Quelling the Cytokine Storms during CAR-T Cell Therapy
2023-11-15ByYANFusheng
By YAN Fusheng
A supercharged immune army can turn against us if left unchecked during CAR-T cell therapy,a form of immunotherapy that involves genetically engineering a patient’s own T cells to recognize and attack cancer cells.The infusion of these engineered T cells can trigger the release of life-threatening flood levels of inflammatory cytokines, disrupting the immune system from within.Recently, scientists from the National Center for Nanoscience and Technology(NCNST) of the Chinese Academy of Sciences have developed a technique to quell cytokine storms before they wreak havoc on patients undergoing this cancer immunotherapy.
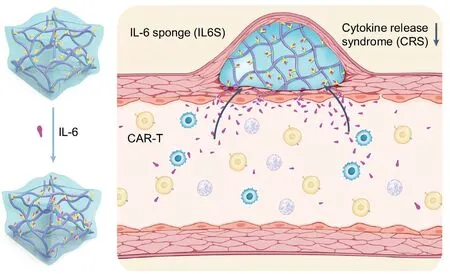
When subcutaneously injected before the infusion of chimeric antigen receptor (CAR) T cells, an antibody-conjugated hydrogel acts as a cytokine sponge (IL6S) that preemptively captures elevated IL-6 levels during cytokine release syndrome (CRS), alleviating symptoms without compromising CAR-T therapy.More importantly, the implanted hydrogel can be easily removed with a syringe following a cooling-induced gel-to-solution transition.(Image by NCNST)
Our immune defenses can spin out of control during a promising new cancer treatment called CAR-T cell therapy.This treatment involves genetically rewiring a patient’s infection-fighting T cells to spot and wipe out cancer cells within the patient.While promising, it also carries the risk of cytokine release syndrome (CRS) – a severe inflammatory response that can be fatal.
The engineered T cells, known as chimeric antigen receptor (CAR) T cells, multiply rapidly after infusion into patients and besiege the tumor.Meanwhile, these T cells usually trigger a copious release of cytokines, signaling molecules that marshal our defenses.But in CRS, excessive cytokines like IL-6 flood the body, acting like hyperactive soldiers, releasing excessive signals that over-mobilize your immune system and attack your organs.As a result,patients may face organ failure or even death.
For example, in a 2017 study on CAR-T therapy for patients with refractory large B-cell lymphoma, more than 40% of patients experienced severe (grade 3 or higher) CRS.The median time from infusion to CRS onset was two days.Severe CRS symptoms included fever, hypotension, and hypoxia.Two patients died from CRS and associated neurotoxicity.In a 2021 study of CAR-T in multiple myeloma patients, CRS occurred in 85% of patients.Grade 3 or higher CRS occurred in 46%of patients at a median onset of 4 days after infusion.One death occurred due to CRS.
Doctors normally control CRS by monitoring patients closely for early signs of the storm, then injecting antibodies to neutralize cytokines afterward.However,this reactive approach has downsides, as timing is critical whilst transient, and complications can arise before antibodies take effect.
Now, scientists at the National Center for Nanoscience and Technology (NCNST) in China propose a preemptive solution.Their technique subdues cytokines before levels peak, avoiding complications proactively rather than reactively.
The team designed a gel plug containing antibodies against IL-6, a major culprit in CRS.Implanted under the skin before CAR-T cell therapy, the inconspicuous plug acts as a cytokine sponge, sopping up extra IL-6 as soon as levels start to rise.
The hydrogel composed of cross-linked polymers resembles jelly in texture.It remains fluid when cool but solidifies at body temperature, allowing easy injection.The gel itself does not stick to cytokines, so the scientists decorated it with IL-6 antibodies that act like molecular Velcro, trapping free-floating IL-6.
Because the antibodies are tethered to the gel, they persist in tissues far longer than injected antibodies.
The team tested their cytokine sponge in mice with lymphoma tumors.It showed that the mice treated with just CAR-T cells experienced high mortality from CRS within days, but those given the sponge beforehand survived far better, with reduced IL-6 levels and milder symptoms.
The sponge works by lowering the IL-6 response across the board, not just around the implantation site.It lowers IL-6 levels in organs distant from the implantation site, alleviating systemic complications – Like a pebble placed in a stream, the effects ripple outward.
Since the gel stays solid at body temperature, it persists for weeks to provide lasting protection.Notably,it can be liquefied on demand by cooling, allowing for removal after the CRS risk subsides.Scientists can eliminate the plug easily with an ice pack and syringe,like dissolving and aspirating a glob of jelly.
The team is now investigating using larger sponges for human testing.Though still experimental, the proactive approach could make CAR-T and other immune therapies safer over the long term.
Rather than weathering the storm, we now have a way to prevent the clouds from gathering in the first place.Or, as the authors put it, we may see “a shift in the management of CRS, from monitoring to prevention.”
杂志排行
Bulletin of the Chinese Academy of Sciences的其它文章
- Could Gut Bacteria Hold a Cure for Sepsis?
- Looking into the Tissue Adjacent to Tumors
- Daya Bay Collaboration Awarded 2023 High Energy and Particle Physics Prize by European Physical Society
- Largest Optical Time-domain Survey Telescope in Northern Hemisphere Goes into Operation
- CAS Paleogeneticist Awarded UNESCO Prize
- “Magnetically Arrested Disk”Revealed by Multiwavelength Observation
