直接竞争ELISA检测拟柱孢藻毒素的方法建立和评价
2023-10-26刘金平赖淑燕肖利娟雷腊梅
杨 丹,刘金平,赖淑燕,肖利娟,雷腊梅
直接竞争ELISA检测拟柱孢藻毒素的方法建立和评价
杨 丹,刘金平,赖淑燕,肖利娟,雷腊梅*
(暨南大学生态学系,广东 广州 510632)
拟柱孢藻毒素(CYN)对人和动物具有高毒性而在世界上广受关注,快速检测方法的建立可为蓝藻毒素的风险评估提供有效的技术手段.本研究基于前期制备的CYN的单克隆抗体N8,旨在建立一种CYN的快速免疫检测方法-直接竞争ELISA.在对HRP-CYN酶标记物和N8单抗的稀释度进行优化后,最适反应条件下建立的直接竞争ELISA在0~200ng/mL CYN浓度下呈现典型的S型反应曲线,该方法的灵敏度为0.069ng/mL,经logit-log拟合的线性范围为0.1~10ng/mL,检出限远低于WHO建议的0.7ng/mL CYN的安全限值.本研究建立的ELISA方法对水库水样和小球藻稀释液中加标回收率分别为80.44%~125.4%和52.97%~155.19%,在测定CYN时与商业Beacon试剂盒的检测结果高度一致.对大沙河水库中CYN的监测表明该毒素在水体中常年存在,但未超过WHO建议的安全限值.本研究发展的ELISA可实现水样和藻样中CYN的快速检测,为供水安全保障提供一种新的技术手段.
直接竞争ELISA;拟柱孢藻毒素;单克隆抗体;HRP-CYN连接物;加标回收率
水体富营养化所导致的蓝藻水华现象在全球范围内普遍存在[1-2],许多水华蓝藻会产生一类次生代谢物-蓝藻毒素,如微囊藻毒素(MCs),拟柱孢藻毒素(Cylindrospermopsins,CYN),节球藻毒素, anatoxin-a,和antoxin,不仅对水体造成污染,还对公众健康构成严重威胁[3-4].近20年来,随着热带特征性蓝藻-拟柱孢藻在全球范围内的快速扩张,其所产生的CYN成为继微囊藻毒素后广受关注的另一类蓝藻毒素[5-6].拟柱孢藻是世界上第一个被发现能产CYN的蓝藻[7],随后陆续发现束丝藻、长孢藻、颤藻、尖头藻等蓝藻也能产生CYN.据报导,除了南极洲,CYN目前已呈全球分布态势[6].
CYN是一种分子量为415Da的生物碱,由三环胍部分与羟甲基尿嘧啶结合形成[8].1992年首次确定了它的化学结构[9],科科辛斯基(Kokociński)等发现了CYN存在5种类似物,分别是CYN、7-deoxydesulfo-CYN,7-deoxydesulfo-12-acetyl-CYN,7-epi-CYN,7-deoxy-CYN[10].研究发现CYN具有肝毒性、细胞毒性、神经毒性、繁殖毒性等多种毒性效应[11-12],其进入细胞后能够抑制蛋白质和谷胱甘肽的合成,通过共价修饰的方式改变DNA和RNA,从而造成基因损伤,具有潜在的致癌作用[8],CYN结构已被证明非常稳定,在可见光和紫外光下、极端pH(pH4-12)以及反复煮沸的高温下都难以降解[7,14],由此有研究者认为CYN比微囊藻毒素对人类和动物的危害更大.鉴于CYN的危害性,澳大利亚首先提出1μg/L作为饮用水中CYN的安全限值[15-16], 2016年美国环保署也发布的CYN安全指导值为:对于6岁以下儿童为0.7ng/mL,对于6岁以上人群则为3ng/mL[16-17];2022年,世界卫生组织(WHO)也对饮用水中CYN限值进行了建议,其长期暴露浓度为不超过0.7ng/mL.
蓝藻毒素的潜在危害使得其检测技术长期以来一直是国内外研究热点[3,6,17],利用蓝藻毒素的产生基础和生理化学特性如分子量、生色团和反应性,已发展了化学分析、分子分析、免疫分析和生物分析等一系列方法用于产毒蓝藻的甄别和蓝藻毒素的定量检测,其中以微囊藻毒素的检测分析体系发展得最为成熟[19-21].在CYN的检测中,HPLC[21](高效液相色谱法)和LC-MS/MS[23-24](液相色谱串联质谱技术)为主要的化学检测方法,它们具有检测结果准确、能区分不同异构体等优点[23],但这类检测技术操作复杂,仪器昂贵,对操作人员要求高,不适合用于检测次数频繁的常规和应急监测[26-29].免疫学分析具有高灵敏、快速度、高通量的优点,近二十多年来在蓝藻毒素尤其是MCs检测领域应用十分广泛,基于MCs的多克隆和单克隆抗体发展的方法有酶联免疫吸附法(ELISA)、时间分辨免疫分析法(TRFIA)、化学发光免疫分析法、现代的电信号免疫分析法、光信号免疫分析法免疫荧光试纸条等[30-33]等,这些免疫检测技术已应用于水体、藻样或食品中微囊藻毒素的定量分析.
与微囊藻毒素相比,拟柱胞藻毒素抗体制备的研究极为稀少,目前仅有两例,2013年 Elliott等[34]在国际上最先报道成功制备了CYN单克隆抗体,并建立了CYN的间接竞争ELISA方法;随后Lei等[35]也成功筛选到多株CYN的特异性单抗,为国内首次,并基于其中的N8单抗建立了TRFIA方法.由于ELISA在蓝藻毒素的检测中应用更为广泛,本研究以N8单抗为基础,发展CYN的直接竞争ELISA方法,以实现对水体中拟柱孢藻毒素浓度的快速测定,为保障供水安全提供一种新的技术手段.
1 材料与方法
1.1 材料和试剂
羊抗鼠二抗购自美国Biodesign公司,BSA(牛血清白蛋白)为MBCHEM公司产品,HRP(辣根过氧化物酶)为Sigma-Aldrich公司产品,CYN标准品购自ALEXIS公司,10kd超滤离心管购自广州普智生物有限公司,96微孔板为Corning公司产品,其他试剂均为国产分析纯.
1.2 方法
1.2.1 酶标记抗原HRP-CYN的制备 HRP-CYN的制备参考文献[34],将辣根过氧化物酶(HRP)溶解在磷酸盐缓冲液(PBS)中,向其中加入CYN和甲醛并将混合物避光室温下搅拌,最后通过0.01mol/L磷酸盐缓冲液对偶联物进行透析,于-20℃冷冻保存备用.
1.2.2 固相包被二抗的制备 将浓度为5μg/mL的羊抗鼠IgG按200μL/孔分装到96孔微孔板中,室温震荡10min,37℃恒温箱放置过夜,倾倒包被液并洗板,加入封闭液进行封闭,4℃过夜,倾去封闭液并洗涤后真空抽干,-20℃保存备用.
1.2.3 抗原抗体效价优化测定 通过棋盘格滴定法对单克隆抗体N8和酶标抗原HRP-CYN的效价进行摸索确定,在包被二抗的微孔板中依次加入50μL不同稀释度的HRP-CYN(1:2000,1:4000, 1:8000,1:16000)和50μL不同稀释度的N8单抗(1:2500, 1:5000, 1:7500, 1:10000, 1:15000, 1:20000), 37℃下反应1h,洗涤6次后加入显色液,37℃反应30min后加入终止液,通过多功能酶标仪测定OD450nm的吸光值.

1.2.5 评价指标 (1)灵敏度
计算8组标准曲线中零浓度点的OD450nm的平均值()和标准差(s),根据-2s从标准曲线计算出相对应的浓度.
(2)稳定性
比较该体系不同时间段的50,根据多次检测判断剂量反应曲线的位置漂移从而考核该方法的稳定性.
(3)加标回收率的测定
收集小球藻裂解上清100倍稀释液和没有发生蓝藻水华的流溪河水库水为空白水样,过滤后加入CYN标准品,终浓度分别为0.1,0.5,5,8ng/mL,随后用直接竞争ELISA进行浓度测定,每个浓度做3个平行测定.
(4)与商业ELISA试剂盒检测结果的比较
用美国Beacon公司的ELISA试剂盒和我们建立的ELISA方法对22份样品(水库水样和产毒拟柱孢藻藻样)同时进行检测,比较两种方法对CYN浓度测定的一致性.
1.2.6 大沙河水库中CYN浓度的测定 大沙河水库位于广东省开平市,前期研究表明该水库拟柱孢藻已形成季节性优势[35],为了解该水库中CYN的发生情况,于2021年9月~2022年8月期间(2021年12月样品缺失)每月定期采集取水口和浮台表层0.5m的水样,经反复冻融处理后采用上述ELISA法测定上清液中的总CYN浓度.
2 结果
2.1 酶标抗原HRP-CYN的稀释度和单克隆抗体效价确定
由图1可知,在相同的HRP-CYN稀释度下, OD450nm的吸光值均随抗体稀释度的升高而降低, HRP-CYN在1:8000的稀释后的吸光值较1:4000显著下降,为保证较高的吸光值,在标准曲线的制作中,酶标抗原选用1:4000的稀释度.竞争ELISA反应中酶标抗原HRP-CYN需与游离CYN竞争性结合单克隆抗体,因此抗体浓度必须适量,因此在进一步研究中选用1:10000的稀释度.

图1 不同稀释度下酶标抗原HRP-CYN和CYN单抗反应曲线
2.2 直接竞争ELISA标准曲线
以CYN为标准品制作的直接竞争ELISA的标准曲线见图2,在0~200ng/mL的范围下,标准曲线呈典型的S型(图2a);采用logit-log法进行线性拟合发现,本研究建立的直接竞争ELISA在0.1~10ng/mL的范围内呈良好的线性(图2b).进一步从图2b可知该标准曲线的的标准差极小,板内3个标样重复测定的变异系数在0.54%~7.8%,说明本研究建立的ELISA标准曲线具有较好的重现性.

a, CYN浓度在0~200ng/mL范围内OD450nm吸光值变化的反应曲线; b,相对应的logit-log 线性标准曲线和批内精确度
2.3 方法的灵敏度与稳定性
CYN浓度为0ng/mL时在OD450nm处的吸光值均值减去2倍标准差的值,在线性标准曲线中所对应的浓度,即为该直接竞争ELISA方法的灵敏度,经计算为0.069ng/mL.IC50在8条不同时间段的均值为(0.48±0.053)ng/mL,SD值相对较小说明该方法的稳定性好.
2.4 加标回收率
将CYN标准品分别加至流溪河水库滤水和小球藻裂解稀释液中,采用本研究建立的ELISA法对加标样品中的毒素浓度进行测定,发现水库滤水的回收率在80.4%~125.4%,CV值小于9.05%(表1),这表明环境水样中的基质对ELISA测定的影响较小.CYN在小球藻稀释液中的回收率在52.9%~ 155.2%间,CV值小于8.16%(表1),表明藻样中的基质对ELISA测定的存在一定干扰.

表1 CYN加标回收率和变异系数
2.5 与商业ELISA试剂盒的比较
采用自行研制的ELISA方法和商业ELISA试剂盒(Beacon公司)同时分析22个样品中的CYN浓度,由图3可知,两种方法所获得CYN浓度间具极显著相关(2=0.9638,<0.05).整体来看商业试剂盒的检测浓度要低于自制直接竞争ELISA的检测浓度,进一步对两组数据进行了one-way ANOVA分析发现它们不存在显著差异(>0.05),表明自行研制的ELISA方法和商业ELISA试剂盒所获得的CYN浓度高度一致.

图3 自制ELISA方法与商业ELISA试剂盒的线性关系
2.6 广东省大沙河水库中CYN浓度的季节变化
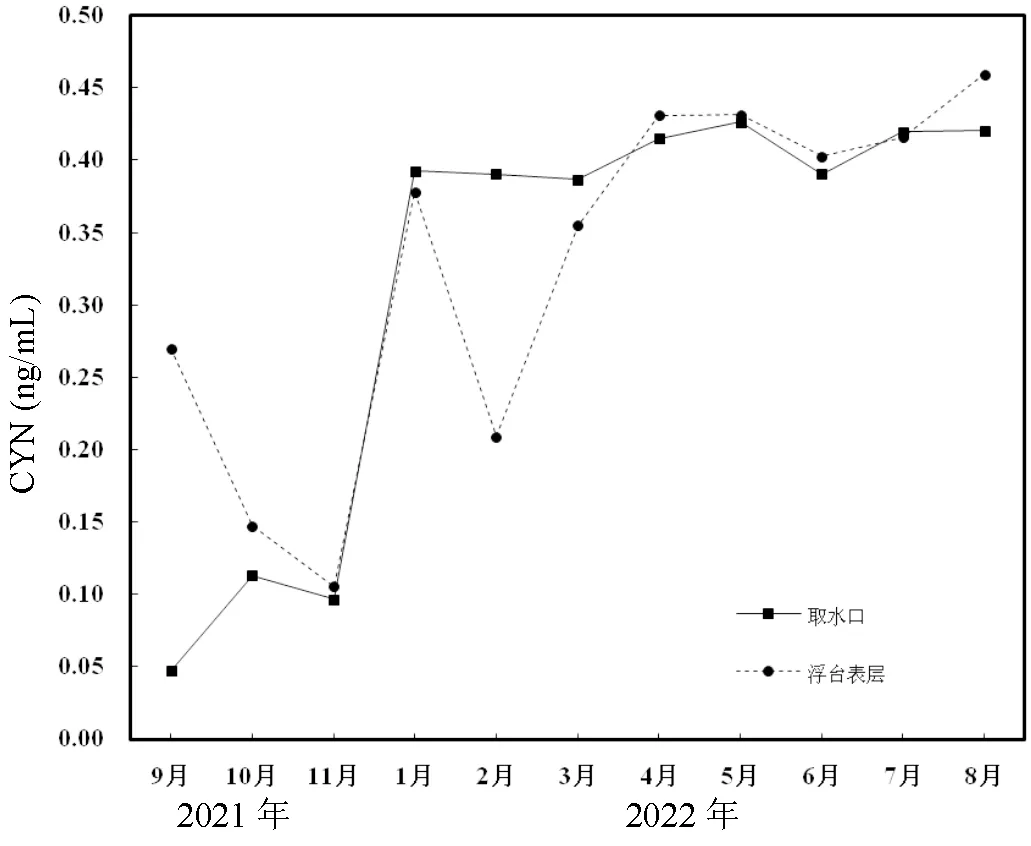
图4 大沙河水库取水口与浮台表层拟柱胞藻毒素浓度
由图4可知,2021年9月~2022年8月间大沙河水库取水口和浮台表层的CYN浓度分别0.1~0.426ng/mL和0.147~0.459ng/mL变化,仅取水口2021年9月的CYN浓度低于ELISA的线性检测限0.1ng/mL.与2021年相比,两个采样点的CYN浓度在2022年1月起即显著上升,随后基本维持在0.4ng/mL的水平;除2021年9月和2022年1月外,大沙河水库两个采样点的CYN浓度基本一致.
3 讨论
作为近年来备受关注的蓝藻毒素,CYN的抗体制备研究极少,因此基于抗体的快速免疫监测方法也相对较少[20,33-34].本研究建立了基于CYN的特异性单克隆抗体N8的直接竞争ELISA,该方法灵敏度为0.069ng/mL,线性检测范围在0.1~10ng/mL,灵敏度与Elliott et al(2013)发展的间接竞争ELISA以及两种商业试剂盒(美国Abraxis和Beacon公司)相当[20].上述两种商业试剂盒的线性范围分别为0.05~ 2ng/mL和0.1~2ng/mL,因此本研究建立的ELISA的线性范围(0.1~10ng/mL)优于两种商业试剂盒,但比同样基于N8单抗建立的时间分辨荧光免疫技术(TRFIA法)的范围窄(0.1~50ng/mL)[33].而以稀土离子作为标记物的时间分辨荧光免疫分析法被认为是更先进的免疫标记方法,是优于酶联免疫吸附法的一种超微量定量免疫分析检测技术,其灵敏度、重复性、检测范围等均要优ELISA[36].
加标准样品回收实验是评价ELISA、TRFIA等免疫学检测方法准确度的一个重要指标,一般认为80%~120%的回收率为正常波动范围[37-39].本研究中水库滤水的加标回收率80.4%~125.4%,此前我们基于N8单抗建立的TRIFA法评价了无水华的池塘滤水的加标回收率[33],与许多基于微囊藻毒素免疫检测的研究结果一致,即自然河流和湖泊的滤水中蓝藻毒素的加标回收率高[30-40],但也有研究发现水样中微囊藻毒素的回收率只有62%[41],这可能是来源不同的水样中存在的干扰物质差异所致[42].而小球藻稀释液在0.1和0.5ng/mL两个低浓度的回收率分别为155.2%和52.9%,比正常波动范围出现较大偏离,这表明小球藻中存在的基质对ELISA检测有更大的干扰,尤其在低浓度时,YU et al.(2001)在藻样中微囊藻毒素的检测中也发现类似的现象.因此采用ELISA分析藻类样品中的CYN时,低浓度的测值可能需要固相微萃取等前处理,以适当的去除藻细胞中的干扰物质[43].
目前市场上主要有两种商业ELISA试剂盒(分别来自美国Abraxis和Beacon公司)在国内外广泛应用于CYN检测[20,22,44].两者的检测模式均为直接竞争模式,与本研究建立的ELISA方法相似.然而Abraxis和Beacon两种商业试剂盒中的抗体都为兔多克隆抗体,而作者建立的ELISA则是基于特异性的单克隆抗体N8,一般认为多克隆抗体特异性相对要低于单克隆抗体[45],这可能是Beacon试剂盒所测得的CYN浓度要稍低于我们建立的ELISA方法的原因,在此前TRFIA与Beacon试剂盒的对比中也发现类似的现象[33].一般认为LC-MS/MS为复杂样品中小分子化合物分析的金标准方法,但由于该方法所需仪器极为昂贵,且需专业人员操作,这极大地限制了质谱技术在蓝藻毒素检测中的推广应用[25,27].基于商业试剂盒在我国的广泛使用[33,44-47],本研究选择与Beacon公司试剂盒所获得的测值进行对比,结果发现尽管两种方法获得的CYN浓度存在差异,但统计分析不显著,这表明本实验研究的ELISA方法是可信赖的,为进一步开发试剂盒奠定了坚实基础.
大沙河水库地处广东省开平市,属典型的亚热带气候.大沙河水库基本常年可检到CYN,但均没有超过WHO建议的0.7ng/mL的安全限值,对饮用水安全不存在威胁.此前我国巢湖夏季水华期的CYN检出率也非常高,在6~7月达100%,最高浓度为0.328ng/mL,均未超出安全限值[46].结合我们前期对广东省水库的研究[47-48],推测CYN在我国的湖泊和水库可能呈广泛分布,但目前除广东省外还缺乏监测数据.我国水体中较低的CYN浓度可能是拟柱孢藻的产毒基因型优势度低所致,Jiang等[49]发现产CYN的拟柱孢藻在我国呈零星分布,本课题组此前的研究也发现广东省水库是非产毒基因型占据优势,CYN浓度与产毒拟柱孢藻丰度和比例的相关性要高于总拟柱孢藻丰度[48].尽管目前大沙河水库中CYN浓度未超过0.7ng/mL,但该水库自2016年以来拟柱孢藻的相对优势度和持续时间不断增加[35],因此需对该水库加强监测,以防范产毒拟柱孢藻优势增加而导致CYN超标问题.
4 结论与展望
4.1 结论
本研究建立基于CYN单克隆抗体N8 的直接竞争ELISA方法,该法的检测限为0.069ng/mL,线性检测范围为0.1~10ng/mL,具较好的稳定性和回收率,与商业试剂盒的检测结果高度一致,因此我们发展的ELISA方法有望替代进口试剂盒,为水样和藻样中CYN的快速检测提供可靠的技术手段,而大沙河水库中CYN的常年发生表明需加强对该毒素的常规监测,以保障水库供水安全.
4.2 展望
本研究表明基于N8单克隆抗体的直接竞争ELISA与Beacon公司试剂盒对CYN的测值高度一致,且我们新建立的ELISA具更宽的线性范围.由于目前国外的Abraxis和Beacon商业试剂盒虽然灵敏度高、稳定性好,但价格极为昂贵,在进行大量样品的监测时成本较高.我们的目标是进一步优化新建立的ELISA方法,提高试剂的稳定性,增加试剂的保存期,逐步将本研究开发的ELISA方法向商业试剂盒的方向推进,最终实现CYN检测的ELISA试剂盒的进口替代.
[1] O’Neil J M, Davis T W, Burford M A, et al. The rise of harmful cyanobacteria blooms: the potential roles of eutrophication and climate change [J]. Harmful Algae, 2012,14:313-334.
[2] Tokodi N, Drobac D, Lazić G, et al. Screening of cyanobacterial cultures originating from different environments for cyanotoxicity and cyanotoxins [J]. Toxicon, 2018,154:1-6.
[3] Dittmann E, Fewer D P, Neilan B A. Cyanobacterial toxins: Biosynthetic routes and evolutionary roots [J]. FEMS Microbiology Reviews, 2013,37(1):23-43.
[4] Berry J P, Lind O. First evidence of “paralytic shellfish toxins” and cylindrospermopsin in a Mexican freshwater system, Lago Catemaco, and apparent bioaccumulation of the toxins in “tegogolo” snails (Pomacea patula catemacensis) [J]. Toxicon, 2010,55(5):930-938.
[5] Rzymski P, Poniedziałek B. In search of environmental role of cylindrospermopsin: A review on global distribution and ecology of its producers [J]. Water Research, 2014,66:320-337.
[6] Moreira C, Azevedo J, Antunes A, et al. Cylindrospermopsin: Occurrence, methods of detection and toxicology [J]. Journal of Applied Microbiology, 2013,114(3):605-620.
[7] Harada K, Ohtani I, Iwamoto K, et al. Isolation of cylindrospermopsin from a cyanobacterium Umezakia natans and its screening method [J]. Toxicon, 1994,32(1):73-84.
[8] Hinojosa M G, Gutiérrez-Praena D, Prieto A I, et al. Neurotoxicity induced by microcystins and cylindrospermopsin: A review [J]. Science of the total environment, 2019,668:547-565.
[9] Ohtani I, Moore R E, Runnegar M T C. Cylindrospermopsin: A potent hepatotoxin from the blue-green alga[J]. Journal of the American Chemical Society, 1992,114(20):7941-7942.
[10] Kokociński M, Cameán A M, Carmeli S, et al. Cylindrospermopsin and congeners [J]. Handbook of Cyanobacterial monitoring and Cyanotoxin analysis, 2016,127-137.
[11] Wimmer K M, Strangman W K, Wright J L C. 7-Deoxy-desulfo-cylindrospermopsin and 7-deoxy-desulfo-12-acetylcylin drospermopsin: Two new cylindrospermopsin analogs isolated from a Thai strain of[J]. Harmful Algae, 2014,37:203-206.
[12] Pichardo S, Cameán A M, Jos A. In vitro toxicological assessment of cylindrospermopsin: A review [J]. Toxins, 2017,9(12):402.
[13] Guzmán-Guillén R, Manzano I L, Moreno I M, et al. Cylindrospermopsin induces neurotoxicity in tilapia fish (Oreochromis niloticus) exposed to Aphanizomenon ovalisporum [J]. Aquatic Toxicology, 2015,161:17-24.
[14] Adamski M, Żmudzki P, Chrapusta E, et al. Effect of pH and temperature on the stability of cylindrospermopsin. Characterization of decomposition products [J]. Algal research, 2016,15:129-134.
[15] Humpage A R, Falconer I R. Oral toxicity of the cyanobacterial toxin cylindrospermopsin in male Swiss albino mice: determination of no observed adverse effect level for deriving a drinking water guideline value [J]. Environmental Toxicology: An International Journal, 2003, 18(2):94-103.
[16] Vaz R, Valpradinhos B, Frasco M F, et al. Emerging optical materials in sensing and discovery of bioactive compounds [J]. Sensors, 2021, 21(17):5784.
[17] D’Anglada L V, Strong J. Drinking water health advisory for the cyanobacterial microcystin toxins [J]. US EPA, EPA-820R15100, 2015.
[18] Huisman J, Codd G A, Paerl H W, et al. Cyanobacterial blooms [J]. Nature Reviews Microbiology, 2018,16(8):471-483.
[19] Poon K F, Lam M H, Lam P K S, et al. Determination of microcystins in cyanobacterial blooms by solid-phase microextraction-high- performance liquid chromatography [J]. Environmental Toxicology and Chemistry: An International Journal, 2001,20(8):1648-1655.
[20] Mountfort D O, Holland P, Sprosen J. Method for detecting classes of microcystins by combination of protein phosphatase inhibition assay and ELISA: comparison with LC-MS [J]. Toxicon, 2005,45(2):199-206.
[21] Moreira C, Ramos V, Azevedo J, et al. Methods to detect cyanobacteria and their toxins in the environment [J]. Applied Microbiology and Biotechnology, 2014,98(19):8073-8082.
[22] Beltrán E, Ibáñez M, Sancho J V, et al. Determination of six microcystins and nodularin in surface and drinking waters by on-line solid phase extraction–ultra high pressure liquid chromatography tandem mass spectrometry [J]. Journal of Chromatography A, 2012, 1266:61-68.
[23] Bláhová L, Oravec M, Maršálek B, et al. The first occurrence of the cyanobacterial alkaloid toxin cylindrospermopsin in the Czech Republic as determined by immunochemical and LC/MS methods [J]. Toxicon, 2009,53(5):519-524.
[24] Guzmán-Guillén R, Prieto A I, González A G, et al. Cylindrospermopsin determination in water by LC-MS/MS: Optimization and validation of the method and application to real samples [J]. Environmental Toxicology and Chemistry, 2012,31(10): 2233-2238.
[25] Li R, Carmichael W W, Brittain S, et al. Isolation and identification of the cyanotoxin cylindrospermopsin and deoxy-cylindrospermopsin from a Thailand strain of(Cyanobacteria) [J]. Toxicon, 2001,39(7):973-980.
[26] Kaushik R, Balasubramanian R. Methods and approaches used for detection of cyanotoxins in environmental samples: a review [J]. Critical Reviews in Environmental Science and Technology, 2013, 43(13):1349-1383.
[27] Van Hassel W H R, Ahn A C, Huybrechts B, et al. LC-MS/MS validation and quantification of cyanotoxins in algal food supplements from the Belgium market and their molecular origins [J]. Toxins, 2022,14(8):513.
[28] Vogiazi V, de la Cruz A, Mishra S, et al. A comprehensive review: Development of electrochemical biosensors for detection of cyanotoxins in freshwater [J]. ACS Sensors, 2019,4(5):1151-1173.
[29] Li R, Carmichael W W, Brittain S, et al. First report of the cyanotoxins cylindrospermopsin and deoxycylindrospermopsin from(Cyanobacteria) [J]. Journal of Phycology, 2001,37(6):1121-1126.
[30] Kalate Bojdi M, Behbahani M, Najafi M, et al. Selective and sensitive determination of uranyl ions in complex matrices by ion imprinted polymers-based electrochemical sensor [J]. Electroanalysis, 2015, 27(10):2458-2467.
[31] Long F, Shi H C, He M, et al. Sensitive and rapid chemiluminescence enzyme immunoassay for microcystin-LR in water samples [J]. Analytica Chimica Acta, 2009,649(1):123-127.
[32] Zhang H, Yang S, Beier R C, Beloglazova N V, et al. Simple, high efficiency detection of microcystins and nodularin-R in water by fluorescence polarization immunoassay [J]. Analytica Chimica Acta, 2017,992:119-127.
[33] Ogungbile A O, Ashur I, Icin I, et al. Rapid detection and quantification of microcystins in surface water by an impedimetric immunosensor [J]. Sensors and Actuators B: Chemical, 2021,348: 130687.
[34] Lei L, Peng L, Yang Y, et al. Development of time-resolved fluoroimmunoassay for detection of cylindrospermopsin using its novel monoclonal antibodies [J]. Toxins, 2018,10(7):255.
[35] Elliott C T, Redshaw C H, George S E, et al. First development and characterisation of polyclonal and monoclonal antibodies to the emerging fresh water toxin cylindrospermopsin [J]. Harmful Algae, 2013,24:10-19.
[36] Xiao L J, Xie J, Tan L, et al. Iron enrichment from hypoxic hypolimnion supports the blooming ofin a tropical reservoir [J]. Water Research, 2022:118562.
[37] Dickson E F G, Pollak A, Diamandis E P, Time-resolved detection of lanthanide luminescence for ultrasensitive bioanalytical assays [J]. Journal of Photochemistry and Photobiology B: Biology, 1995,27(1): 3-19.
[38] Zhang Y, Ding X L, Guo M M, et al. Quantitative and rapid detection of microcystin-LR using time-resolved fluorescence immunochromatographic assay based on europium nanospheres [J]. Analytical Methods, 2017,9(45):6430-6434.
[39] Barna-Vetró I, Solti L, Téren J, et al. Sensitive ELISA test for determination of ochratoxin A [J]. Journal of Agricultural and Food Chemistry, 1996,44(12):4071-4074.
[40] Heussner A H, Winter I, Altaner S, et al. Comparison of two ELISA-based methods for the detection of microcystins in blood serum [J]. Chemico-Biological Interactions, 2014,223:10-17.
[41] Sheng J W, He M, Shi H C. A highly specific immunoassay for microcystin-LR detection based on a monoclonal antibody [J]. Analytica Chimica Acta, 2007,603(1):111-118.
[42] Yang H, Dai R, Zhang H, et al. Production of monoclonal antibodies with broad specificity and development of an immunoassay for microcystins and nodularin in water [J]. Analytical and Bioanalytical Chemistry, 2016,408(22):6037-6044.
[43] Díez-Quijada L, Prieto A I, Guzmán-Guillén R, et al. Occurrence and toxicity of microcystin congeners other than MC-LR and MC-RR: A review [J]. Food and Chemical Toxicology, 2019,125:106-132.
[44] Yu F Y, Liu B H, Chou H N, et al. Development of a sensitive ELISA for the determination of microcystins in algae [J]. Journal of Agricultural and Food Chemistry, 2002,50(15):4176-4182.
[45] Tan F J, Xiao P, Zuo J, et al.Genetic Diversity of Raphidiopsis raciborskii, a Toxic Species Produced in Shidou Reservoir, Fujian Province [J] Journal of Ecotoxicology, 2022,(3):56-67.
[46] Lipman N S, Jackson L R, Trudel L J, et al. Monoclonal versus polyclonal antibodies: distinguishing characteristics, applications, and information resources [J]. ILAR Journal, 2005,46(3):258-268.
[47] Zhu C, Yang X R, Zhao B, et al.Temporal and spatial variation characteristics of Phytoplankton and cyanobacterial toxins during Chaohu Lake bloom in summer 2017 [J]. China Environmental Monitoring, 2018,34(6):103-112.
[48] Lei L, Peng L, Huang X, et al. Occurrence and dominance ofand dissolved cylindrospermopsin in urban reservoirs used for drinking water supply, South China [J]. Environmental Monitoring and Assessment, 2014,186(5):3079-3090.
[49] Lei L, Lei M, Lu Y, et al. Development of real-time PCR for quantification ofcells and potential cylindrospermopsin-producing genotypes in subtropicalreservoirs of southern China [J]. Journal of Applied Phycology, 2019,31(6): 3749-3758.
[50] Jiang Y, Xiao P, Yu G, et al. Sporadic distribution and distinctive variations of cylindrospermopsin genes in cyanobacterial strains and environmental samples from Chinese freshwater bodies [J]. Applied and Environmental Microbiology, 2014,80(17):5219-5230.
Establishment and assessment of a competitive direct enzyme-linked immunosorbent assay (cdELISA) for the determination of cylindrospermopsin.
YANG Dan, LIU Jin-ping, LAI Shu-yan, XIAO Li-juan, LEI La-mei*
(Department of Ecology, Jinan University, Guangzhou 510632, China)., 2023,43(10):5543~5549
Cylindrospermopsins(CYN) has been widely concerned in the world for its high toxicity to humans and animals. The establishment of a rapid detection method can provide an effective tool for the risk assessment of CYN. Based on the monoclonal antibody N8 against CYN prepared, a direct competitive ELISA has been developed. After optimization of the dilution of HRP-CYN conjugate and N8 monoclonal antibody, the newly developed ELISA exhibited a typical sigmoidal response for CYN at concentrations of 0~200ng/mL, with a wide quantitative range between 0.1 and 10ng/mL by using logit-log method for fitting the curve. The detection limit of the method was calculated to be 0.069ng/mL, which is well below guideline value of 0.7ng/mL CYN recommended by WHO. Recoveries ranging from 80.44% to 125.4% and 52.97% to 155.19% were observed upon analyzing CYN-spiked water samples anddiluent, respectively. Moreover, CYN concentrations obtained from our cdELISA method through testing different samples was highly consistent with the Beacon ELISA kit. It was found that CYN can be detected year around in Dashahe reservoir but never beyond the guideline value of 0.7ng/mL.Therefore, the developed ELISA will achieve the rapid detection of CYN in water samples and algae samples and may provide a reliable technical tool for drinking water safety.
direct competitive ELISA;cylindrospermopsin;monoclonal antibody;HRP-CYN;spiked recovery
X171.5;X835
A
1000-6923(2023)10-5543-07
2023-02-20
广东省自然科学基金项目(2023A1515012361);国基自然科学基金项目(31770507)
* 责任作者, 副教授, tleilam@jnu.edu.cn
杨 丹(1997-),女,湖南邵阳人,暨南大学硕士研究生,主要从事蓝藻毒素免疫学检测相关研究.914884242@qq.com.
杨 丹,刘金平,赖淑燕,等.直接竞争ELISA检测拟柱孢藻毒素的方法建立和评价 [J]. 中国环境科学, 2023,43(10):5543-5549.
Yang D, Liu J P, Lai S Y, et al. Establishment and assessment of a competitive direct enzyme-linked immunosorbent assay (cdELISA) for the determination of cylindrospermopsin [J]. China Environmental Science, 2023,43(10):5543-5549.
