Magnesium alloys as extremely promising alternatives for temporary orthopedic implants – A review
2023-11-18NirnjnRghvnrMhuriRoSirjuGuptVikrmKumrJinAishwry
C.A. Nirnjn, T. Rghvnr, Mhuri P. Ro, C. Sirju, M. Gupt,Vikrm Kumr S. Jin, R. Aishwry
a
Department of Industrial Engineering and Management, Ramaiah Institute of Technology, Bengaluru-54, India
b Department of Mechanical Engineering, National Institute of Engineering, Mysuru-08, India
c Department of Chemistry, B.M.S. College of Engineering, Bengaluru-19, India
dDepartment of Mechanical Engineering, Ramaiah Institute of Technology, Bengaluru-54, India
eDepartment of Mechanical Engineering, NUS Singapore-75, Singapore
fDepartment of Metallurgical and Materials Engineering, Indian Institute of Technology, Madras -36, India
Received 28 February 2023; received in revised form 27 July 2023; accepted 13 August 2023
Available online 9 September 2023
Abstract Mg alloys are emerging as potential and very promising alternatives for replacing permanent metallic implant materials such as steels and titanium in applications where the implants need to be removed following healing through revision surgery. Use of Mg alloys for implant application is seen as a game changer and Mg alloys are almost perfect materials for the future in both engineering and biomedical applications. Present review therefore focuses on highlighting significance of Mg alloys in biomedical field and risks of using permanent metallic implants particularly when the implants are no longer required after the injury is healed. In this review, importance of orthopedic implants in present scenario, serious concern related to accidents that are causing permanent disabilities, demand in orthopedic implant market worldwide, potential applications of Mg based materials and their compatibility in biological environment is presented and discussed.In addition, degradation rate, major reactions associated with Mg based materials and effect of alloying elements on implant performance are also discussed based on in-vivo results. Recent advances in development of Mg alloys through various techniques and their performance in in-vitro conditions are also outlined. Possible ways to eliminate the limitations of Mg alloys include alloying, melt purification, surface alterations, surface modifications, chemical treatment, secondary processing etc. are discussed. Challenges and opportunities for Mg alloys to become ideal implant material is also addressed.
Keywords: Accidents; Fractures; Burden of fracture; Magnesium; Ultimate alternative; Degradation rate; Alloying; Melt purification; Coating.
1. Introduction

Fig. 1. Importance and bio compatibility of magnesium in human body: release of Mg ions from magnesium based implants can be effectively diluted and utilized in body fluids and carried to other soft tissues and bones through blood and tissues. Then excess magnesium ions are excreted via urine and feces[1,8].
Currently magnesium alloys are considered to be the most promising materials for wide range of applications where light weight is concerned. During past decades magnesium alloys have proved their importance in automotive, aerospace, medical and electronics sectors as results of their attractive properties such as mechanical strength, corrosion resistance, high specific strength, thermal conductivity and electrical conductivity. As a result, some of the traditional metals such as titanium,steel and cobalt are being replaced by magnesium based alloys in many engineering and biomedical applications[1–6].In recent years, owing to their biodegradable property magnesium based alloys are becoming attractive choice for temporary orthopedic implants. In addition to light weight property magnesium alloys are abundantly available in nature and it is considered to be the 8th most abundant element of the earth’s crust [7]. Another very important and essential property of magnesium alloys is their excellent compatibility with human body. Unlike steel, cobalt and titanium, magnesium is nontoxic to human body.To note that magnesium is the fourth most abundant cation present in human body and it plays a vital role in development of bone and soft tissue [1–6]. Human adult blood plasma contains a total magnesium concentration of 0.65 mmol/L to 1.05 mmol/L [1] and recommended intake is approximately 300 mg for adults [7]. Their enhanced biodegradability, promising bio-compatibility and antibacterial properties in physiological conditions have attracted researchers and engineers to develop magnesium based material to serve as temporary implants. Fig. 1 shows importance and biocompatibility of magnesium in human body [1,8] and that if Mg intake exceeds the permissible limit then it will be carried via circulatory system and will be excreted through urine,without creating adverse effects [9]. Therefore, Mg based materials are considered to be a novel absorbable metallic implant materials. When Mg based screw implants were used to fix tendon graft into bone tunnel in anterior cruciate ligament(ACL) reconstruction, one of the most challenging clinical issues, Mg screw implants demonstrated reasonable strength and significantly improved the graft healing quality. This improvement is most likely attributed to the higher concentration of Mg ions (>15 × 10-3mol·L-1) and high pH that promoted mineralization at tendon graft enthesis. Mg screws also promote bone formation in peri–screw region at the very early healing stage. Thus as a biodegradable metal, Mg has appropriate mechanical integration and better osseointegration required for an ideal implant material, thus may be considered as a promising alternative to existing orthopedic implant materials.
However, Mg based materials are also known for their complex degradation behavior in aqueous solutions of the human body due to the presence of inorganic salts, bioorganic molecules (e.g. protein, glucose, amino acids, vitamins and polypeptide etc.) and bio-organic macromolecules. Unfortunately, Mg base materials have a tendency to degrade too quickly at physiological pH (7.4 – 7.2) and in physiological media containing higher concentrations of aggressive ions,which decreases the mechanical integrity before the healing.Therefore degradation of Mg based materials have become major limitation in the clinical field and needs to be addressed.
Though in recent years, many reviews have been published on Mg based implant materials, majority of reviews focused on enhancement of corrosion resistance and mechanical integrity of Mg based materials through improved existing and advanced techniques. Present review, covers important areas including accident reports across different parts of the world,fracture due to accidents, cost of bone fracture treatments and melt purification techniques that are less covered in the previous reviews.
Based on recent studies on Mg based materials used as implants (in-vivo and in-vitro) this work provides significant outcome on advantages, future scope of magnesium based materials for implant applications and also limitations of permanent metal implants such as fixation plates, screws and other plates made of steel, cobalt and titanium alloys. This study also highlights different techniques for optimizing the properties of Mg based materials for implant applications.
2. Importance of implant materials in present scenario
Millions of people worldwide suffer with different kinds of fractures every year such as femur fracture, humerus fracture,spine fracture, hand, ribs etc. According to the recent studies most of the fractures are associated with femur in male and humerus in women [10]. These bone fractures are generally a medical condition of a patient in which there will be a partial or complete destruction in the continuity of bone.One of the major factors responsible for bone fracture is road traffic accidents (RTA) and most of the roads accidents are reported with death cases of people from age 5–29 [11]. The number of RTA has been raising on a daily basis, as increase in population is increasing the number of vehicles on road and which in turn raising the likelihood of accidents. As an example, in Saudi Arabia, 3, 52,464 accidents were reported during 2017, out of which 30,217 cases resulted in injuries and 6025 deaths [12].
Recent statistics from ministry of road transport and highways (MORTH) of India shows a total number of accidents during 2019 leading to 1, 51,113 deaths and 4, 51,361 injuries [13]. Among different accidents happened so far, most of the injuries are reported with permanent disabilities and hospitalization. From the literature it is observed that, most common bone fracture in men was femur(28.2%)followed by hemerus (20.8%) and most common fracture found in women was humerus (20.8%) followed by ribs (17.11%). Hand and feet fractures were observed as common fracture in both men and women (1.9%) [10]. Fig. 2 shows common bone fracture areas.
Ministry of Road transport and highways transport research wing also indicated that road accidents lead to death and injuries across the world and over 1.35 million death cases were reported in the year of 2018 [21]. Unfortunately majority of the (90%) accidents and casualties are taking place in developing countries.Most of the serious fractures that are happening due to accidents or diseases requires external medication and are mostly fixed by using implants through surgeries.
In 2019, Global Burden of Diseases (GBD) conducted systematic efforts to quantify the status of fractures in collaboration with GBD fracture collaborators. Burden of fractures due to accidents and diseases worldwide was investigated. Data were collected from 21 GBD regions and from 204 countries& territories by sex, age and year (1990–2019) by considering 95% confidence level. Following were the key findings of analysis, which shows shocking and strange facts.
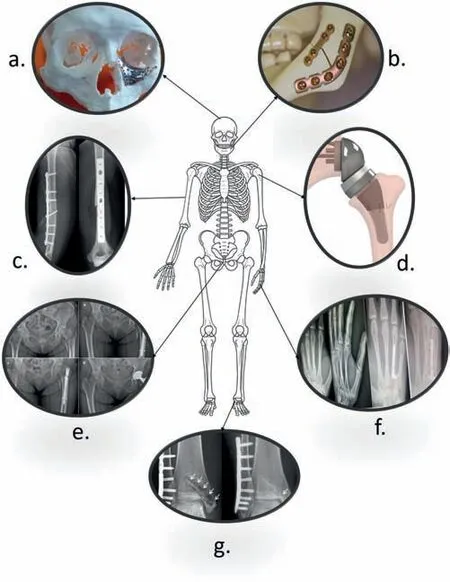
Fig. 2. Common bone fracture areas affected by disease/accident. a) maxillofacial [14] b) mandibular [15] c) humeral midshaft [16] d) shoulder [17] e)hip [18] f) metacarpal [19] g) ankle [20].
a. 178 million new fracture cases (33.4% increase in cases since 1990)
b. 455 million frequent cases of acute and long term symptoms of a fracture (70.1% increase since 1990)
c. 25.8 million years lived with disability (YLD) (65.3% increase since 1990).
d. Patella fractures (lower leg fracture), tibia/fibula fractures and ankle fractures were observed as most common and worrisome fractures.
Traditional fixation of implants through surgery is done by either osteosyntheis or oesteotomy in which permanent metallic implants such as fixation plates and screws made of titanium alloys, cobalt alloys and steel alloys are used and exercised.More than 1 million patients undergo hip replacement surgeries to get rid of ache and repair damages in hip joints caused by osteoarthritis every year using permanent metal implants[22].However permanent implants are removed by secondary surgery after 2–3 years of first surgery and this cause serious impact on patient’s mental health and physical health.Moreover removal of permanent implants is very expensive.For example Traumatic brain injuries (TBI) that are caused by accidents is considered to be the most expensive medicaltreatment after heart related surgeries. TBI related surgery cost comprise about 56 billion dollars annually in United states alone and rest of the bone injuries consume $32 billion annually, Bone fractures which require grafts/transplants undergo surgeries of 3 million per year worldwide [23].

Table 1 Disadvantage of permanent metallic implants.
According to recent research survey, the global orthopedic implants market size has reached USD 50.3 billion in 2022 and it is expected to reach USD 72.1 billion by 2030. Rapid increase in road traffic accidents and high velocity trauma cases have significantly raised the demand for orthopedic implants in recent years [24].
Thus it is important to focus on bone fracture treatments by providing most economic, efficient and quality implant materials, which can significantly increase the mental and physical health status of a patient. However healing of bone fractures is a very complex mechanism associated with anatomical, biological, biodegradable and most importantly bio mechanical processes that are essential for developing bone materials.
Present review therefore focuses on highlighting the importance of Mg based materials for temporary implant applications and hurdles for Mg implants to reach ideal implant material status. Literature has indicated magnesium based materials as new generation biodegradable materials that can eliminate limitations associated with use of permanent metal implant materials. Moreover, it is required to focus on some of the major limitations of Mg based materials so that they can evolve as most suitable temporary implant materials.
3. Current trends in magnesium based implants
Biocompatibility and cytotoxicity of any biomaterial should undergo adequate in-vitro laboratory tests in accordance with ISO 10993-5/-12 [25] and ASTM F3268-18a/ASTM G1–03 (E1:2017) [26] prescribed standard regulations as a partial basis of biomaterial registration. In addition to in-vitro laboratory experiments it is highly essential to ensure the biosafety and effectiveness of the developed biomaterials under in-vivo clinical trials. Implant material made of metals such as titanium, stainless steel are inert in nature and may increase the health risk in human body. Due to increase in demand for safe biomaterials for different orthopedic surgery, magnesium based materials have gained tremendous attention from global orthopedic device making industries, as they offer excellent bio-compatibility, bio degradability and also possesses similar mechanical properties as that of bone in both in-vitro and in-vivo conditions.Unlike titanium,stainless steel, cobalt-chromium alloys, magnesium have low elastic modulus (41 GPa-45 GPa) and density (1.74 g/cc) similar to that of natural bone natural bone 1.8 g/cc and moreover magnesium based materials are available abundantly at a reasonable price [27,28]. Currently magnesium based materials are being used as temporary implants that degrades completely in biological environment (in-vivo) and also being replaced by newly developed bone. Thus magnesium based materials eliminates second surgery risk that are usually associated with permanent implant materials.In addition to secondary surgery risk permanent metallic implant demonstrated several disadvantages and adverse reactions. Though Mg implants have several advantages over conventional implant materials, Mg alloys are too expensive than conventional implants made of Titanium and steel [20]. Initial costs of surgery are comparatively higher for Mg implants. However, according to the analysis carried out by Juutilainen et al. [29] bioadsorbable screws made of Mg for fixation is more economical than traditional metal implants when all expenses are considered(initial surgery cost and implant removal cost). Klauser suggested that, use of Mg bioabsorbable screws in treatment of all hallux vegas fracture would save about €9 million in Germany[30]. When secondary surgeries are considered, bio absorbable screws are also cost effective in ankle fracture treat-ments [20]. Table 1 shows the disadvantages of permanent metallic implants.

Table 2 Commercially available Mg implants and their applications.
During 2000–2009 considerable research was conducted on Mg based materials worldwide. Compared to previous studies (before 2000) interest in magnesium based materials has significantly increased to 491% and research interest in developing biodegradable materials for orthopedic applications has also been increased to eliminate secondary surgery needs and to produce cost effective implant materials. Therefore magnesium is considered as the most valuable alloy of 21st century and also considered as the frequent structural material in the world [55]. Table 2 shows list of commercially available and clinically proven Mg-based temporary implants, their current applications.
4. Mechanistic issues of Mg alloys
Mg based implant materials has gained significant attention in the field of biomedical engineering due to its favorable mechanical and biocompatible properties. However, corrosion of Mg alloys remains one of the major mechanistic issues that need to be addressed in order to ensure their successful clinical application. Poor corrosion resistance of Mg based materials in saline and physiological environments are considered to be a threat for Mg alloys as potential orthopedic implants.From the literature it was also established grain refinement is useful in improving the corrosion resistance by alloying or mechanical treatments [59,60]. It has been found that corrosion resistance in aqueous environments such as body fluids is lower than in atmospheric environments. This is attributed to the development of quasi-passive magnesium hydroxide byproducts on the surface, which reduce the corrosion rate. The magnesium hydroxide formed can react with chloride ions to form highly soluble magnesium chloride. On the other hand,Mg can develop a protective passive oxide layer in an atmospheric environment, which can protect some magnesium alloys even in the marine environment [61]. It has been found that high- purity alloys such as AZ91E have up to 100 times higher corrosion resistance than a normal grade alloy . The corrosion of magnesium and its alloys mainly depends on(i) the content of deleterious impurities, namely Fe, Ni, and Cu, (ii) the composition, size, and distribution of secondary phases, (iii) the grain size, (iv) the environment, and (v) the type and thickness of the coating [61].
4.1. Galvanic corrosion
Mg is one of the most active metals and has the least noble in the galvanic series. This makes Mg based alloys very vulnerable to galvanic corrosion. Galvanic corrosion takes place in the presence of impurities or agglomerated cathodic secondary phases in the microstructure. It can also be triggered when the material gets in contact with a superior material inside a conductive environment which leads to indigenous corrosion around the contact area. The influence of secondary phases was found to be reduced by heat treatment that changes the lamellar and spherical secondary phase into finely dispersed precipitates [62,63]. Besides, the inclusion of alloying elements like Mn and Zr was found to enhance the corrosion resistance of Mg-Zn based alloys by dissolving the insoluble impurities such as Fe and Ni in to less reactive phases [64].
Galvanic corrosion, also known as coupled corrosion, normally occurs in physical or electrical contact where two types of metals with different electrochemical potentials interact in an ion-conducting liquid environment [65]. It is one of the major problems to the use of Mg components in chemically active environments. Galvanic corrosion in Mg-based alloys can generally be attributed to poor quality alloys resulting from noble metal enrichment [66,67], poor device design, or inadequate assembly procedures[68,69].Due to the relatively low solubility of elements in Mg, second phase particles can form when the alloy exceeds the solubility limit[70]. Subsequent inter galvanic effects can strongly influence the initial stages of corrosion in Mg alloys [71,72].The growth of precipitate phases directly influences corrosion rates in Mg-based alloys because of limited solubility of magnesium[70]. These secondary phases are normally nobler than pure Mg, causal to the formation of internal galvanic couples that enhance the rate of degradation.
4.2. Pitting corrosion
Pitting corrosion is localized in nature and often affected due to grain size, solution environment, chemical composition, impurities and secondary phase [73]. Mg alloys with finer grain structure have comparatively higher pitting corrosion resistance than the course grain alloys [74]. In general pitting corrosion involves mechanism of oxygen reduction reactions at cathode, however in Mg implant materials pitting corrosion depends on hydrogen evolution reactions [75] and oxygen may not play significant role [76].Researchers noticed that, the secondary phases (βphases) in traditional series of Mg alloys such as AZ, AM, found more nobler than Mg matrix (αphase) [73,77] and could accelerate the corrosion rate of Mg alloys due to micro-galvanic couple effect [78]. Literature has indicated that addition of RE elements can enhance the corrosion resistance due to scavenging effect, which restrict the harmful impurities and transforms them in to intermetallic compounds and thus reduces the detrimental effect on corrosion [79]. The secondary phases in Mg alloys containing RE are more active than Mg matrix and promotes bio-corrosion resistance [73,80]. Secondary phases of these Mg-RE alloys also play significant role in enhancing the strength of Mg alloys [81]. During recent years Mg-RE alloys have gained tremendous attention because of their good corrosion resistance combined with better bio compatibility. Best example for such alloy is commercially available implant material MAGNEZIX® containing Mg-Y-RE- Zr [82]. Apart from MAGNEZIX® RE based alloy Mg-Nd-Zn-Zr was demonstrated with better corrosion resistance properties in clinical trials [83] . In both these alloys such as MAGNEZIX® and Mg-Nd-Zn-Zr presence of Zr is the reason for increased corrosion resistance, since Zr element is considered to be one of the powerful grain refiner for Mg-RE alloy to enhance both pitting corrosion resistance and mechanical properties [84,85]. However, pitting corrosion resistance of Mg-RE alloys developed through primary processes are still unclear and not satisfactory. Therefore several investigators recommended secondary processing of Mg-RE alloys to further enhance the pitting corrosion[86–92].
4.3. Stress corrosion cracking
Mg-based implants perform well and are resistant to failure even when placed in corrosive bodily fluids, especially when under mechanical stress The kind of burden may either be fixed or recurring, and this may result in the complexities of abrupt breaking of implants because of the effects of stress corrosion cracking (SCC) and corrosion fatigue (CF)[93,94]. Typically, SCC is connected to the dissolution of anode and the fragility caused by hydrogen. Microscopic reactions between the second phases found at the boundary of grains and the surrounding matrix may cause anodic dissolution, along with stress corrosion due to inter-granular factors. This is a hypothesis derived from sources [95–99]. Electrochemical thermodynamic activity, surface layer disruption,and accumulation of dislocations at grain boundaries or inside inclusions can all increase the risk of corrosion. Localized corrosion or pitting is frequently present in conjunction with this. According to the process of hydrogen embrittlement,the matrix has the ability to absorb hydrogen produced during anodic dissolution, which can then help cracking. Transgranular stress corrosion cracking is frequently attributed to hydrogen interactions [100,101]. The precise role of hydrogen in the stress corrosion cracking of magnesium alloys has not yet been fully understood. New research has shown that most of the hydrogen was made up of the corrosion products and not the matrix. CF is type of metal deterioration,is caused by the accumulation of cyclic loads in conditions of high strain. The outcome is caused by the combination of localized chemical or electrochemical reactions and permanent cyclic plastic changes. In the interim, it has a notable impact on the longevity of metal implants. Especially when used frequently, equipment designed for orthopedic or cardiovascular purposes needs to have better resistance against corrosion fatigue. The lifespan of implants can be greatly impacted by corrosive phases. Kannan et al. assert that the creation of passive coatings can decrease the initiation of cracks as well as their propagation [102]. Also, Jafari et al. suggested [103] that the formation and spreading of CF cracks,which commonly start in pits, are influenced by the electrochemical conditions and the amount of applied stress. Therefore, enhancing the ability to resist pitting is of utmost importance in the augmentation of CF resistance of Mg based alloys[104].
5. Current status of clinical applications of Mg implants in animal and human body
Fig. 3 shows potential applications of Mg implants in animal mode [9]. In most of the cases, Mg alloys were used as pins, screws and plates. Majority of these animal models were tested to observe degradation behavior and biological response of peri–implant bone tissues [105–107]. Compared to traditional implants Mg based implants demonstrated better results and significantly promoted recovery/healing of bone fracture without affecting the biological and bio efficacy properties. The history of Mg alloys dates back to almost a century. Germany[82], South Korea [108] and China[109] were the first countries to conduct clinical trials on Mg alloys. First implant was introduced in hallux valgus surgery using MgYReZr alloy screw. This was the first implant in human body produced by Germany. Fig. 4 shows the use of Mg screws in different parts of the body [9,82,108]. Mgbased implants were further successfully developed and introduced in a series of clinical trials owing to their superior biocompatibility [9]. Table 3 summarize some of the recent application of Mg alloys in both human and animal modes.
From the Table 3, advantages of Mg implants to tissues growth can been fully validated through clinical indications obtained by different tests such as computer tomography(CT), histological analysis, X-Ray analysis and fluorescent imaging in both human and animal modes. Compared to traditional implant materials Mg based implants found to promote bone fracture healing significantly by maintaining both bio efficacy and bio safety. In accordance with the in vivo studies it has also been noted that, Mg ions released from the Mg based implant materials during in vivo degradation may potentially prevent collapse of femoral head. Mg based implants proven to provide a strong mechanical strength to potentially support the fractured bones during initial stages of healing, such as inflammatory phase and soft callus phase.However, in order to further accelerate transitional research on novel Mg implants, clinical use of Mg based implants requires modification in the process, composition and advanced surface treatments to reduce degradation rates and hydrogen release rates. Currently these two issues are considered to be the major concerns.
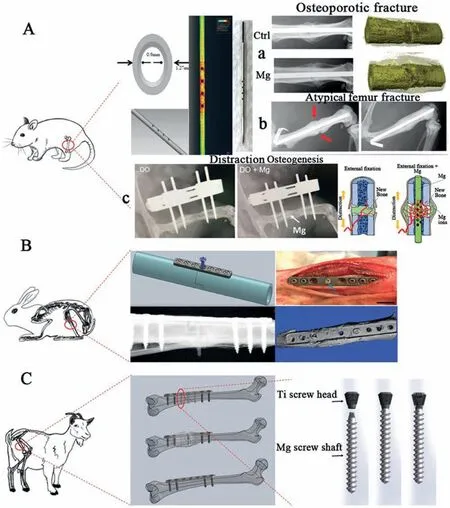
Fig. 3. Animal modes of implants made of Mg alloys: (a) Rat model, (b) Rabbit Model and (c) Goat Model [9].
6. Challenges for Mg based implants

Fig. 4. Use of Mg screws in human body: A. hallux valgus fracture, B. distal radius fracture and C. fixation of bony flap (Hip) [9,82,108].
From the literature, it has been observed that in most of the implant cases Mg alloys were used as the screws,pins and fixation plates. Magnesium plays crucial role in forming new bone with comparatively lesser degradation rate. Magnesium based implants eliminates second surgery as Mg-based implant materials disappears completely over the time.No major reactions were observed during healing process. No patients were reported with swelling near joints, bone destruction and high osteolytic abnormalities, which are common in implants made of steel, titanium and chromium based materials. However,few literature have also indicated partial success of magnesium based alloys in several implant applications (both invitro and in-vivo). Degradation rate and biocompatibility still appears to be deficient for in-vivo applications which is not recommended for ideal biodegradable implant.Though significant research has been carried out on improving corrosion resistance, some of the alloying elements and impurities present in magnesium lead to increase corrosion resistance. Increase in corrosion resistance can be attributed due to low solubility of some of the alloying elements in magnesium [61,129]Even small percentage of some of the alloying elements such as iron, nickel and copper can significantly increase corrosion rate. On the other hand corrosion rate also increases degradation rate which in turn increases pH value in body fluid and creates unnecessary risk to the patient’s health. [130]. In body fluids,Mg alloys degrade with different corrosion modes suchas through micro-galvanic and pitting corrosions. This leads to formation of hydroxides, oxides and H2. Especially formation of hydrogen bubbles between the implant and damaged bone interface cause delay in bone healing process [131,132]. Fig. 5 shows X-Ray images after implantation (in-vivo) of as-cast and extruded Mg–Sr alloy. The X-ray image clearly indicates formation of bubbles after 4 weeks and 8 weeks of implantation [133].
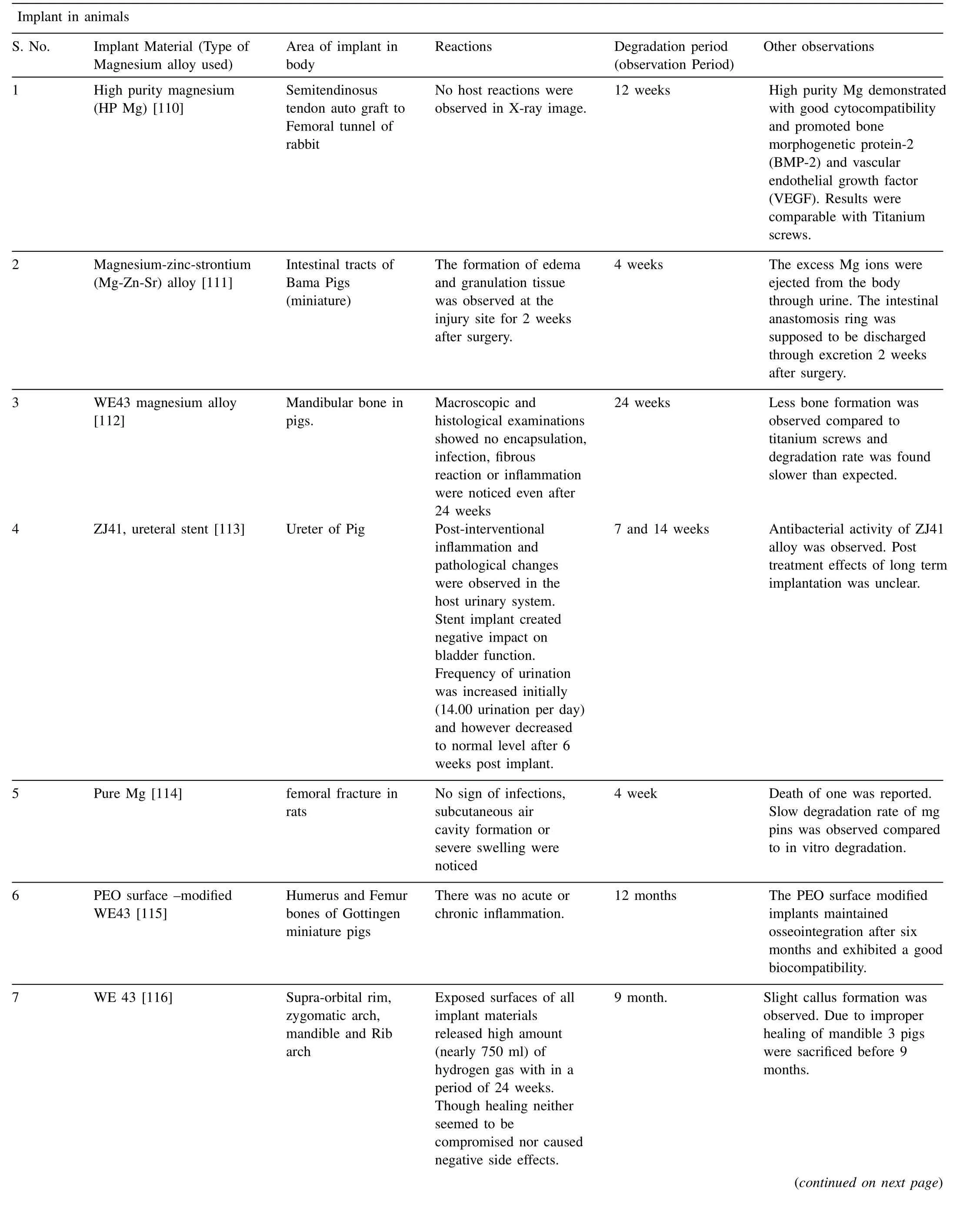
Table 3 Magnesium based implants in animals and human body.
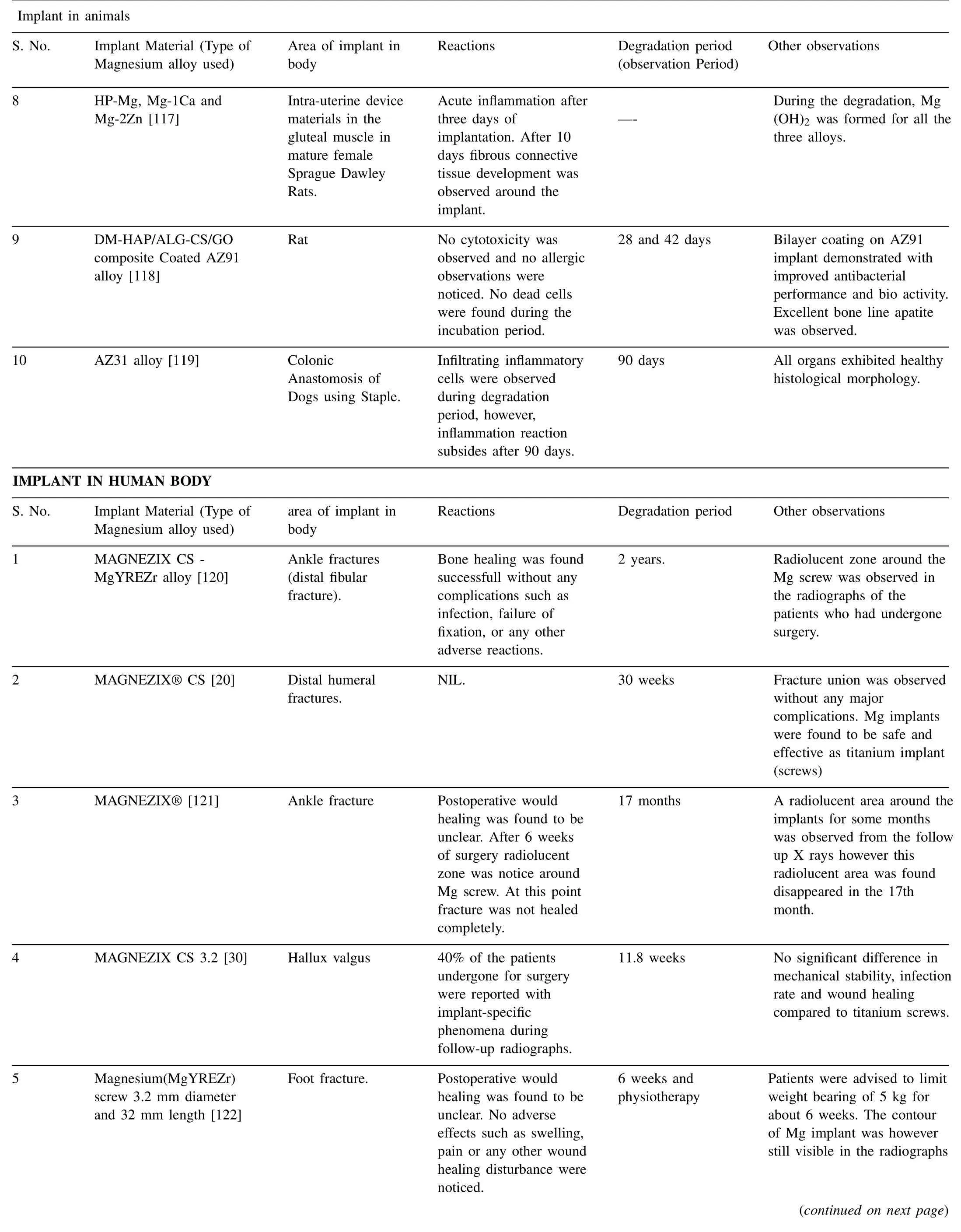
Table 3 (continued)
Table 3 (continued)
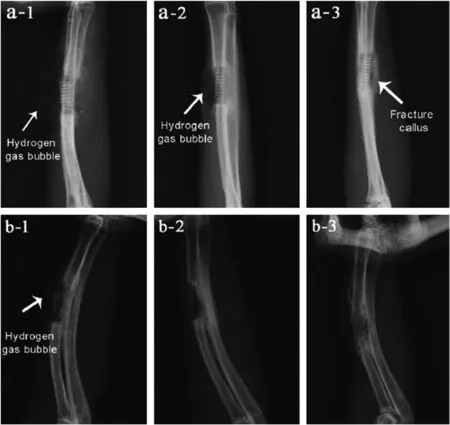
Fig. 5. X-ray image after implantation: a) 4 weeks, b) 8 weeks. 1. As cast alloy 2. As extruded alloy & 3. Cast alloy with coating [133].
Majority of the in-vivo studies have indicated that formation of hydrogen bubble is the major concern for magnesium based materials.When magnesium plate was used in first clinical application it was removed immediately after implantation as extensive gas cavities, heavy pain and swelling was noticed [134]. Therefore it is very essential to focus on elimination of hydrogen bubbles through proper selection of alloying elements of magnesium and researchers must be aware of unintentional introduction of contaminants during the manufacturing of magnesium implant materials such as copper,nickel, iron and cobalt as these impurities lead to substantial increase in degradation rates which in turn increases corrosion rate leading to accelerated formation of hydrogen bubbles. However, limiting the percentage of impurities below their tolerance limit, will significantly reduce the corrosion rate as well as accumulation of hydrogen bubbles. Following are some of the important observations that need be considered while selecting primary manufacturing methods for magnesium based implant materials.
1. Crucible and stirrer made of steel contains small percentage of nickel and iron may contaminates magnesium melt[135,136].
2. Adding aluminum to Mg decreases the iron tolerance limit[137].
3. Addition of impure aluminum in magnesium melt may introduce copper as a impurity [138].
4. Exemption of materials containing iron and nickel to make dies, stirrer and crucible [139].
5. Even small amount (<1%) of Mn can effectively reduce the inclusion of impurities of heavy metals such as Fe, Ni and Cu [140].
6. Refining the flux to eliminate various metal and nonmetallic inclusions in magnesium melt [141].
7. Blowing purification (Non-flux purification) to remove H2inclusion by introducing a gas into Mg melt [142].
Along with the above considerations, researchers should exploit different methods that can eliminate iron, nickel, copper, cobalt and other metal & nonmetallic impurities from the magnesium melt and increase the corrosion resistance. If it is impossible to eliminate these impurities, tolerance limits are suggested. Table 4 shows tolerance limits for few magnesium alloys in binary composition with Fe, Ni and Cu.
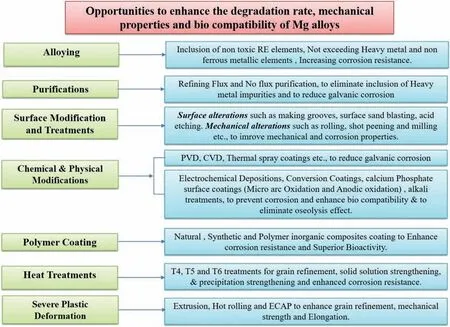
Fig. 6. Opportunities for Mg implant materials.
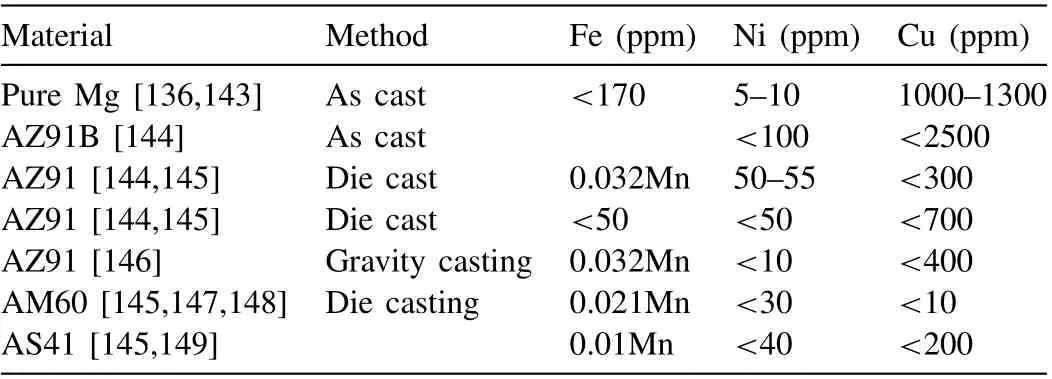
Table 4 Tolerance limits for magnesium based materials.
7. Opportunities and future prospects
There are numerous approaches available for researchers to overcome challenges associated with the Mg based materials for orthopedic applications. During past decade, extensive research was carried out on various methods such as alloying, melt purification, surface modifications, mechanical alterations, chemical & physical modifications through coatings,heat treatment, plastic deformations etc., to regulate/enhance the degradation, mechanical and bio-compatible properties of Mg alloys.Fig.6 shows the current challenges and opportunities available for Mg alloys to become ideal implant materials for implant applications. In the present section, a brief review on a aforementioned approaches has been made and advantages and opportunities of the same are discussed.
7.1. Alloying
Mg and Mg based alloys are generally used in three major groups: the 1st group constitutes of pure Mg; the 2nd group containing aluminum (Al) such as AZ31, AZ91 alloys and rare earth elements (RE) namely AE21alloy and the 3rd group consists of Aluminum free alloys such as WE, Mg-Ca,MZ and WZ series alloys. Researchers have further classified alloying elements into 3 groups namely Toxic, Allergic and Nutrient elements [150–152]
(1) Nutrient elements: Ca, Mn, Zn, Sn, Sr.
(2) Allergic elements: Al, Co, V, Cr, Ni,Ce, La,Cu,Pr
(3) Toxic elements: Cd, Be, Pb, Ba, Th
Based on previous studies, alloying elements such as Ca,Al,Y,Li,Mn,Zr,Zn and RE in Mg can significantly enhance the physical and mechanical properties of the Mg by: 1) grain size refinement 2) Enhancing the corrosion resistance 3) Formation of intermetallic phases that can improve the strength and 4) Ease of manufacturing and shaping of Mg alloys.
Nutrient elements such as Ca, Mn, Zn, Sn and Sr are vital trace elements for human body and Few RE elements with anti-carcinogenic properties can be the better choice for incorporation into Mg. Inclusion of these nontoxic alloying elements significantly improves the mechanical stability and degradation rate of magnesium based implants [153,154]. Ca plays crucial role in wide range of physiological functions such as oocyte activation, building strong bones, transmission, fluid balance with in cells etc., [155]. Addition of Ca significantly increases the grain size of AZ91 Mg alloy. Ca also reported with a spheriodizing theαphase in the alloy matrix [156]. Mn is added to many commercial alloys to improve corrosion resistance and decrease the harmful effects of impurities [61]. Addition of Zn has a positive impact on performance of Mg. Zn is one of the chief secondary elements in majority of the Mg alloys. Zn in Mg leads to increase yield strength from 1 to 6% due to its better solubility with Mg [157]. In order to balance the strength and corrosion behavior of Mg alloys it is recommended to limit Zn to 4wt%[158–160].From the previous studies it was indicated that,Sn in Mg has numerous advantages such as improved castability,reduces die sticking during melting of Mg-Zn-Al and Mg-Al-Mn Mg alloys, flow ability (when maintained between 0.3%to 0.5%) and reduces casting cracks (for>0.2 wt.%). Other very important alloying element for Mg is Strontium (Sr).Inclusion of Sr to Mg-Zn alloys have demonstrated with better biocompatibility and bio activity with comparatively lower degradation rate. Sr is known to be a bone-seeking element,which can stimulate new bone formation without affecting the mineralization of bone. Sr helps in protein synthesis, stimulating replication of bone cells, depressing bone resorption,increasing strength and density of bones and inhibiting osteoporosis [161]. Researchers have varied Sr wt.% in Mg-Zn,Mg-Zn-Ca alloys from 0.1 to 1 wt.% and suggested inclusion of smaller amount of Sr in Mg alloys to accelerate bone tissue growth during degradation. However the optimal percentage of Sr in mg alloys remains unclear.
Mg is possibly an ideal biocompatible implant choice because it is nontoxic to humans, however, it is important to thoroughly research how long an Mg-based alloy may be used safely. For instance, after being implanted into a number of rabbit models’ femurs, AZ91 alloy rods deteriorated and replaced by new bone tissue after three months [162,163].Most of the alloying elements, including Al, would have been released into the rabbits’ bodies by the time this degrading process was completed. Although the long-term health repercussions on the rabbits are unclear, the release of Al into the body will have adverse impacts on human health[164]. Al is a neurotoxin (human) and has been linked,through long-term build up in brain tissues, to neurological conditions such Alzheimer’s disease, dementia, and senile dementia [165].
Investigations by Song have recommended that very small amounts of RE elements and other alloying metals for example Mn and Zn can be tolerated in the human body and can also enhance corrosion resistance [166]. Mg alloys comprising rare earth elements have also been found to increase the resistance to the diffusion of Mg2+ions out of the Mg matrix via the Mg oxide layer [167]. Marva et al. investigated impact corrosion behavior of commercially available Mg-Zn-Zr-RE alloys i.e. ZE41 (contains 0.41 wt.% Ce, 0.12 wt.% La, 0.11 wt.% Nd) and EZ33 (contains 1.16 wt.% Ce,0.47 wt.% La, 0.26 wt.% Nd) under simulated physiological conditions. In vitro biocompatibility is evaluated by indirect cytotoxicity tests using NIH3T3 cells. Immersion test indicated that, corrosion resistance of EZ33 was found 3 times greater than ZE41 at shorter immersion times.And both alloys possessed microstructure with T–Phase precipitates along the grain boundary with Zr-rich precipitates in the middle of Mg grains. Due to higher volume fraction of RE in EZ33, more continuous and interconnected T-phase precipitates were observed along grain boundaries and thus increased corrosion resistance. Fig. 7 shows microstructure of as received ZE41 and EZ33 Mg alloys [168]. The main differences between microstructures of two alloys are the morphology and distribution of both T-Phase and Zr rich precipitates. T-phase in EZ33 (Fig. 7d) is comparatively thicker than ZE41 (Fig. 7b)due to higher wt.% RE in EZ33 alloy (7.09% v/s 4.96% in ZE41). However ZE41 reported with better yield and ultimate strength with double tensile elongation. Post-immersion test both alloys experienced loss in mechanical strength. Cytotoxicity results indicated EZ33 was comparatively more biocompatible compared to ZE41.
Witte et al. recently studied the degrading behavior of polymer-based control rods (poly (lactic acid)) and Mg-based alloy rods in animal models. Guinea pig femurs were implanted with 20 mm length, 15 mm diameter rods, and the deterioration profile of the rods was tracked. The Mg alloys under investigation were 3% Al and 1% Zn (AZ31) and 9%Al and 1% Zn (AZ91) with the remaining alloys being made entirely of pure Mg. In addition, two RE containing alloys were researched: WE43, which had 4% yttrium and 3% of a rare earth combination made up of neodymium, cerium, and dysprosium, and LAE442, which contained 4% lithium, 4%aluminum, and 2% of a rare earth alloy made up of cerium,neodymium, lanthanum and praseodymium [5,169]. The implants were removed at 6 and 18 weeks. Radiographs were routinely acquired during this period, and the deterioration of the implant was studied using a micro-tomography-based approach with X-ray synchrotron radiation. All Mg-based alloy implants were shown to be advantageous and encouraged the creation of new in situ bone tissue, whereas the impact of the polymer control rods was less pronounced. All the alloys exhibited comparable, but lower values of corrosion resistance whereas the LAE442 alloy exhibited the highest corrosion resistance. However neodymium and yttrium has limited solubility in Mg(Nd,3.6 wt.%and Y 11.4 wt.%)and apart from WE43 series,Mg contains Gadolinium(Gd)has reported with better solubility of 23.49% which is higher than commonly used RE elements in Mg alloys. Mg containing Gd is considered to be the most promising ultra-high strength material with better thermal stability, toughness and formability [170].In addition to solubility, mechanical properties of Gd-Mg alloys can be altered in wide range through solid solutioning and aging [171]. Gd addition to Mg slow down corrosion rates in in vivo conditions due to scavenger effect (effect of RE on Mg impurities such as (Fe, Ni and Cu) [172,173].Bogdan Istrate et al. [174] in their studies varied Y percentages in Mg contains 0.5% Ca. Y was varied at 0.5%–3.0%.From this study it was observed that, increasing the percentage of Y more than 1% leads to increase in electro-corrosion.2%–3% increase in Y resulted with improved biodegradability with lower degradation rate and better cytocompatibility.From the corrosion point of view, Y significantly influences on corrosion resistance when>1.5%. Cytocompatibility was found more than 70% for all compositions. Authors recommended researchers to further increase of Y wt% to explore the cytocompatibility and corrosion resistance.
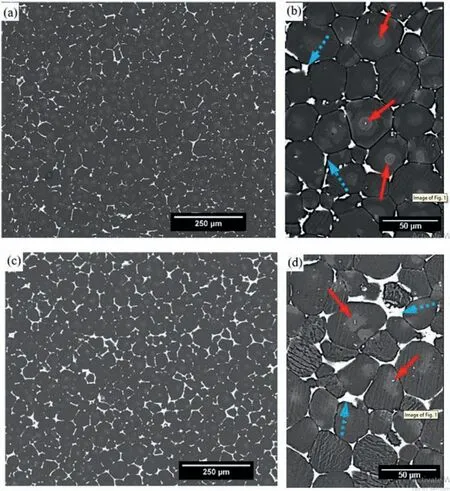
Fig. 7. Micrographs of as received (a, b) ZE41 and (c,d) EZ33 [168].
Additionally, administering RE elements including cerium,praseodymium, and yttrium to rats caused significant liver toxicity [175]. Due to their (RE elements) capacity to create stable complexes and interfere with the regular molecular processes of enzymes,DNA and proteins,utilizing heavy metallic elements as alloying components is also potentially hazardous to the human body[13].To mitigate potential toxic effects,researchers and manufacturers take measures to minimize REE release from Mg implants. This includes optimizing the alloy composition, surface treatments, and coatings to reduce corrosion and control the release rate of REE ions.
It is unquestionably important to choose alloying elements that are nontoxic to humans. Considering the toxicity of Al and RE elements in Mg, Dong et al. developed AL-RE free Mg-Li alloys for stent application. Mg containing 8.5% Li was extruded to a dimension of 3.2 mm (outside) and 2.5 mm (inside) tested for invitro and in vivo degradation and biocompatibility.Developed stent indicated with decent degradation rate (< 0.15 mm/y) in invitro condition. However addition of Li resulted in significantly higher degradation rate(>0.6 mm/y) in in vivo conditions [176]. Nontoxic alloying components such as Ca [5] and Zr [150] have the potential to dramatically increase the Mg alloy’s corrosion resistance and decrease its degradation rate, making it a feasible implant material [177]. Ongoing research on adding Ag and Cu in Mg has indicated better antibacterial resistance and better anti-biological properties [178,179] and Mg containing La reported with anti-cancer and anti-tumor properties[180].Addition of Ag and Cu suppresses the bacterial infection rate of Mg alloys by releasing metal ions that kills bacteria [181].Treating bacterial infection is of the foremost concerns for implants in biomedical application since a bacterial infection may have an adverse effect on the bone repair process [182].However,addition of Ag and Cu increases degradation and researchers have suggested to obtain homogeneous distribution of Ag to enhance the corrosion resistance and more detailed in-vivo trials should be carried out concerning biocompatibility of Mg implants.
Form all these observations it is important to note that the selection and concentration of alloying elements in Mg alloys should be carefully considered to achieve the desired balance of mechanical properties, corrosion resistance, and biocompatibility. The specific application and requirements of the Mg alloys will determine the optimal alloying elements to be incorporated. Extensive research and testing are ongoing to develop Mg alloys with improved properties for various biomedical applications, including orthopedic implants and cardiovascular devices.
7.2. Melt refining and purification
Majority of the Mg alloys used in orthopedic applications are produced through casting route. Casting remains one of the dominant production routes for producing net shape Mg alloys, representing 98% of overall structural components[183]. Therefore melt refining and purification plays significant role in production of quality Mg alloys for orthopedic applications. Purification of Mg melt can be done in 2 ways, either by flux refining method or by non-flux purification method. Refining the Mg melt under protection of flux offers numerous advantages such as degassing and protection from self-ignition, oxidation and in avoiding inclusion of reactive elements by forming protective oxide films [184–187].From the perspective of effectiveness of melt refinement and elimination of CO2emissions, flux refining method is convenient and cost effective. Flux material with variety of chemical compositions have been used in casting process such as MagRexR○[188], RJ-2 [189], carnallite flux [190], salt flux[191], JDMF refining flux [192], JDMR-1,2 [193] and flux containing varying amount of chlorides, fluorides and oxides[194]. Selection of flux materials mainly depends on alloying elements. As an example, RE elements such as Y and Gd react with flux containing MgCl2and results in reduction of RE.In these cases in order to suppress/compensate loss of Y and Gd, special refining agents should be used with RE elements such as YCl3and GdCl3[195]. However use of these protective flux agents still produces some sort of harmful gasses and thus oxidation is unavoidable.
Another popular melt purification is blowing purification in which instead of using flux, gas is used mainly to remove H2and to adsorb bubble rise during melting. Non-flux blowing purification offers many advantages such as ease of operation,effective removal of inclusion of H2&other impurities[196].Argon is generally used as a gas and the efficiency of this method strongly depends on stability of gas, size of bubble formed,gas flow rate,aeration time and melt temperature etc.,Wu et al. investigated influence of blowing parameters on purity of AZ91 Mg alloy, in which impurities were adequately removed when melt temperature was maintained at 740 °C,gas flow rate at 2.0 L/min and aeration time at 30 min. From this purification methods,AZ91 Mg alloy obtained highest ultimate strength of 183.9 MPa and elongation of 3.54% [196].However,it was suggested to increase the temperature of melt and blowing speed of rotator since low melting temperature led to increased impurities. Zhang et al. in their study eliminated limitation of flux protection method, i.e. loss of RE alloying elements in Mg due to reaction between flux material and RE. Loss rates of magnesium, alkaline and RE found to decrease by 8.3%, 10.1% and 32.5%, respectively. Overall loss of alloy was decreased by 11% and formation of slag also significantly reduced.This result was obtained when flow rate of gas was maintained at 450 N·cm3/s under top-blown argon agitation method. The method of top-blown argon agitation refining is found to be the better method to refine Mg alloys as this method has shown promising results with significant energy saving. However, cautions must be taken while selecting the blowing parameters. If pressure of injection is too high and bubble ascent velocity is very high, splashing of melt may take place while gas is escaping and it may damage the protective film on melt surface and if the gas flow is high and bubble flow rate increases, the benefits of degassing effect will be reduced due to reduction in contact area between the bubble and melt [197]. Due to limited studies available on optimizing the process and process parameters of melt purification methods,there is lot scope for researchers to explore an effective method to improve the quality of magnesium alloys.
Apart from flux or non-flux purification, literature has also witnessed purification of Mg melt by adding RE elements to melt. Addition of very small amount of RE elements purifies the Mg melt by combining the impurities (Fe, Ni and Cu)and deposit it to the bottom of melt [198]. Mercer and Hill et al. discovered that, RE such as Ce, La, Nd and Pr can trace harmful impurities (Fi, Ni, Cu) and decreases cathode activity by forming intermetallic compounds [199]. This can be attributed to scavenger effect of Mg due to addition of RE elements to the melt [79]. It is believed that addition of RE in very small percentages to Mg melt combine the impurities in Mg rapidly, deposit it to the bottom of melt and will not allow impurities to exceed tolerance limit [198]. REE addition not only segregate impurities but also significantly enhance the corrosion resistance of Mg alloys. However researchers should be aware of solubility limit and toxicity of RE elements. The solubility limit is typically expressed as a maximum percentage by weight of the REE in the alloy.For most REE, such as cerium (Ce), lanthanum (La), and neodymium (Nd), the solubility limit in Mg alloys is typically less than 5%. This means that the total weight percentage of REE in the alloy should not exceed this limit to ensure that the REE can be fully dissolved in the magnesium matrix.
7.3. Surface modifications and treatments
Due to the high deterioration rate of Mg and Mg alloy implants in the physiological medium, the mechanical integrity of the implant would decrease before the bone tissues had time to recover [200]. There are two ways to lower the deterioration rate; the first was covered in Section 6.1 and includes alloying magnesium with biocompatible elements that can withstand the corrosion process. The second technique,which is covered in this section, involves altering the implant’s surface properties through a procedure that creates a protective barrier against corrosion. For ideal implant materials, the healing or regenerating processes of bone and other related bodily tissues are a crucial consideration and that must be made before any surface therapy is done. Researchers divide bone healing into 3 processes, i.e. inflammatory, reparative, and remodeling [201].
The initial inflammatory phase, which normally lasts 3 to 7 days, is the immune system’s natural response to the biomedical device or implant in the body. During the reparative phase, which usually lasts 3 to 4 months, the implant is integrated with the newly formed and regenerated tissues.The final stage, which is the lengthiest, might take years to complete [202]. The rate of degradation is the major problem associated with Mg based implant materials and thus needs to be slowed down to allow for the healing process complete and once the wound area is healed, new tissues will be formed to provide their support before the implant’s structural integrity is destructed. It must take place for at least 12 weeks to occur [200]. The ideal biodegradable implant materials must have good wettability for adhesion of cells on the surface and dissolve in the body after performing necessary bio functions. Therefore, surface modifications of implant materials and therapies may have a significant role in deciding how rapidly the implant deteriorates. Various surface modification techniques can now be used to alter the surface characteristics of biomaterials. Many of these strategies have been used to increase the corrosion resistance and bio degradable properties of Mg alloys. Moreover, these mechanical alterations do not introduce foreign materials and contributes to the surface features of Mg alloys without affecting their bulk properties [203]. A brief overview of a handful of these surface modification methods is discussed in the following sections.
7.3.1. Surface roughness
The surface features of any implant material plays vital role in deciding initial response of the surrounding tissues after the surgery. Most of the time, the effectiveness of the integration between implant material and tissue is determined by the surface characteristics of the implant material. Literature has witnessed some of the surface alterations that resulted in growth of surrounding cells and integration with implant material that includes surface alteration by means of surface roughness, creating grooves, columns, pits and other types of depressions [204,205]. These alterations have shown superior cell adhesion and encourage more cell proliferation in Ti alloy implant materials [206]. Features such as roughness provide beneficial mechanical locking for implants for initial stages of cell adhesion [207]. During past decade extensive research has been carried out on different Mg alloys to verify the effect of various surface morphological profiles on to response of cells. However, to date how tissues will react to surface alterations in biological environment is still unresolved for Mg implant materials.
Zhou et al. investigated impact of surface gradients on bio efficacy of Mg vascular implants (in-vitro). Surface gradients on Mg-Nd-Zn-Zr Mg alloy with uniform roughness(both micro scale and nano scale) was created and immersed into 0.25 mol/L citric acid solution. Post chemical treatment specimens were subjected to thin coating (GR-MgF2-PDA).It was observed that, increase in surface roughness of implant material from nano scale to sub micro, micro texture significantly promoted endothelial cell’s proliferation but affected the degradation rate. Optimum surface roughness was identified in micro-scale ranging from 1.0 μm to 2.0 μm .Authors suggested to tailor the surface morphology features to enhance endothelialization and enhance bio-efficacy of Mg implant materials [208].
In another study, Singh et al. reported the impact of soda lime-shot blasting (SBSL) of AZ31 alloy on surface roughness and bioactivity (in-vitro)[209]. Initial surface roughness was maintained at 6.23 μm ± 0.86 μm. No surface coating was made on AZ31 alloy post SBSL. Micro hardness of SBSL Mg alloy increased by 19.5% with increase in corrosion resistance. Surface roughness of AZ31 alloy was refined by SBSL technique to 1.98 μm which resulted in excellent cell adhesion and growth characteristics during 3rd and 5th day of implant surgery.
Recent two important methods of surface alterations include ball burnishing and laser surface melting. Unlike earlier traditional methods,no material is removed in these processes from the surface of the implant materials. Ball burnishing method looks similar to conventional machining process but produces a uniform work hardened surface. Implant surfaces are altered by means of creating depressions on the surfaces using rotating balls to induce plastic deformation. As the material gets deformed plastically the altered or burnished surface gets work hardened and the micro hardness increases[210]. Although, the ball burnishing method is being applied in automotive and aerospace industries since several years,it is new in the field of biomedical applications. Especially for Mg based materials limited studies are available. From the available literature, ball burnishing method has proven to be one of the better methods of surface alterations by offering several advantages such as improved surface roughness,improved micro hardness, and improved corrosion resistance with more uniform corrosion pattern.However,functional performance of ball burnished Mg alloys should be systematically evaluated in biological environment to access the functional performance.
Laser surface melting is one the important surface modification techniques in which surface of the implant material is altered by melting using different laser densities. Here also no material is removed or no chip is formed during surface alterations, but the properties of the surface is altered by uniformly refining the microstructures, increasing homogeneity by rapid solidification under the different intensities of laser beam [211]. So far few works have been reported on laser melting of Mg alloys such as AM60B [212], AZ31[213], WE43 [213], AZ61 [213], and AZ91D [214] to evaluate the corrosion resistance and generally CO2laser has been employed for surface alterations. Homogeneous distribution ofβ-phase with refined grains, enriching of alloying elements, microstructural transitions and enhanced corrosion resistance were some of the favorable advantages reported. In-vitro studies also indicated better surface wettability, cell adhesion, high degree of homogenization and fine microstructure due to increase in laser energy density of Mg alloys [215]. The drawbacks of Laser melting include, severe pitting mode of corrosion and discontinuous corrosion layer under lower energy density conditions of Laser. Since very few works have been reported on laser melting of Mg alloys for surface modifications, it is difficult to draw conclusions on the properties obtained in in-vitro conditions. As indicated in literature, researchers can focus on optimizing the laser energy densities for different range of Mg alloys for better understanding of corrosion resistance and biological response.
Additionally, the impact of mechanical alterations such as mechanical processing techniques for Mg alloys has the ability to significantly affect surface and subsurface characteristics of implant materials [216,217]. Machining and mechanical working such as rolling, drawing, extrusion, forging etc.,are a few examples of such mechanical processing techniques.Machining involves removal of the material from the surface to shape the medical implants. Machining has a great influence on surface and subsurface of the implant material. Friction induced during machining may influence the corrosion rate. Therefore achieving the defined subsurface properties of implant materials through proper machining process is decisive. In the instance of milling, the surface produced by honed cutting tools tends to generate a rougher surface than those with sharp cutting tools at moderate cutting speeds.Additionally, it is unclear exactly how metal chip removal during milling and related procedures affects the sub-surface below [218], whereas chip removal from the surface during machining can directly affect the surface topography [219].Traditional machining and milling can significantly affect the surface properties of Mg implant materials. Most importantly when using traditional machining, magnesium chips tends to ignite due to their high flammability. Literature has witnessed ignition risk of Mg alloys when contact temperature of tool and Mg alloy reaches to 450 °C during turning and milling processes and even some cases are reported with fire hazard[220,221]. Generation of high temperatures can significantly affect the properties of implant materials and can affect the corrosion resistance. Therefore, researchers are now focusing on use of non-traditional machining (NTM) processes such as Electric Discharge Machining (EDM), Laser Beam Machining (LBM) and Water Jet Cutting (WJC). EDM eliminates mechanical stresses, chatter and vibrations during cutting as there is no direct contact between tool and work piece. EDM is considered as one of the most suitable methods for machining profiles with high aspect ratio [222]. However, toxicity was observed in EDM Mg implant surface [223] and that should be eliminated by optimizing the process parameters of EDM. LBM is one of the most preferred methods to create micro and complex geometries in vascular stent materials[224].One major challenge associated with LBM is poor surface integrity due to generation of high heat. As LBM is subjected to high energy plasma it may cause thermal damage in the form of HAZ (Heat Affected Zone), oxide layers and micro cracks in Mg alloys and resulting in poor corrosion rates [225–227]. WJM offers numerous advantages for implant materials over other NTM processes such as no detrimental effects on the machined surface as this process do not involve chatter and heat and is flexible, inexpensive, versatile and environmental friendly [228,229]. When used with abrasive particles, abrasive water jet cutting can significantly increase the machining capability of target materials[228]and most importantly unlike other NTM process such as WEDM and LBM, AWJC offers risk free machining for Mg based materials [230].
In addition to using machining methods to remove chips,rolling processes can also produce strong passive pressures that act normal to the surface and cause the subsurface to harden. The compressive forces created during rolling alter the sub-surface grain structure,which has a substantial impact on the surface’s micro-topography[231].An Mg-Ca alloy that was deep rolled exhibited much lower corrosion rates than an alloy that was machined, according to a recent research by Denka et al. (corrosion studies showed a factor of 100 reduction in corrosion rates) [232]. Another benefit of having residual compressive stresses after rolling is that it prevents micro cracks from forming from crack nucleation spots already present in the substrate. The improvement of the fatigue life cycle of a material being examined for biomedical applications depends in part on the suppression of fracture development [232,233].
Von Der Hoh et al. [234] recently looked at the significance of surface and sub-surface treatments on Mg alloy implants. Three geometric types of samples were used on Mg-Ca (0.8% wt calcium) alloy during their investigation.Three various geometric sample types were created using the alloy. The first test sample was a smooth 3 mm-diameter machined cylinder, while the second was similar to the first but had its surface topography changed to a threaded cylinder and the third was threaded and sandblasted for 30 s with particles ranging in size from 300 m to 400 m. The smooth cylinders were machined without applying any further surface treatments, therefore the cutting tool’s microsurface topography was preserved. The smooth cylinders reported with good integration with the surrounding tissues and showed the least structural degradation after six months implantation in adult New Zealand white rabbits. The threaded cylinders fell somewhere in the middle of these two extremes, whilst the sand-blasted cylinders saw the most material loss with the original cylindrical form entirely destroyed. The outcomes showed that the test samples having rougher surfaces encouraged greater degradation rates, but the cylinders’ smoother micro-topographic surface characteristics were acceptable for resorbable Mg alloys. The findings of this investigation made it abundantly evident that variations in the test materials’ surface roughness may have a major impact on the rates of in-vivo degradation. The findings also made clear the necessity for more research into how various surface alterations affect other biocompatible Mg alloys.
7.4. Deposition or coating techniques
From an engineering perspective, isolating the metal from the environment by coating the metal component with a protective barrier is the most efficient technique to avoid corrosion. The protective coating must be consistent, firmly attached, and free from flaws like pits, scratches, and cracks in order to be effective against corrosion. As it was already indicated, the main issue with magnesium is its reactivity when exposed to air or an aqueous environment, which causes an oxide/hydroxide layer to develop over the metal surface. The coating’s ability to adhere to the metal surface and produce a homogenous protective layer will be negatively impacted by the existence of the oxide/hydroxide layer. In order to achieve a successful surface coating, the metal surface must first be cleaned and given the proper pre-treatment. Surface coatings play a significant role in improving the corrosion resistance of Mg alloys by acting as a physical barrier between the substrate and physiological media without disturbing grain morphology. However these protective coatings do not enhance the mechanical properties of the implant materials but efficiently minimizes the degradation of Mg implant materials and extend the implant life [235,236].
Surface deposition on Mg alloys can be obtained mainly by five methods such as physical, mechanical, thermal, biological and chemical methods [237] and further classified into numerous groups based on conversion and deposition techniques. Physical deposition methods include plasma spraying[238], radio frequency magnetron sputtering [239], Ion beam assisted deposition (IBAD) [240] and pulsated laser deposition (PLD) [241]. The alterations of surface using either mechanical or thermal or sometimes combined methods are categorized under physical depositions. Depositions involving biological methods such as self–healing biomimetric coatings,bio-inspired functional surface coating and molecular recognition methods are classified under biochemical coatings [242].Lastly chemical coatings, one of the most popular and most investigated coatings for Mg implant materials are further classified into conversion and deposition coatings. Conversion coating involves oxidation of substrate surface resulting in formation of either oxides or hydroxide layers which serves as a barrier against corrosion. Deposition coating sometimes refers to non-conversion coating involves depositing thin coatings on Mg alloys by means of different physical vapor deposition (PVD), magnetron sputtering, electrophoretic and cold gas dynamic spraying. Apart from these well-known techniques, researchers have conducted and developed umpteen methods on advanced coating on Mg based implant materials to fulfill the bio-functionalities. Still selection of appropriate method of coating is challenging to meet the diverse biological requirements. Fig. 8 shows detailed generic classification of coating methods [242]. In forthcoming sections, challenges and opportunities associated with some of the popular and latest conversion and deposition coating methods are discussed.
7.4.1. Micro arc-oxidation (MAO)
So far, many approaches have been made to coat bio materials using variety of coating techniques. Among these approaches, MAO or plasma electrolytic oxidation (PEO) is one of the most trusted and efficient approaches for biomaterials, since MAO reduces initial degradation rates up to 90%throughout the healing period [243,244]. Unlike other methods MAO not only protect the surface of biomaterials against corrosion but also resist bacterial infections. Resisting bacterial infections is one of the major requirements of an ideal Mg implant material.More interesting thing about MAO approach is manipulation of coating to introduce antibacterial agents to already existing coating structure. Recent studies have shown that micro and nano size particles in the electrolytes bring a strong antibacterial activity in implant materials.
MAO is basically a substrate conversion process similar to anodizing, a nontoxic alkaline electrolyte is typically used but higher potential is applied (150 v – 600 v). In recent years,MAO coatings have been successfully applied (in-vitro) on different Mg alloys such as AZ31, AZ91 [245] AM50 [246],ZM21 [247], ZK60 [248], Mg-Ca [249], WE43[115], Mg-Zn-Ca-Mn [250] etc. Majority of the researchers have used silicate, aluminate, and phosphate based electrolytes on Mg alloys. From the recent studies MAO coating demonstrated improved corrosion resistance,enhanced adhesion strength between coating and Mg alloy surface, enhanced antibacterial properties, cyto-compatibility and bioactivity and it is indicated that the main advantage of MAO of Mg alloy lies in its morphology and thickness of coating that can be controlled over a range of intrinsic and extrinsic parameters such as composition of electrolyte, pH, concentration, temperature,electrical parameters, and treatment time etc. [251]. The main drawback of MAO coating reported in the literature is presence of deep porosities and pores on the coated surface that can significantly affect the corrosion resistance of Mg alloys and needs secondary treatment to enhance the corrosion resistance [252,253]. However, researchers can further increase the efficiency and effectiveness of the MAO method by optimizing the composition of electrolyte, combining MAO with other popular methods such as EPD, conversion coatings, solgel etc., which can significantly improve bioactivity, antibacterial properties, biocompatibility and corrosion resistance of Mg implant materials.
7.4.2. Sol gel coating
Sol-gel deposition is a popular wet chemical technique for producing inorganic or organic ultra-structures generally at room temperature or low temperature [254]. In ceramic engineering and material science, chemical solution deposition,sometimes referred to as the sol-gel technique. Generally, this technology is used to create materials for an integrated network where a chemical solution acts as a precursor. The process of hydrolysing organometallic compounds (alkoxides) or reacting inorganic precursors produces a gel of hydrous oxides that will sinter into a dense and homogeneous ceramic body [255]. This method employs the hydrolysis and condensation processes to prepare the coating. The process of creating a solid phase from a liquid colloidal solution known as "sol" falls within the broad range of activities known as"sol-gel" [256]. Usually sol-gel is coupled with few deposition techniques such as dip-coating, spray coating and spin coating [257].

Fig. 8. Generic classifications of coating methods [242].
Sol-gel method is very suitable for applying anti-corrosive and biocompatible coatings on Mg alloys for orthopedic purposes due to its excellent compatibility with the environment and body [258]. Additional capabilities that might be added to the coatings include medication transport, precursors for boosting bioactivity and biocompatibility, etc. Several sol gel methods are used but the deposition of bioactive glass or glass-ceramic sol-gel coatings, such as bio glass 45S57, 58S,etc.,is one excellent example[258–260].To achieve an appropriate interfacial bonding with bone, these compounds react with the physiological fluid to generate a layer of hydroxyapatite (HA). Utilizing inorganic or hybrid sol-gel coatings is another method to improve the corrosion resistance of magnesium alloys [261]. In this situation, the majority of articles describe the coatings’ densification at lower or around 300 °C in order to protect the substrate’s mechanical qualities. Though they may be used as biodegradable implants,these sol-gel coatings are often produced to create enough barriers for enhancing corrosion resistance over extended periods of time [262].
Castro and Duran [263] in their recent studies developed AZ31 and AZ91 magnesium biodegradable implant materials as substrates and modified the surfaces of Mg alloys using inorganic and organic silica sol-gel coatings. Silica was used as they possess better corrosion resistance and biocompatibility.On both alloys, two distinct silica sols (Hybrid silica gels denoted by MTL and TG sols) were created using dip coating,one with and one without colloidal silica particles. Utilizing three distinct in vitro tests—hydrogen evolution, pH variation, and potentiodynamic polarization curves—the coatings were immersed in SBF (Simulated Body Fluid). The findings demonstrate that the corrosion behavior was influenced by the composition of the coating and the alloy. After 8 days of SBF immersion, coatings on AZ91D-MTL were able to stop the alloy from degrading, however coatings on AZ91D-TG could only keep their stability for 3 days. The encouraging corrosion findings coated AZ91D samples are confirmed by the electrochemical testing [263]. Apart from enhanced corrosion resistance, bio-compatibility and bio activity, sol-gel technique provide excellent advantages such as low cost, easy preparation, low synthesis temperature, chemical and physical homogeneity and high product purity [264,265]. However,when sol-gel is applied on Mg alloys, it must be defect free and adherent so as to achieve adequate corrosion resistance[266]. Sol-gel coating may develop porous and cracky structure due to immediate evaporation of the solvent and residual water during heat treatment. Achieving defect free sol-gel films on Mg based substrates is still considered to be a challenging. Another major drawback of this technique is when sol solution interact with Mg alloy formation of numerous small hydrogen gas bubbles results in unacceptable defects and poor adhesion [267–269]. Nevertheless, Mg alloys are sensitive for high temperatures (400 °C to 450 °C). Literature has witnessed densification of the layers at high treatment temperatures and may lead to degradation of microstructure of Mg alloys [270–272]
To overcome these major limitations of sol-gel coating researchers have suggested few advanced techniques such as multi-layer sol-gel coatings [273], composite films [274],nano particles doping[275],corrosion inhibitors in the sol-gel films [276], application of primer such as anodic oxide layer before sol-gel films [277], hybrid sol-gel coating [278], MAO combined with sol-gel [279] etc.,
7.4.3. Polydopamine (PDA) coating
PDA coating technique have attracted researchers for its efficient strategy to promote cell-adhesion on to substrates with different types of biomaterials under nontoxic condition.With excellent adhesive function PDA technique is a very useful biomimetic surface modifier and biomolecule immobilizer[280]. PDA is also known for its improved bioactivity, antibacterial activity and corrosion resistance [281–283]. From these invitro investigations, coating Mg and its alloys with PDA and composite significantly improved corrosion resistance, degradation rate and more importantly better cytocompatibility [284–286]. Studies have also indicated that the PDA induces a substantial amount of protein observation,that helps in promoting endothelial cell (EC) attachment and proliferation [287]. The Mg alloy AZ31B heated with alkali (NaOH)was treated by Pan et al. [288] using the self-assembly technique to generate a PDA coating. Electrochemical studies showed that the PDA sample’s icorr was lowered by three orders and its ecorr increased from -1.174 V to -0.178 V,indicating the PDA coating’s strong corrosion resistance. Additionally, as compared to ECs grown on the unmodified Mg alloy, ECs had an enhanced proliferative profile, suggesting that the PDA film-coated Mg alloy showed superior cytocompatibility with ECs. These outcomes may be explained by the ability of the various chemical groups (such as amine) introduced by the self-assembly technique to further interact with other biomolecules with various roles in order to increase biocompatibility. Unfortunately, utilizing the conventional procedure to create self-polymerizing PDA coatings on Mg alloys takes a long time and results in a lot of PDA agglomeration and susceptibility to rapid corrosion of Mg alloys can result in breaking of PDA coating and results in excessive roughness[289,290]. Guan et al. [291] used the galvanostatic technique in sodium salicylate (SS) aqueous solutions to create an electropolymerized dopamine(ePDA)coating on the ZE21B alloy to solve this issue. The ePDA coatings were found more uniform than the dip-polydopamine (dPDA) coatings, according to SEM pictures. It was required to further explore the underlying mechanism and improve the ePDA coating method on Mg alloys since the corrosion resistance of ePDA coatings was marginally lower than that of dPDA coatings.
From the recent studies it was concluded that, PDA can be successfully utilised as an intermediary layer for extra surface functionalization of Mg alloys. However, because the imino and quinine groups will quickly adsorb proteins, undesirable events including platelet adhesion, aggregation, and coagulation will ensue, resulting in very poor blood compatibility of PDA coatings [292,293]. For better blood compatibility, researchers have tried to link functional biomolecules (such as heparin) to PDA coatings [294].
7.4.4. Polymer coatings
Many implanted biomedical devices and implants have a thin, adhering polymeric coating that effectively shields them from the body’s aqueous environment. To alter the surface characteristics of biomedical implants and increase their biocompatibility, functionality, and therapeutic efficacy, polymer coatings are commonly utilized. For the right immunological response to be elicited, the interaction between the implant surface and the bodily environment is crucial. As a result, selecting the right polymer coating is essential for defining the implant’s biocompatibility and offers a greater variety of design possibilities that may be utilized to enhance the surface characteristics of the original implant surface. For instance,choosing a polymer coating with a moderate biodegradation rate may be able to prevent the corrosion of a magnesium implant and preserve its mechanical integrity for a longer period of time. Two main kinds of biomedical coatings may be distinguished: 1) short term, which includes disposable or single patient usage, and 2) long term use for prosthetic implants and reusable laboratory equipment [295]. A successful polymer coating needs to stick to the Mg implant and be sturdy and flexible enough to endure the implant’s typical movement in order to meet the bio-functional criteria and provide protection. The coating should also have the ability to be sterilized and be strong enough to fulfill its protective function in the circumstances anticipated for the specific application [296]. The benefit of polymer coatings is that they may be specialized chemically, physically, and mechanically to fit a particular purpose. Polymeric materials may now be employed in a variety of coating applications, including protection, increased lubricity, antibacterial, adhesion resistance,ultrasonic imaging, and blood compatible coatings for drug delivery [297], thanks to the ability to choose and fine-tune the different features.
The Mg implant’s surface must first undergo a suitable pre-treatment procedure before coating. The end result of this procedure need to be a clean, dry, and contaminant-free surface that can offer the most adhesive strength between the polymer and implant surface. Degassing and the development of holes in the coating during the curing process might be caused by trapped air and moisture on the surface. The resultant microstructure and morphology of the polymer coating can be influenced by the coating procedure and related coating factors. This explains why surface characteristics can differ between coatings with similar compositions [298].
A Mg implant may get a polymer coating by means of a solvent, an aqueous solution, or from the vapor phase. Since there are no harmful solvent wastes created,water-based coating processes have the advantages of lowering or eliminating solvent impacts on the substrate and being more environmentally friendly. vapor-deposition polymer coating can provide coatings that are consistent and devoid of flaws. These coatings’ qualities and chemical composition work together to both improve the coating’s protective qualities and act as an effective barrier against the bodily environment. For instance,a polymer covering called Parylene (poly (p-xy lylene)) has been utilised to shield implanted sensors and other biomedical devices [298].
The adhesion between the polymer covering and the implant surface must also be improved,thus Mg demands a good surface preparation or binding agent. Unfortunately, metallic ion primers, components of binding agents, and nearly all organic solvents present in paints and similar surface preparations are poisonous or harmful to the human body and cannot be employed in biomedical applications.Huang et al.coated a pure Mg substrate with degradable poly (lactic acid) utilizing dip coating technique after priming the substrate with a silane coupling agent recently [299]. A Mg-based implant’s surface characteristics may be changed by polymeric coatings, which would greatly increase the implant’s performance, longevity,and usage. It is possible for polymeric materials used to coat implants to be carefully engineered to give physical, chemical, and mechanical reactions that may be fine-tuned to elicit and amplify particular biological responses inside the bodily environment. Finding polymeric materials that can provide thin, trustworthy, multifunctional coatings with regulated degradation rates, however, would still require a large amount of study. These coatings might be utilized to extend the mechanical efficiency of implants made of magnesium.
7.4.5. Atmospheric plasma spraying (APS)
The APS has gained significant attention in recent years as a promising technique for the fabrication and surface modification of Mg alloys.This technique involves the use of a hightemperature plasma jet to melt ceramic and metal particles,which are then deposited onto a substrate to form a solid alloy or ceramic coating. This process allows the preparation of large-scale coatings that exhibit good adhesion on substrates of complex shape.Plasma sprayed ceramic coatings have been found to greatly improve the corrosion, oxidation, and wear resistances of magnesium alloys, making them more suitable for various applications.Furthermore,the formation of a thick and dense oxide coating on the surface of magnesium alloys through APS significantly enhances their corrosion resistance[300]. In addition, APS allows for the fabrication of relatively thick coatings, which provides greater protection and durability to Mg alloys. APS has emerged as a commercially viable method for the surface modification and fabrication of magnesium alloys [300]. Bogdan et al. [301] investigated properties and in vitro response of Mg-Ca and Mg-Ca-Zr alloys coated by APS. Alloys were coated with two types of coating materials i.e. ZrO2-CaO ZrO2-CaO (95% ZrO2and 5%CaO, in wt.%) and ZrO2-Y2O3(92% ZrO2and 8% Y2O3, wt.%). In vitro examination indicated that, Mg alloys coated with ZrO2-Y2O3found to be more cytocompatible (80%) than Mg alloys coated with ZrO2-CaO indicating good cell viability.Non uniform trend was observed in values young’s modulus, hardness and stiffness for both ZrO2-Y2O3and ZrO2-CaO coated alloys.
Thirumalaikumarasamy et al. [300] in their studies investigated influence of chloride ion concentration on immersion corrosion behavior of plasma sprayed alumina coatings on AZ31B Mg alloy. The uncoated and alumina coated AZ31B demonstrated with superior corrosion resistance in lower chloride ion concentration NaCl solutions (0.01 M NaCl). However corrosion resistance found declined with increased chloride ion concentration. Both coated and uncoated AZ31B alloys reported with higher susceptibility to localized damage and could not provide effective corrosion protection.
Though APS technique found to be effective in preparing bio active coating and demonstrated with better osseointegration of implant devices made of stainless steel (316 L and 304 L), Co-Cr alloy, and Ti-6Al-4V alloy [302,303], researchers are required to further analyze the effect of APS coated Mg alloys under in vitro conditions to enhance the bio compatibility and bio efficacy.
7.5. Heat treatment
Fig. 9. Microstructures of (a) As received ZM 21 alloy, hot rolled at different temperatures (b) 250 °C (c) 300 °C and (d) 350 °C [310].
Heat treatment of Mg alloys is most commonly used method to enhance mechanical properties by considering solubility of certain alloying element with respect to temperature. In contrast to other processing methods, heat treatment often alters the microstructure of the materials rather than a chemical makeup. The two primary methods for enhancing the mechanical characteristics of magnesium alloys are grain strengthening by grain refinement and second phase strengthening. Solid solution treatment (T4), age treatment(T5), and solid solution + aging treatment (T6) are the most commonly applied thermal treatments to magnesium alloys(T6) [304,305]. Due to the high solubility of zinc in magnesium, Mg-Zn alloys are often subjected to heat treatment to enhance their mechanical characteristics [306,307]. Because of the persistence of residual Mg51Zn20and Mg12Zn13second phases at grain boundaries, Lotfabadi et al. [308] discovered that T4 treatment at 340 °C for 6 h increased the strength and elongation of Mg-1.5Zn alloy only marginally while significantly improving the properties of Mg-9Zn alloy.Zhang et al. [309] discovered that the precipitation strengthening might be primarily responsible for the significant improvement in the mechanical characteristics of the extruded Mg-3Nd-0.2Zn-0.4Zr alloy after 10 h of aging at 200 °C.Final tensile strength, yield strength, and elongation all increased, reaching 243 MPa, 189 MPa, and 21%, respectively. According to Maier et al. [304], the precipitation hardening of the Mg-Y-Nd alloy with yield strength of 133.3 MPa, ultimate tensile strength of 235.5 MPa, and elongation of 15.4% might be improved by T6 treatment. Overall, heat treatment is an effective technique for enhancing the mechanical and biodegradable qualities of magnesium alloys. After heat treatment, the microstructure and second phase distribution will change, both of which have a direct impact on the mechanical characteristics of magnesium alloys.
Sriraman et al. [310] investigated ZM21 Mg alloy subjected to hot rolling (HR) at different temperatures (250, 300,and 350 °C) with a 75% reduction, followed by short annealing at 200 °C for 10 min. The researchers performed in-vitro tests to evaluate the bio-corrosion behavior of the thermo-mechanically processed ZM21 alloys. Fig. 9 shows the microstructure of the as-received ZM21 alloy contains a single-phase alpha-Mg with larger coarse grain size and some micro-porosities throughout the matrix. However, after HR at different temperatures (Fig. 9b-d), the microstructure exhibited deformed twins with fewer micro-porosities.As the rolling temperature increased, grain growth and recrystallization were observed, and the density of twins was significantly reduced. These HR samples were further subjected to short annealing (HRA) treatment, which resulted in a relatively coarser grain structure and a reduction of 30%–50% in twins compared to HR samples as shown in Fig. 10.Corrosion behavior was conducted on these sample conditions in simulated body fluid during immersion tests. The results showed that the ZM21 alloy HR250 °C exhibited a higher corrosion rate than the other sample conditions. This can be attributed to higher deformation twin energy than the matrix,leading to a local reduction of the equilibrium potential in the twin region, accelerating the corrosion rate [311]. Additionally, the fine grain structure of the ZM21 alloy HR 250 °C enhances corrosion activity because corrosion mainly occurs along surface defects such as grain boundaries[312,313]. On the other hand, the HRA350 °C sample with a lower density of twins and coarser grain structure exhibited a lower corrosion rate of about 30%–50% compared to other processed alloys. The presence of micro-porosity in the matrix, both in the as-received and ZM21A conditions, acts as an active center for corrosion, resulting in a higher corrosion rate (about 10%–20%) compared to the ZM21 alloy hot rolled at 350 °C and hot rolled and short annealed at 350 °C. The HR350A sample also exhibited a much lower pH (close to 30%) than the other processed ZM21 Mg alloys, confirming its good corrosion resistance.
Major challenges associated with the heat treatment of Mg alloys are volume fraction and morphology of the secondary phases. Depending upon type and chemical composition of alloying elements the mechanical and corrosion properties can be varied by selecting suitable heat treatment. Some of the important and general effects of T4 and T6 heat treatments are:
a. T4 heat treatment eliminates the dentric structure and correlated segregations. More importantly, it reduces the density of dislocations, twins and other defects by reducing the internal energy of cast Mg alloy [308,314].
Fig. 10. Optical micrographs of hot rolled followed by short annealed (a) ZM21 alloy (b) 250 °C (c) 300 °C (d) 350 °C[310].
b. T4 heat treatment homogenously distributes the alloying elements and increases their solubility [315].
c. Uniform distribution of alloying elements in turn improves the corrosion resistance effectively and size of uniformly distributed secondary phase particles can significantly increases the performance of bio degradable magnesium alloy [316].
d. T6 heat treatment helps to achieve better precipitation strengthening and it mainly depends on important factors such as precipitate shape, precipitate volume fraction precipitate size and distribution, the nature of the interface with the surrounding matrix etc., [317,318].
Corrosion resistance of the Mg alloys can be further enhanced by optimizing the time and temperature of heat treatment. Analyzing techniques such as X-ray diffraction(XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM) and electrical conductivity can be employed to determine optimum time and temperature of heat treatment. In addition to experimentation trials, researchers have suggested to use models and simulation techniques to further predict the optimum conditions of heat treatment. Some of the recently developed models include novel microstructure model to predict microstructure evolution during T6 heat treatment [319,320], Precipitation simulation using Pan-Precipitation module of PandatTMsoftware to predict the yield strength and micro hardness of Mg alloys [321], CALPHAD (Calculation of Phase diagram) approach for phase diagram calculations and materials property simulation [322], phase field models for microstructure evolution [323] etc., can be utilized for future investigations.
7.6. Plastic deformation
In addition to all mentioned mechanical procedures in the previous sections, plastic deformation as one of the important mechanical processing technique has gained tremendous attention over years. Plastic deformation plays significant role in improving mechanical properties, refining grains of Mg alloys, texture modifications and more importantly enhancing the corrosion resistance. Plastic deformation include mechanical processing techniques such as extrusion [309], rolling[324,325],forging[326],drawing[327,328]and severe plastic deformation(SPD)[329].During recent years SPD techniques have witnessed remarkable microstructure refinement and texture modification with significant enhancement in strength than other methods. SPD has become effective tool for processing of Mg based implant materials. SPD can be implemented using variety of techniques developed recently such as equal channel angular pressing (ECAP) [330,331], multi directional forging (MDF) [332], parallel tubular channel angular pressing (PTCAP) [333], high pressure torsion (HPT)[334], cyclic extrusion and compression (CEC) [335], severe shot peening (SSP)[336], and accumulative roll bonding(ARB) [337].
Among different SPD techniques ECAP has gained tremendous attention during past 5 years. ECAP is an effective method that has great potential to develop bulk material for Mg based implant materials [338,339]. ECAP of Mg alloys are usually carried out at temperatures ranging from 200 °C to 350 °C [340,341]. Recrystallization play important role in ECAP since it involves elevated temperatures. Size and distribution of precipitates can be manipulated and hardness of the Mg based alloys can be increased using ECAP.ECAP offers dual advantage of enhancing the mechanical integrity and bio-corrosion resistance. Recent investigations has revealed use of two step ECAP for better grain refinement and mechanical properties [342]. Researchers have advised to focus on following aspects of ECAP process to further enhance the performance of Mg alloys [338,343].
a. Analyzing the combined effect of crystallographic orientation and grain refinement on mechanical properties and corrosion properties.
b. Performance of Mg based materials through ECAP in-vivo models needs be further studied to develop commercial bio-degradable implants.
c. Design of experiments can be used to effectively model the ECAP process.
d. Response surface methodology can be adapted to obtain mathematical relationship between process variables and also to predict the Vickers hardness and average grain size.
8. Concluding remarks
Considering the serious impact of accidents across the world, it is very much required to develop cost-effective implant material for betterment of society. Mg based materials are invaluable candidate to mankind with immense biocompatibility benefits and should be exploited discreetly. Therefore in the present study the inherent features of Mg alloys for orthopedic implant applications has been reviewed on the basis of their desirable mechanical properties, biodegradability,mechanical integrity and biocompatibility. Researchers indicated incorporation of Zn, Zr, Ca alloying elements in Mg with minute wt.% of RE elements such as Y, Gd and Ce produce promising and compatible implant material for biomedical application. However, the specific application and requirements of the Mg alloys will determine the optimal alloying elements to be incorporated. Taking into consideration, demands of enhancing the corrosion resistance this review highlights the importance of eliminating heavy metal impurities in Mg alloy, melt purification techniques, surface alterations, coatings and deformation techniques. Most of the methods are reported with exiting results and rapid growth in extending the biocompatibility and degradability has been achieved in last two decades. Literature has indicated ample of opportunities available in each methods discussed in this review to exploit the unique characteristics of Mg alloys for implant application. Currently Mg based materials are used as screws,pins and fixation plates,hence future studies should be focused on expanding the application areas of Mg implants in other surgical and orthopedic implants. In order to expand the application areas of Mg based materials, more number of in-vivo studies should be conducted to evaluate the biological performance,antibacterial properties of Mg alloys and also reducing the degradation rate and investigating combined effects of coating, optimizing the surface alteration parameters, models and simulation techniques to further predict the optimum conditions of heat treatment are important to progressively achieve an ideal implant material status.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jma.2023.08.002.
杂志排行
Journal of Magnesium and Alloys的其它文章
- NaH doped TiO2 as a high-performance catalyst for Mg/MgH2 cycling stability and room temperature absorption
- Microstructure, mechanical properties and fracture behaviors of large-scale sand-cast Mg-3Y-2Gd-1Nd-0.4Zr alloy
- Enhanced initial biodegradation resistance of the biomedical Mg-Cu alloy by surface nanomodification
- Elucidating the evolution of long-period stacking ordered phase and its effect on deformation behavior in the as-cast Mg-6Gd-1Zn-0.6Zr alloy
- Effect of long-period stacking ordered structure on very high cycle fatigue properties of Mg-Gd-Y-Zn-Zr alloys
- The mechanism for tuning the corrosion resistance and pore density of plasma electrolytic oxidation (PEO) coatings on Mg alloy with fluoride addition
