LAEAC)/esophagogastric junction with PD-L1-positive tumors,especially CPS≥10,had better OS and recurrencefree survival (RFS).This finding suggests that the post cCRT PD-L1 status may predict the survival of patients who receive neoadjuvant CRT combined with adjuvant PD-1/PD-L1 inhibitors and may help to select patients who would most likely benefit from postoperative immunotherapies.
In addition,PERFECT attempted to use a six-gene IFNy signature to predict which kind of esophageal cancer patients will respond to iCRT (14);this cytokine is released by T cells and may lead to upregulation of PD-L1 in tumor microenvironment (71,72).Compared to non-responders,responders had a higher baseline expression of an established IFNy signature.This means that neoadjuvant iCRT is mainly beneficial for cases with an already inflamed microenvironment.
Cell subsets
T-cell factor 1+(TCF-1) CD8+T cells have recently been proven to be the essential subpopulation for a successful response to immune checkpoint blockade (73,74).According to the related analysis of patients with ESCC who received preoperative pembrolizumab combined with CRT,it was found that the presence of CD8+T cells was statistically comparable between the non-pCR and pCR patient groups and that the percentage of TCF-1+cells was significantly higher in the pCR group than in the non-pCR group (P=0.01) (15).The percentage of TCF-1+cells was significantly higher in the pCR group than in the non-pCR group.The findings are consistent with several reports (75-77),but whether TCF-1+cells play a role as potential biomarkers for prediction of the response to the combination of iRT for ESCC needs further investigation.
Overall,the diversity of peripheral CD8+T-cell receptors (TCRs) at baseline and the dynamic changes in TCRs within a tumor may serve as effective biomarkers for the combination of RT and immunotherapy for esophageal cancer.Results have shown that RT combined with PD-1 blockade greatly promotes spatial and temporal changes in TCR sequences between tumor tissues and peripheral blood.
One study showed that CD4+and CD8+T cells in the peripheral blood were associated with survival;specific Tcell subsets,such as Ki67+CD8+,interferon-γ+CD8+,Ki67+Treg,and cytotoxic T-lymphocyte-associated protein 4-positive CD4+T cells,were also closely associated with patient survival.Recently,the NCT03222440 trial (9)found that treatment with RT combined with camrelizumab can disrupt T-cell subsets in tumor tissues.These results suggest that PD-L1+CD4+tumor-infiltrating lymphocytes (TILs) and PD-1+CD8+TILs contribute to the inhibitory immune microenvironment in ESCC.These results are consistent with several recent studies which suggested that the combination of RT and an anti-PD-1 antibody may result in a synergistic antitumor effect by both priming and reactivating the immune response(39,78).In addition,comprehensive characterization of the tumor immune microenvironment will help in identifying responders and assessing potential synergy between perineoadjuvant chemo(radio)therapy and ICIs for resectable EAC patients.These findings from the tumor microenvironment and systemic immune status provide candidates for predictive biomarkers.
The NCT02639065 trial (23) focused on macrophages of LA-EAC by immune deconvolution and suggested that a higher relative proportion of M2 macrophages and CD4 memory-activated T cells is associated with improved RFS.One possible explanation is that M2 tumor-associated macrophages (TAMs) are associated with high PD-L1 expression on tumor cells and immune cells and may contribute to improved efficacy of PD-L1 inhibitors.Intriguingly,a higher proportion of M1 TAMs was associated with an increase in the proportion of resting dendritic cells and inferior RFS with durvalumab,possibly due to the induction of peripheral CD8 T-cell tolerance(79).Nevertheless,this study was limited by the size of the patient groups,and validation in a multicenter study with a large sample size is needed.
Future directions of EC-iRT
The overwhelming majority of data on esophageal cancer studies combining RT with immunomodulation focus on the context of PD-1/PD-L1 or cytotoxic T-lymphocyte antigen-4 inhibitors,but several other immune checkpoints and targets are currently being prospectively evaluated.
With the development of immunotherapy,RT has made great progress through the introduction of new technologies,such as proton or heavy ion therapy.Although little is known about the immune response after heavy ion particle irradiation,combining heavy ion particle irradiation with immunotherapy may be a good option.Compared with other RT methods,heavy ion particle therapy has two synergistic advantages,namely,high specificity for tumors and large cytotoxic effects (80).A retrospective cohort observation of advanced hepatocellular carcinoma has suggested that PBT combined with anti-PD-1/PD-L1 could improve overall tumor control and survival without unexpected AEs.Concurrent PBT and ICIs could provide advantages for PBT with palliative purpose because the unirradiated tumors probably could be effectively treated by anti-PD-1/PD-L1 by synergistic effect (81).Another animal study also showed that sequential administration of carbon ion therapy and immunotherapy could reduce the number of lung metastases more efficiently than the X-ray group (48).These findings suggested that particle therapy combined with immunotherapy might be a promising regimen for advanced cancer,regardless of the sequence of combination therapy.Although the therapeutic effects of particle therapy combined with immunotherapy in esophageal cancer have not been reported,this is still a promising research direction.Additional RT techniques to reduce radiation to surrounding tissues should be further explored.
Conclusions
Many clinical studies of iRT for esophageal cancer have been conducted from 2021 to the present.For this review,we integrate the latest data to analyze and assess the efficacy and safety of iRT for esophageal cancer.Many studies have demonstrated that RT/CRT combined with immunotherapy has a synergistic effect and leads to a good outcome for esophageal cancer,warranting further research.We also describe esophageal cancer trials of chemoradiotherapy combined with immunotherapy from iRT to explore efficacy and safety.In this paper,we explore different kinds of biomarkers in preclinical and clinical studies to better identify patients who may benefit from iRT for esophageal cancer.In conclusion,the combined application of RT and immunotherapy in the comprehensive treatment of esophageal cancer has bright prospects and will play an increasingly important role.More related studies are needed to assess this combination treatment.
Acknowledgements
This study was supported by National Natural Science Foundation of China (No. 82172865) and Clinical Research Special Fund of Wu Jieping Medical Foundation(No.320.6750.2021-02-51 and 320.6750.2021-17-13).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
杂志排行
Chinese Journal of Cancer Research的其它文章
- Epidemiology of pancreatic cancer: New version,new vision
- MRD-directed and risk-adapted individualized stratified treatment of AML
- Inspired by novel radiopharmaceuticals: Rush hour of nuclear medicine
- Genetic susceptibility loci of lung cancer are associated with malignant risk of pulmonary nodules and improve malignancy diagnosis based on CEA levels
- A novel multimodal prediction model based on DNA methylation biomarkers and low-dose computed tomography images for identifying early-stage lung cancer
- Baseline radiologic features as predictors of efficacy in patients with pancreatic neuroendocrine tumors with liver metastases receiving surufatinib
