Effects of acupuncture at Feishu (BL13) and Tianshu(ST25) on pulmonary function and tissue inflammation in asthma model rats
2023-10-27LAIYitian来奕恬ZHOUJingying周竞颖DINGPanting丁攀婷LIUMi刘密PANJiang潘江LINan李南ZHANGGuoshan张国山QIURanran邱冉冉
LAI Yitian (来奕恬), ZHOU Jingying (周竞颖), DING Panting (丁攀婷), LIU Mi (刘密), PAN Jiang (潘江), LI Nan (李南),ZHANG Guoshan (张国山), QIU Ranran (邱冉冉)
1 School of Acupuncture-moxibustion, Tuina and Rehabilitation, Hunan University of Chinese Medicine, Changsha 410208, China
2 The First Hospital of Hunan University of Chinese Medicine, Changsha 410007, China
Abstract
Keywords: Acupuncture Therapy; Point, Feishu (BL13); Point, Tianshu (ST25); Lung Being Connected with Large Intestine;Respiratory Function Tests; Inflammation; Asthma; Rats
Bronchial asthma (referred to as “asthma”) is a chronic airway inflammatory disease with recurrent attacks, wheezing, phlegm, and airway hyperresponsiveness as the main symptoms, involving many cells and cell components[1]. Drugs such as leukotrienes receptor antagonists are usually used in modern clinics to control and alleviate asthma attack symptoms, to maintain the normal living standard of patients, and to prevent disease aggravation. However,the long-acting specific drugs still need further research,development, and verification[2-3]. Acupuncture, as a traditional external treatment of Chinese medicine, can not only effectively relieve asthma attack symptoms,but also significantly reduce asthma attack frequency[4-5].Based on the traditional theory of “lung and large intestine in an exterior and interior relationship”[6]and the modern theory of “gut-lung axis”[7], if we simultaneously take into account the condition of intestinal organs, that is, “simultaneous treatment of lung-intestine” when lung diseases such as asthma are treated with acupuncture, it will effectively improve the traditional Chinese medicine (TCM) symptoms of patients with asthma and improve their pulmonary function and quality of life[8-10]. Through animal experiments, XU N,et al[11]confirmed that acupuncture and moxibustion at Feishu (BL13), Dazhui (GV14), and Fengmen (BL12) regulated the immune inflammatory indicators such as eosinophils in the lung tissue and interleukin (IL)-13 in the blood of asthma model rats[12];LI L S,et al[13]found that acupuncture at Feishu (BL13)and Shenshu (BL23) inhibited the expression of matrix metalloproteinase-9 in asthmatic rats, thus alleviating the inflammatory reaction in asthma. Unfortunately, the idea of “simultaneous treatment of lung-intestine” has not been widely used in clinical and animal experiments on asthma treatment with acupuncture[14-15]. Therefore,we used asthma model rats as the experimental model and acupuncture as the intervention means to observe and compare the difference between Feishu (BL13) plus Tianshu (ST25) and Feishu (BL13) alone in improving the pulmonary function and the tissue inflammation of asthma model rats by acupuncture, and to discuss the significance of “simultaneous treatment of lungintestine” in improving the pulmonary function and the tissue inflammation of asthma rats by acupuncture, so as to provide experimental basis for the clinical application and popularization of “simultaneous treatment of lung-intestine” in asthma management by acupuncture.
1 Materials and Methods
1.1 Animals and groups
Forty-eight specific-pathogen-free grade Sprague-Dawley rats, half male and half female, 7-week-old, with a body mass of 150-170 g, were provided by Hunan Slack Jingda Experimental Animal Co., Ltd., China, with the production license number of SCXK (Xiang)2019-0004. Rats were raised in the Experimental Animal Center of Hunan University of Chinese Medicine at a temperature of 20-25 ℃ and humidity of 50%-70%.After one week of adaptive feeding, the rats were randomly divided into a normal group, a model group, a lung treatment group, and a lung-intestine treatment group according to the random number table method,with 12 rats in each group. The operation and treatment of animals during the experiment complied with theGuiding Opinions on the Treatment of Experimental Animals. This study was approved by the Animal Experimental Center of Hunan University of Chinese Medicine (Approval No. LL2021032407).
1.2 Main equipment and reagents
Ovalbumin (OVA, Cat. No. A5503) was from Sigma,USA; aluminum hydroxide gel (Cat. No. 77161) was from Thermo Fisher, China; IL-25 (Cat. No. JM-10874R1),IL-17 (Cat No. JM-10601R1), IL-4 (Cat. No. JM-01598R1),IL-5 (Cat. No. JM-01501R1), IL-13 (Cat. No. JM-01492R1),IL-33 (Cat. No. JM-10775R2), leukotrienes (LT, Cat. No.JM-11585M1), thymic stromal lymphopoietin (TSLP, Cat.No. JM-10881R1), and prostaglandin D2(PGD2, Cat. No.JM-02211R1) enzyme-linked immunosorbent assay(ELISA) kits were from Wuhan Bioswamp Biology Company, China.
SPECTRAMAX multifunctional microplate reader(Molecular Devices Corporation, USA); YP1002 small animal electronic scale (Shanghai Youke Instrument Co.,Ltd., China); small animal pulmonary function tester(Beijing Baianji Company, China); self-made atomization box (60 cm × 60 cm × 40 cm); ultrasonic atomization inhaler (Haier Company, China).
1.3 Model preparation and evaluation criteria
The asthma rat model was made by OVA sensitization and stimulation according to the literature[16-17].
Sensitization stage: Five grams of OVA and 50 g of aluminum hydroxide gels were weighed, and then 500 mL mixed solution was prepared with 0.9% sodium chloride solution for later use. On the first day of the experiment, 0.2 mL of antigen solution was injected subcutaneously into the back and groin, and 0.8 mL of antigen solution was injected intraperitoneally in rats.On the 9th day of the experiment, sensitization was repeated with the same dose and method. From the 15th day of the experiment, rats in each group except for the normal group were placed in a self-made glass atomization box and were atomized with 1% OVA solution to stimulate for 20 min each time, once a day,continuously for one week.
It suggested that the model was successful if the rats appeared nose/face scratching, irritability, shortness of breath, abdominal breathing, or cyanosis of lips during atomization.
1.4 Intervention methods
After successful modeling, rats in the model group were fixed on the rat plate for 30 min every day; rats in the lung treatment group were fixed on the rat plate,with bilateral Feishu (BL13) disinfected with 75% alcohol,and then Hwato brand stainless steel needles (0.25 mm in diameter and 25 mm in length) were inserted into 3-5 mm, rotated for 20 s, retained for 15 min, and rotated for another 20 s during treatment and at needle withdrawal, respectively. The same method was used in the lung-intestine treatment group: 15 min of acupuncture at bilateral Feishu (BL13) and Tianshu(ST25), respectively (for a total of 30 min of acupuncture). Rats in the three groups were atomized with 1% OVA solution for 20 min after acupuncture. All the interventions were conducted once a day for 14 consecutive days. The experimental flow is shown in Figure 1.
The locations of Feishu (BL13) and Tianshu (ST25) are referred to theExperimental Acupuncture Science[18]and anthropomorphic point location method.

Figure 1 Experimental flow chart
1.5 Observation items and detection methods
1.5.1 Behavioral observation
Observed and recorded the mental state, reaction ability, mobility, food intake, water intake, urine, feces,and other general behavioral changes of rats every day,and whether there were acute asthma attack-like reactions such as nose and face scratching, irritability,shortness of breath, abdominal breathing, and lip cyanosis.
1.5.2 Pulmonary function test
After 14-day interventions, the experimental rats were anesthetized. Ten items, including peak expiratory flow (PEF), lung resistance (RL), dynamic lung compliance (Cdyn), forced expiratory flow (FEF) 75%,FEF50%, FEF25%, forced vital capacity (FVC), forced expiratory volume in the first second (FEV1), forced expiratory volume in the first second/forced vital capacity (FEV1/FVC), and maximal mid-expiratory flow(MMEF), were measured by a small animal pulmonary function tester. The airway pulmonary function was measured using computer pulmonary function analysis software.
1.5.3 Histopathological observation of lung
After the pulmonary function test, the thoracic cavity was opened to cut out part of the right middle lung tissue, quickly immersed into 4% paraformaldehyde,and fixed for 24 h. After being dehydrated by ethanol,the lung tissue was soaked in wax at 60 ℃, embedded,sliced (4 μm in thickness), stained in hematoxylin and eosin solution, dehydrated, and sealed with neutral gum. The pathological changes of lung tissue were observed under the light microscope.
1.5.4 Observation of collagen deposition in lung tissue
After fixation, dehydration, waxing, and embedding,the right middle lung tissue was sectioned (4 μm in thickness), and the collagen was stained by Masson method. After antigen repair, the lung tissue was stained with hematoxylin-ferric chloride mixture for 5 min, then an acidic ethanol differentiation solution was added dropwise. After differentiation for 5 s, the lung tissue was rinsed with PBS buffer solution for 1 min,subjected to blue return for 1 min with alkaline ammonia solution, rinsed with PBS buffer solution for 1 min, stained with Ponceau S staining solution for 5 min, sucked out the liquid with filter paper, dropwise added phosphomolybdic acid solution and kept for 1 min, added toluidine blue dye solution dropwise and kept for 1 min, dehydrated with gradient ethanol,transparentized with xylene, and sealed with neutral gum.
The same 200× field of view for each slice was captured under consistent background light by the Case Viewer. The blue color was used as a unified standard to judge the positivity of all photos, and the collagen area was obtained by analyzing each photo with Image J software. Four slices from different angles were obtained from each rat to determine the collagen area.The collagen fibers were blue, and the muscle fibers and red blood cells were red after Masson staining. The distribution of collagen fibers (blue) was observed under the light microscope, and the collagen area of each group was analyzed using Image J image processing software. The degree of collagen deposition= Collagen area ÷ Total tissue area × 100%.
1.5.5 Collection of the bronchoalveolar lavage fluid(BALF) for cell classification count
After anesthesia, the neck of rats fixed in a supine position was cut along the midline, the trachea was separated, a 1 mL syringe was inserted into the trachea,and the right lung was lavaged with 1 mL PBS 3 times.The recovery rate of lavage fluid was more than 80%.The lavage fluid was centrifuged at 3 500 r/min for 10 min, and the supernatant was subpackaged and frozen in the -20 ℃ refrigerator for later use. The precipitated cells were smeared, fixed with 4%paraformaldehyde after natural withering, and stained by Wright’s-Giemsa staining, and the number of eosinophils and neutrophils was counted under a light microscope at 200×[19].
1.5.6 Expression levels of IL-4, IL-5, IL-13, IL-17, IL-25,IL-33, LT, TSLP, and PGD2in lung tissue
After the airway pulmonary function test, part of the right lung tissue was collected and mixed with the lysate in the homogenizer according to the weight of 1:9 for homogenization. After 30 min, the lysate was transferred into a 1.5 mL centrifuge tube and centrifuged at 4 ℃ at 12 000 r/min for 5 min. The supernatant was subpackaged in 0.5 mL centrifuge tubes and stored at -20 ℃. The levels of IL-4, IL-5, IL-13,IL-17, IL-25, IL-33, LT, TSLP, and PGD2in the lung tissue were detected strictly according to the operation instructions of ELISA kits.
1.6 Statistical methods
All data were analyzed using the SPSS version 20.0 statistical software. The measurement data were expressed as mean ± standard deviation (±s). First,the normal distribution and the variance homogeneity tests were carried out. Between-group comparisons were performed with one-way analysis of variance for the normal distribution data. The least significant difference method was used for data with homogeneity of variance. Dunnett T3 method was used for data with heterogeneity of variance. When the normality was not satisfied, the rank-sum test was used. The difference was statistically significant whenP<0.05.
益生菌能直接改变肠道菌群的种类和数量,而益生元能刺激肠道有益菌的生长,提高活性。降糖药物二甲双胍和小檗碱通过改变肠道菌群的结构促进肠促胰岛素分泌,减少炎症反应,改善胰岛素抵抗,降低血糖。
2 Results
2.1 Comparison of the general conditions of rats
During the whole experiment, rats in the normal group were sensitive with neat hair, a strong body, neat and uniform breathing rhythm, and normal urine and feces without abnormal secretions in the mouth and nose. After OVA atomization stimulation, rats in the model group gradually appeared asthma attack-like reactions such as nose/mouth scratching, nodding frequently, shortness of breath, sneezing, white sticky secretions in the mouth and nose, obvious abdominal breathing, and light-colored thin feces around the anus.
2.2 Comparison of the pulmonary function of rats
Compared with the normal group, PEF, Cdyn, FEF25%,FEV1/FVC, and MMEF in the model group were significantly decreased (P<0.01 orP<0.05), while RL was significantly increased (P<0.01). Compared with the model group, only FEF25% and FEV1/FVC were significantly increased in the pulmonary function of rats with lung treatment (P<0.01,P<0.05); PEF, FEF25%, and FEV1/FVC were significantly increased (P<0.01 orP<0.05), while RL was significantly decreased (P<0.05) in the lung-intestine treatment group. There was no significant difference in the pulmonary function indicators between the lung treatment group and the lung-intestine treatment group (P>0.05). See Table 1.
2.3 Comparison of the pathomorphology of rat lung tissue
The hematoxylin-eosin staining showed that the lung tissue structure was normal, the alveolar wall was intact,and the cells were arranged orderly in rats in the normal group. Compared with the normal group, the model group showed severe abnormal lung tissue structure, thickened alveolar walls, severe parenchymal lung, inflammatory cell infiltration and proliferation of interstitial fibrous tissue around the bronchus.Compared with the model group, the alveolar wall thickening was improved, the structure was substantially complete, inflammatory cells around the bronchus and alveolar tissue were obviously reduced,and the cells were arranged neatly in the lung treatment group and the lung-intestine treatment group (Figure 2).
2.4 Comparison of the collagen deposition in rat lung tissue
Masson staining showed a small amount of collagen fiber deposition under the microscope in the airway epithelium and around the airway of normal rats.Compared with the normal group, the airway mucosa in the model group showed thickened smooth muscles,collagen fiber deposition, and a significantly increased blue area of positive collagen. Collagen fiber deposition could still be seen in rat lung tissue of the lung treatment group and the lung-intestine treatment group, but the collagen fiber deposition was obviously reduced compared with the model group (Figure 3).
Compared with the normal group, the collagen fiber deposition in the lung tissue of the model group was significantly higher (P<0.01). Compared with the model group, the collagen fiber depositions in rat lung tissue of the lung treatment and the lung-intestine treatment groups were significantly reduced (P<0.01). There was no significant difference in the degree of collagen fiber deposition between the lung treatment group and the lung-intestine treatment group (P>0.05). See Table 2.
Table 1 Relative pulmonary function in each group (±s)
Note: PEF=Peak expiratory flow; RL=Lung resistance; Cdyn=Dynamic lung compliance; FEF75%=Forced expiratory flow 75%;FEF50%=Forced expiratory flow 50%; FEF25%=Forced expiratory flow 25%; FVC=Forced vital capacity; FEV1=Forced expiratory volume in the first second; FEV1/FVC=Forced expiratory volume in the first second/forced vital capacity; MMEF=Maximal mid-expiratory flow; compared with the normal group, 1) P<0.01, 2) P<0.05; compared with the model group, 3) P<0.01, 4) P<0.05.
Item Normal group(n=12)Model group(n=12)Lung treatment group(n=12)Lung-intestine treatment group(n=12)PEF/(mL·s-1) 12.33±2.02 6.20±0.861) 9.58±2.84 11.42±3.033)RL/(cmH2O·mL-1·s-1) -1.68±0.16 -1.04±0.141) -1.41±0.31 -1.52±0.344)Cdyn/(cmH2O·mL-1·s-1) 0.20±0.03 0.06±0.021) 0.11±0.07 0.13±0.08 FEF75%/(mL·s-1) 11.90±3.80 5.17±0.80 9.71±2.27 11.58±3.49 FEF50%/(mL·s-1) 9.84±3.07 4.94±0.28 7.92±1.61 9.46±2.81 FEF25%/(mL·s-1) 6.54±1.13 2.29±0.291) 4.95±0.673) 5.62±1.143)FVC/mL 2.14±0.34 3.23±0.55 1.75±0.22 1.93±0.17 FEV1/mL 0.19±0.08 0.13±0.03 0.16±0.05 0.17±0.05 FEV1/FVC (%) 8.61±2.72 4.25±1.382) 9.04±3.404) 8.76±1.864)MMEF/(cmH2O·mL-1·s-1) 11.24±2.70 6.22±1.361) 8.21±1.73 8.80±1.74

Figure 2 Microscopic observation and comparison of rat lung tissue in each group after hematoxylin-eosin staining (×100)

Figure 3 Microscopic observation and comparison of lung tissue after Masson staining (×200)
Table 2 Comparison of collagen fiber deposition degree in rat lung tissue (±s)
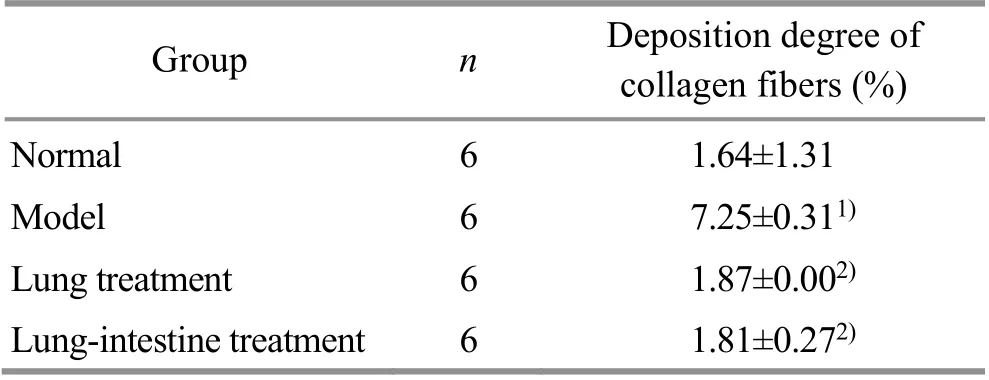
Table 2 Comparison of collagen fiber deposition degree in rat lung tissue (±s)
Note: Compared with the normal group, 1) P<0.01; compared with the model group, 2) P<0.01.
Group n Deposition degree of collagen fibers (%)Normal 6 1.64±1.31 Model 6 7.25±0.311)Lung treatment 6 1.87±0.002)Lung-intestine treatment 6 1.81±0.272)
2.5 Comparison of the eosinophil and neutrophil levels in rat BALF
Compared with the normal group, the neutrophil proportion in the BALF of the model group was significantly increased (P<0.05); the eosinophil and neutrophil proportions in the lung treatment group were not significantly different from those in the model group (P>0.05), but the neutrophil proportion in the lung-intestine treatment group was significantly lower than that in the model group (P<0.05); there was no significant difference in the eosinophil and neutrophil proportions between the lung treatment group and the lung-intestine treatment group (P>0.05). See Table 3.
2.6 Comparison of the inflammatory factors in rat lung tissue
Compared with the normal group, the levels of IL-4,IL-5, IL-13, IL-17, IL-33, LT, TSLP, and PGD2in the lung tissue of the model group were significantly increased(P<0.01). Compared with the model group, the levels of IL-4, IL-5, IL-13, IL-17, LT, TSLP, and PGD2in the rat lung tissue of the lung treatment group and the lungintestine treatment group were significantly decreased(P<0.01 orP<0.05); in addition, the IL-33 level in rat lung tissue of the lung-intestine treatment group was also significantly decreased (P<0.05). Compared with the lung treatment group, the IL-5 level in rat lung tissue in the lung-intestine treatment group was decreased(P<0.05). See Table 4.
Table 3 Comparison of the inflammatory cells in rat alveolar lavage fluid among groups (±s)

Table 3 Comparison of the inflammatory cells in rat alveolar lavage fluid among groups (±s)
Note: Compared with the normal group, 1) P<0.01; compared with the model group, 2) P<0.01.
Group n Proportion of eosinophils (%) Proportion of neutrophils (%)Normal 6 2.92±1.93 1.44±1.75 Model 6 2.55±0.97 3.70±1.831)Lung treatment 6 1.14±0.38 2.88±2.13 Lung-intestine treatment 6 1.65±1.12 1.26±1.212)
Table 4 Comparison of the relative inflammatory factor expression in rat lung tissue among groups (±s) Unit: pg/mL

Table 4 Comparison of the relative inflammatory factor expression in rat lung tissue among groups (±s) Unit: pg/mL
Note: IL=Interleukin; LT=Leukotrienes; TSLP=Thymic stromal lymphopoietin; PGD2=Prostaglandin D2; compared with the normal group, 1) P<0.01; compared with the model group, 2) P<0.01, 3) P<0.05; compared with the lung treatment group, 4) P<0.05.
Item Normal group(n=12)Model group(n=12)Lung treatment group(n=12)Lung-intestine treatment group(n=12)IL-4 57.20±16.27 108.78±17.291) 65.93±10.802) 57.63±4.042)IL-5 42.98±7.07 82.84±11.011) 57.82±7.442) 43.15±2.872)4)IL-13 15.14±2.53 35.65±5.831) 20.57±2.602) 22.03±2.772)IL-17 70.31±12.16 119.10±21.751) 90.83±18.602) 83.47±7.132)IL-25 97.47±22.75 106.40±26.46 121.05±21.07 105.45±18.39 IL-33 27.54±10.84 58.16±12.731) 39.04±9.12 32.81±2.663)LT 1 049.61±242.39 2 837.78±592.611) 1 699.31±387.093) 1 699.32±339.473)TSLP 7.87±2.38 17.49±2.121) 11.10±2.902) 9.94±2.032)PGD2 149.30±41.31 278.38±56.621) 165.18±28.972) 149.54±26.452)
3 Discussion
3.1 Effect of acupuncture at Feishu (BL12) and Tianshu(ST25) on pulmonary function in asthma model rats
Asthma is a common respiratory disease in clinics. In addition to clinical symptoms, objective criteria such as respiratory hyperresponsiveness and reversible airflow obstruction also support the diagnosis of asthma[20].Therefore, it is of great significance to evaluate airway resistance, reversibility, and airway hyperresponsiveness by pulmonary function tests for diagnosis and treatment evaluation and mechanism analysis of asthma[21].
Among many pulmonary function evaluation indicators, FEV1, FEV1/FVC, FEF25%, FEF50%, and FEF75% are all parameters to evaluate effective vital capacity of asthma[22-23]. Cdyn and RL are affected by multiple factors, such as lung elasticity, thoracic volume,and airway resistance. FEV1, PEF, and FEV1/FVC mainly reflect the ventilation function of airway, and the variation rate of PEF can judge whether airflow limitation is reversible or not[24]. FEF25%, FEF50%,FEF75%, and MMEF reflect the ventilation function of small airways, indicating the independent forced expiratory part of routine ventilation pulmonary function[25]. Our results showed that compared with the normal group, PEF, Cdyn, FEF25%, FEV1/FVC, and MMEF were significantly decreased (P<0.01 orP<0.05),while RL was significantly increased (P<0.05) in the model group, suggesting that the large and small airway ventilation function of the model rats were limited to varying degrees, and the airway function was obviously damaged, reflecting the severity of asthma. Compared with the model group, the FEF25% and FEV1/FVC of rats in the lung and the lung-intestine treatment groups were significantly increased, which indicated that acupuncture at Feishu (BL12) alone or at Feishu (BL12)combined with Tianshu (ST25) could regulate the effective vital capacity and the limited ventilation function of large and small airways in asthma model rats. Compared with the model group, the PEF was significantly increased, and the RL was significantly decreased in the lung-intestine treatment group,suggesting that the combination of Feishu (BL12) and Tianshu (ST25) can improve the expiratory airflow limitation and reduce LR when acupuncture regulates the pulmonary function of asthma model rats. However,there were no significant differences in the PEF and RL between the lung treatment group and the model group. It is suggested that the effect of combined Feishu(BL12) and Tianshu (ST25) is better than that of Feishu(BL12) alone in trend when acupuncture regulates pulmonary function in asthma model rats.
3.2 Effect of acupuncture at Feishu (BL12) and Tianshu(ST25) on pulmonary inflammation in asthma model rats
Asthma is a chronic airway inflammatory disease. It is caused by excessive activation of immune cells and damage of airway epithelium due to excessive inflammatory factors, leading to continuous progress of inflammatory reactions and finally causing airway hyperresponsiveness and airway remodeling[26]. Airway immune inflammation runs through the whole process of asthma; the degree of eosinophil inflammation is positively correlated with the severity of asthma, and neutrophils may also promote the occurrence of asthma[27]. Therefore, regulating the balance of inflammatory factorsin vivoand controlling immune inflammatory reactions are the key to managing asthma[28].
Helper T cell 1/helper T cell 2 (Th1/Th2) imbalance is an important cause of asthma. In the pathogenesis of asthma, Th2 secretes IL-4, IL-5, and IL-13, and airway epithelial cells produce IL-25, IL-33, and TSLP[29]. Mast cells release bronchoconstrictor mediators such as LT and PGD2, participate in the inflammatory reaction process of the respiratory tract, and promote the aggregation of inflammatory cellsin vivo[30-31]. The TSLP levels in the airway epithelial cells and mast cells of asthma patients are increased significantly, which directly stimulates CD4+T cells to release Th2 cytokines and induce allergic inflammation[32]. IL-13 induces inflammation by stimulating the expression of various chemokines, including promoting goblet cell proliferation and airway smooth muscle proliferation[33-34]. IL-17A and IL-17F induce airway epithelial cells and smooth muscle cells to release neutrophils, aggravate airway inflammation and airway hyperresponsiveness in asthma mouse models, and are also related to neutrophil-mediated inflammation[35].IL-25 (also known as IL-17E) increases the number of Th2 cells, induces the expression of IL-4, IL-5, and IL-13,and produces the inflammatory reaction mediated by eosinophils[36]. IL-33 promotes Th2 cell differentiation by affecting chromatin structure and encourages the release of many inflammatory mediators through cytokines such as IL-4, IL-5, IL-13, and IL-25, which makes an abnormally increased airway sensitivity and induces airway inflammation[37].
Studies have shown that electroacupuncture at Tianshu (ST25) reduces the expression of serum inflammatory factors, inhibits the release of pro-inflammatory factors, regulates immune balance,and has a holistic regulatory effect on postoperative intestinal paralysis mice[38-39]. Based on the “gut-lung axis” theory of intestinal flora, the close relationship between airway inflammatory factors and intestinal organs in asthma is clearer. The importance of the intestinal flora, as one of the highest biomass microbial communities in the metabolism and immunity of the organism, is well known[40]. According to the concept of“gut-lung axis”, intestinal microorganisms and their metabolites can not only regulate gastrointestinal immunity but also affect pulmonary immune response through lymph or blood circulation[41-42]. The results of this study showed that the levels of IL-4, IL-5, IL-13,IL-17, LT, TSLP, and PGD2in rat lung tissue of the lung treatment group and the lung-intestine treatment group were significantly lower than those in the model group (P<0.05), which indicated that acupuncture at Feishu (BL12) alone or at Feishu (BL12) and Tianshu(ST25) could effectively regulate the inflammatory reaction of lung tissue in asthma model rats. In addition,the IL-33 level in rat lung tissue of the lung-intestine treatment group was still significantly reduced. The IL-5 level in rat lung tissue of the lung-intestine treatment group was significantly lower than that of the lung treatment group. It is suggested that when acupuncture regulates the inflammatory reaction of lung tissue in asthma model rats, the lung-intestine treatment may play a better role by regulating the Th2 cell differentiation, controlling eosinophils, and reducing the secretion of related inflammatory factorsin vivothrough multiple targets in regulating the IL-33 and IL-5 inflammatory factors.
3.3 Theoretical analysis of the effect difference between “simultaneous treatment of lung-intestine”and “treatment of lung”
Based on the theory of “lung and large intestine in an exterior and interior relationship”, the meridians of lung and large intestine are interlinked, useful for each other in physiology, and influencing each other in pathology.The respiratory tract communicates with the outside environment through the nose and ends at the lung,while the digestive tract communicates with the outside environment through the mouth and ends at the large intestine. The two tracts meet at the pharynx. Therefore,the common intestinal and lung-related diseases in the clinic, especially the intestinal reactions in lung diseases such as asthma, can be reasonably explained. In addition, mucosal immunity may be the bridge between the lung and large intestine, which are also involved in the mucosal immune system, and the intestinal-lung network relationship may be formed through mucosal immunity[43]. Therefore, although asthma is in the lungs,it is closely related to the large intestine.Ling Shu(Miraculous Pivot) says that the Qi of Zang-Fu organs is closely related to the Back-Shu points and the Front-Mu points through Qi streets. Therefore, in this experiment,Feishu (BL12), the Back-Shu point of the lung, and Tianshu (ST25), the Front-Mu point of the large intestine, were used as typical matching points for lung-intestine treatment, and the two points are matched in tandem to regulate the Qi movement of the Zang-Fu organs. At the same time, the application of Chinese medicine at Tianshu (ST25) can effectively improve lung compliance and airway resistance in patients with ventilator-associated pneumonia[44].
It can be observed in acupuncture clinics that“treatment of lung”, “treatment of intestine”, and“simultaneous treatment of lung-intestine” improve the pulmonary function of asthma patients, and the effect of “simultaneous treatment of lung-intestine” is more obvious[10,45]. Acupuncture and moxibustion can not only treat intestinal symptoms of constipation patients but also improve lung symptoms[46]. Here, we found that the simultaneous lung-intestine treatment significantly reduced the neutrophil proportion in the BALF of asthma model rats, significantly improved PEF and RL in the pulmonary function of asthma model rats,and significantly reduced the IL-33 level in the inflammatory factors of lung tissue, compared with the model group. However, there was no statistical difference in the above indicators between the lung treatment group and the model group. Moreover, the IL-5 level was significantly lower in the lung-intestine treatment group than in the lung treatment group.
In conclusion, our current study suggests that acupuncture at Feishu (BL12) alone or Feishu (BL12)and Tianshu (ST25) can regulate pulmonary function and tissue inflammation in asthma model rats, while the combination of Feishu (BL12) and Tianshu (ST25)has a better effect than Feishu (BL12) alone. It may be necessary to increase the sample size to obtain a more stable trend in the future. Meanwhile, clinical application and generalization of the diagnosis and treatment idea of “simultaneous treatment of lung-intestine” also need the support of multi-center and large-sample evidence-based medicine evidence,and the mechanisms still require further research and discussion.
Conflict of Interest
The authors declare that there is no potential conflict of interest in this article.
Acknowledgments
This work was supported by the Project of Natural Science Foundation of Hunan Province (湖南省自然科学基金项目, No. 2021JJ30513); Scientific Research Project of Hunan Education Department (湖南省教育厅科学研究项目, No. 20B444); Project of National Natural Science Foundation of China (国家自然科学基金项目, No.81603705); China Postdoctoral Science Foundation (中国博士后科学基金项目, No. 2107M612567).
Statement of Human and Animal Rights
This study was approved by the Animal Experimental Center of Hunan University of Chinese Medicine (Approval No. LL2021032407). The treatment of animals in this experiment conformed to the ethical criteria.
Received: 28 March 2022/Accepted: 22 June 2022
