Laparoscopy-assisted gastrectomy for advanced gastric cancer patients with situs inversus totalis: Two case reports and review of literature
2023-10-21HongBoLiuXiaoPengCaiZhaoLuBinXiongChunWeiPeng
Hong-Bo Liu, Xiao-Peng Cai, Zhao Lu, Bin Xiong, Chun-Wei Peng
Abstract
Key Words: Situs inversus totalis; Laparoscopy-assisted gastrectomy; Advanced gastric cancer; Surgery modality; Lymphadenectomy; Case report
INTRODUCTION
Situs inversus totalis (SIT), a rare autosomal recessive disorder characterized by a complete congenital mirror-image malposition of the thoracic and abdominal organs, has an incidence of about 1/10000-1/20000[1]. Due to the inverted anatomical disposition of organs in patients with SIT, surgeons are required to adopt unfamiliar operating habits, which also increases the difficulty of identifying anatomical structures. Despite the rarity of encountering this anomaly, focusing on SIT may compensate for the lack of experience in surgery.
Laparoscopic radical gastrectomy has been performed in specialized centers in recent years for its minimal invasiveness and association with uneventful recovery. However, this operation is still challenging due to the change in traditional operating habits and increased difficulty in identifying anatomical structures under the local visual field, which are also difficulties encountered in patients with SIT. Moreover, no tactility can be of significant influence during surgery, making it more complicated to implement laparoscopic radical gastrectomy in patients with SIT. In light of such patients, it is still worth summarizing experience and exploring the most feasible ways of optimizing surgery.
In this report, we present the cases of two patients having advanced gastric cancer (GC) with SIT, both of whom underwent laparoscopy-assisted gastrectomy and standard D2 lymphadenectomy (LND). We also review the literature to discuss the current surgical strategies for GC patients with SIT.
CASE PRESENTATION
Chief complaints
Case 1: A 65-year-old Chinese man presented with intermittent abdominal pain and distension, occasional belching, and acid reflux with no obvious cause.
Case 2: A 55-year-old Chinese man presented with upper abdominal distension with pain and discomfort after eating.
History of present illness
Case 1: In April 2022, the patient presented at a local hospital with intermittent abdominal pain and distension, occasional belching, and acid reflux of no obvious etiology for 4 mo, all of which were unrelated to eating. He denied nausea, vomiting, hematemesis, black stool, diarrhea, and constipation. Upper gastrointestinal endoscopy identified poor motility of the gastric antrum, with a large ulcer covered with black scabs and blood clots. Furthermore, tissue biopsy with histopathology revealed the presence of poorly or moderately differentiated adenocarcinoma. The patient did not receive any anti-tumor treatment before hospitalization. Since the onset of the disease, his spirit, appetite, sleep, physical strength, and weight did not change significantly. The patient was admitted to our hospital for further treatment.
Case 2: In December 2019, the patient visited a local hospital for postprandial upper abdominal distension with pain and discomfort for 3 mo. He denied nausea, vomiting, hematemesis, black stool, diarrhea, and constipation. Also, the selfadministered gastric protective medicine was ineffective. Upper gastrointestinal endoscopy identified a gastric cardia ulcer, which tissue biopsy identified as poorly differentiated adenocarcinoma and partly signet-ring cell carcinoma. The patient did not receive any anti-tumor treatment before hospitalization. Since the onset of the disease, his spirit, appetite, sleep, and physical strength did not change significantly, except for his weight which decreased slightly. Then, the patient was admitted to our hospital for further treatment.
History of past illness
Case 1: The patient denied any history of a chronic illness. He had no history of abdominal surgery.
Case 2: The patient acknowledged a history of hypertension for ten more years that was properly controlled with regular medication. He had no history of abdominal surgery.
Personal and family history
Case 1: The patient denied any family history of malignant tumors.Case 2: The patient denied any family history of malignant tumors.
Physical examination
Case 1: Physical examination revealed an apical heartbeat on the right side.
Case 2: Physical examination revealed mild lower abdominal tenderness and an apical heartbeat on the right side.
Laboratory examinations
Case 1: No obvious abnormal tumor markers were observed.
Case 2: Carbohydrate antigen CA199 level was 365.2 (normal range: 0–37.0) U/mL.
Imaging examinations
Case 1: The detailed imaging results are presented in Figure 1. Abdominal contrast-enhanced computed tomography (CT) revealed mirror transposition of all organs (Figure 1A) and thickening of the antral wall (Figure 1B). Besides, positron emission tomography-CT performed in a local hospital revealed increased metabolism in the gastric antrum and surrounding lymph nodes. No abnormal course of vascularity was found in this process.
Case 2: Abdominal contrast-enhanced CT revealed mirror transposition of all organs (Figure 1G), localized thickening of the cardia and smaller curvature of the stomach, and increased numbers of peri-gastric small lymph nodes (Figure 1H). No abnormal course of vascularity was found in this process.
FURTHER DIAGNOSTIC WORK-UP
Case 1
A preoperative pathological examination was performed. The analysis of the biopsy segment resected from the gastric antrum ulcer revealed poorly to moderately differentiated adenocarcinoma (cT3N1-2M0). Immunohistochemical analyses revealed that the tumor cells tested positive for MSH2 and MSH6 and negative for HER-2, MLH1, and PMS2, which signifies a loss of MMR expression.
Case 2
No preoperative pathological examination was performed.
FINAL DIAGNOSIS
Case 1
The patient was diagnosed with adenocarcinoma of the gastric antrum (cT3N1-2M0) with SIT.
Case 2
The patient was diagnosed with gastric cardia malignancy (cT3N1M0) with SIT.
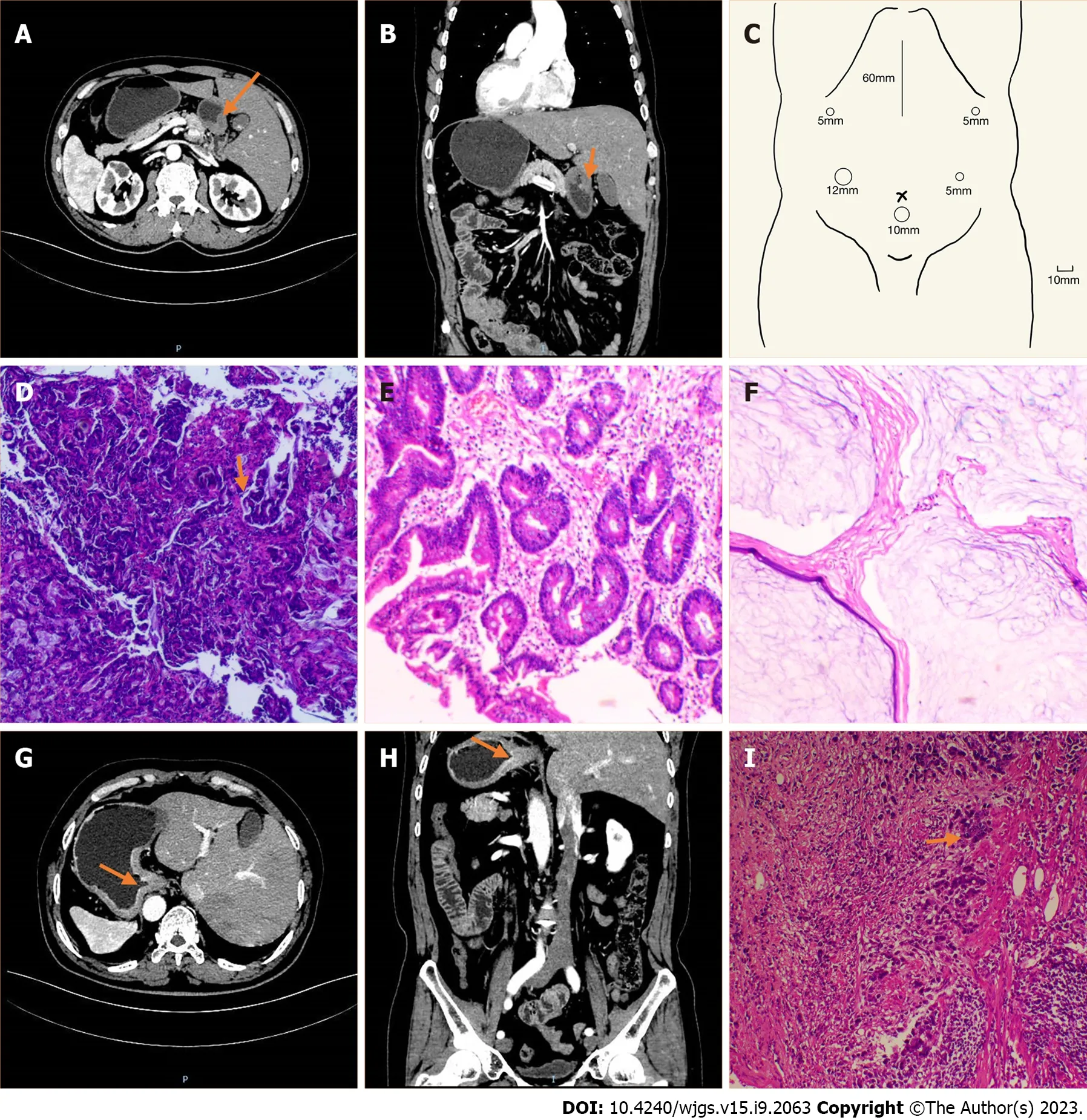
Figure 1 Imaging and pathological features of the two patients. A–F: Case 1; A: Computed tomography (CT) image showing the inverse positioning of intra-abdominal organs; B: CT image showing antral wall thickening; C: Sites of trocar placement. A 12-mm trocar was placed in the right hypochondriac region, and the other three 5-mm trocars were placed (one each) in the right subcostal region, the right lateral abdominal region, and the left lateral abdominal region; D:Preoperative (after neoadjuvant chemotherapy) pathologic biopsies showing that tumor nests still existed; E: Postoperative pathologic biopsies showing that there were no nidi; F: Postoperative pathologic biopsies showing no nidi; G–I: Case 2; G: CT image showing the inverse positioning of all intra-abdominal organs; H: CT image revealing the thickening of the cardia and smaller curvature of gastric tissue and enlarged peri-gastric small lymph nodes; I: Postoperative pathologic biopsies showing that nidi still existed.
TREATMENT
Case 1
Before surgery, four cycles of neoadjuvant chemotherapy with the S-1 and oxaliplatin (SOX) regimen (oxaliplatin 230 mg on day 1, tegafur 60 mg twice daily on days 1–14, and sintilimab 200 mg once every 3 wk) and immunotherapy was given. The preoperative (after neoadjuvant chemotherapy) pathologic biopsies are presented in Figure 1D.
Laparoscopy-assisted distal gastrectomy with standard D2 LND and Billroth II reconstruction were performed. During surgery, the surgeon stood on the right side of the patient (opposite to the usual side for patients undergoing laparoscopic gastrectomy). A 10-mm trocar was created 10 mm below the umbilicus, and carbon dioxide was injected into the peritoneal cavity at 10 mmHg. The other four trocars were placed in the bilateral subcostal and lateral abdominal area, arranged in a “U” shape (Figure 1C). No implantation metastasis or vascular variants were found (Figure 2).
The procedure lasted for 240 min, and the estimated blood loss was 50 mL. A total of 34 lymph nodes were retrieved. The lesion was located in the antrum, and there were enlarged lymph nodes; also, there was no visible invasion of the serous layer. The final pathological stage was ypT0N0M0. None of the 34 retrieved lymph nodes (Figure 1E and F) showed metastasis.
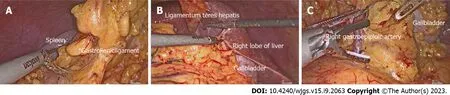
Figure 2 Images of surgery. A: The surgical field showing that the spleen in the right side of the patient; B: The surgical field showing that the gallbladder and right lobe of the liver in the left side of the patient; C: The key procedure during surgery: Dissecting station 6 lymph nodes.
Case 2
The patient accepted laparoscopy-assisted total gastrectomy with standard D2 LND and Roux-en-Y reconstruction. The position of the surgeon and placement of trocars were the same as those in Case 1. No implantation metastasis or vascular variants were found.
The procedure lasted for 168 min, and the estimated blood loss was 50 mL. A total of 34 lymph nodes were retrieved. The lesion was located at the bottom of the gastric body and cardia, and it measured 2.5 cm × 2.3 cm × 0.3 cm. It was identified as poorly differentiated adenocarcinoma invading nerves, vasculature, and fibroadipose tissue in the serous membrane of the stomach wall. The final pathological stage was pT3N3aM0. Eleven of the 34 retrieved lymph nodes showed metastasis (Figure 1I).
OUTCOME AND FOLLOW-UP
Case 1
We implemented enhanced recovery after surgery on the patient, and his postoperative course was uneventful. The patient was able to resume drinking water on postoperative day (POD) 3 and eating liquid food on POD 5. Thereafter, he was discharged on POD 9. Eight months after the operation, he is doing well without recurrence.
Case 2
Without any complication, the patient was discharged on POD 10. Notably, the patient was administered six cycles of postoperative adjuvant chemotherapy with the SOX regimen. The patient had metastasis of supraclavicular lymph nodes in the twelfth postoperative month; however, there has been no sign of local recurrence.
DISCUSSION
Literature review
SIT may be caused by a genetic mutation; however, its specific mechanism remains unknown. A study conducted by Reishet al[2] suggested that the homozygousNME7mutation results in the deletion of amino acids essential for its interaction with the γ-TuRC, which is associated with the impaired left-right asymmetry that manifests as SIT. Although the majority of patients with SIT lead normal lives, a subset of individuals (15%–25%) exhibit accompanying respiratory anomalies (Kartagener syndrome), cardiovascular anomalies (Fallot tetralogy), and digestive anomalies[3]. In addition to these malformations, patients with SIT may also be at an increased risk of cancer due to malfunction of the KIF3 complex[4]. Reports indicate that SIT can be associated with multiple cancers[5], including lung cancer, esophageal cancer, gallbladder cancer, and colon cancer[6-9]. In recent years, laparoscopic radical surgery, including laparoscopic cholecystectomy, laparoscopic colectomy, laparoscopic fundoplication, and laparoscopic gastric band surgery, has increasingly been adopted for cancer patients with SIT[10].
In 1936, Allen[11] described the first case of gastrectomy in a GC patient with SIT. In 2003, the first case of laparoscopic gastrectomy performed on a GC patient with SIT was reported[12], followed by the first case of laparoscopic gastrectomy combined with D2 LND in 2015[10].
In terms of our surgery and summary, we searched PubMed for previously reported cases of GC with SIT in which the patients underwent gastrectomy within the last 11 years (from January 1, 2012 to February 31, 2023). The terms used for topic searches of PubMed were “situs inversus totalis” AND “gastric cancer” or “situs inversus totalis” AND “gastrectomy”. Two cases[13,14] in which gastrectomy was introduced as previous surgery and a case[15] in which a noncancer patient underwent prophylactic gastrectomy were excluded. Consequently, a total of 33 cases were identified, including the two cases that we had previously introduced (from 30 articles). The detailed profiles of the patients in the reported cases are presented in Table 1. Among the patients in the included cases, 22 were men and 11 were women; the patients were aged 40–84 years. According to the articles identified, modified surgical procedures to cope with anatomical malposition are what surgeons concentrate on the most, including the surgery modality, extent of LND, and position of the surgeon.
Table 1 Reported cases of gastric cancer patients with situs inversus totalis in the previous 11 years
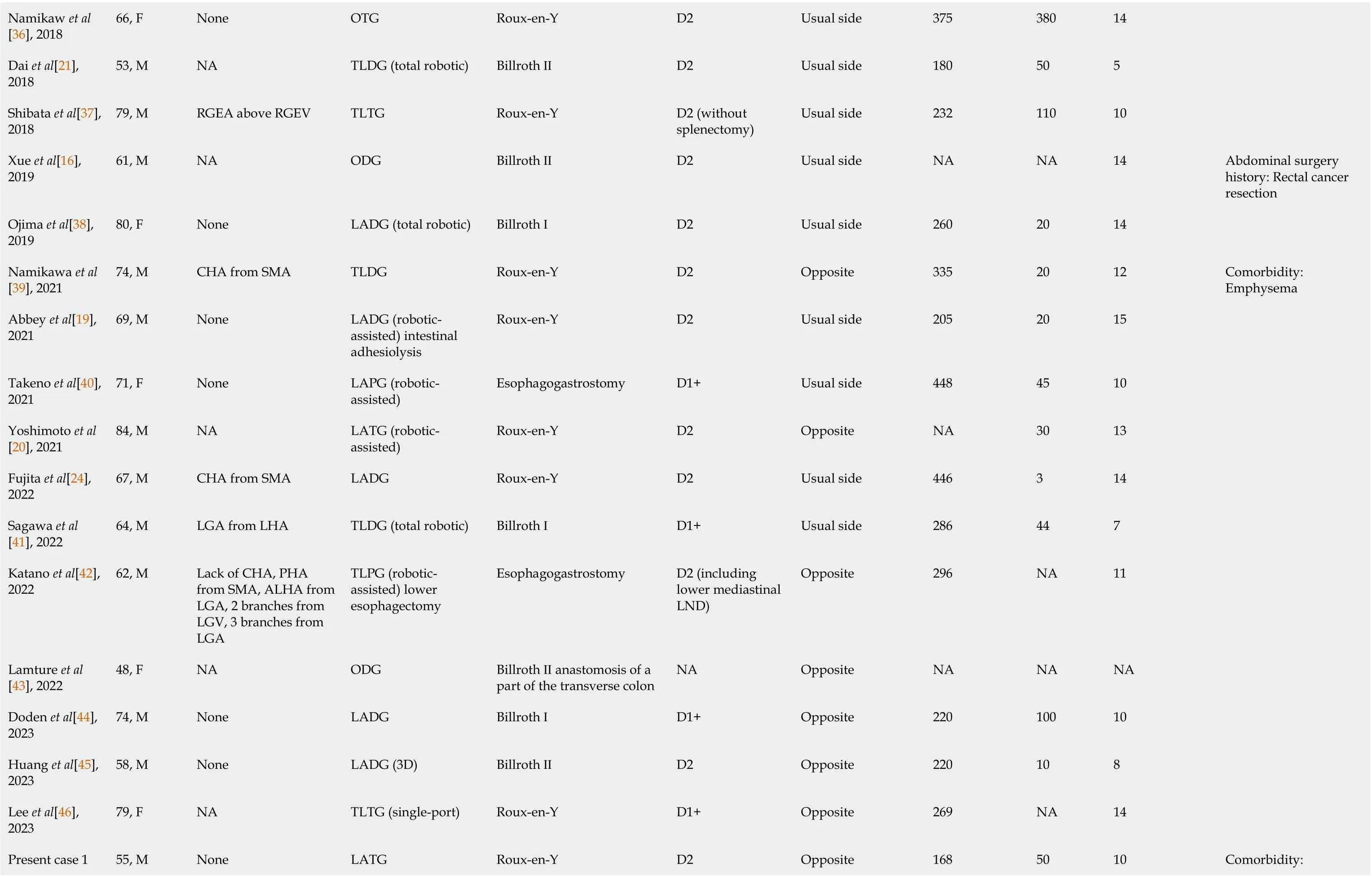
Namikaw et al[36], 2018 66, F None OTG Roux-en-Y D2 Usual side 375 380 14 Dai et al[21], 2018 53, M NA TLDG (total robotic)Billroth ⅡD2 Usual side 180 50 5 Shibata et al[37], 2018 79, M RGEA above RGEV TLTG Roux-en-Y D2 (without splenectomy)Usual side 232 110 10 Xue et al[16], 2019 61, M NA ODG Billroth ⅡD2 Usual side NA NA 14 Abdominal surgery history: Rectal cancer resection Ojima et al[38], 2019 80, F None LADG (total robotic)Billroth ⅠD2 Usual side 260 20 14 Namikawa et al[39], 2021 74, M CHA from SMA TLDG Roux-en-Y D2 Opposite 335 20 12 Comorbidity: Emphysema Abbey et al[19], 2021 69, M None LADG (roboticassisted) intestinal adhesiolysis Roux-en-Y D2 Usual side 205 20 15 Takeno et al[40], 2021 71, F None LAPG (roboticassisted)Esophagogastrostomy D1+Usual side 448 45 10 Yoshimoto et al[20], 2021 84, M NA LATG (roboticassisted)Roux-en-Y D2 Opposite NA 30 13 Fujita et al[24], 2022 67, M CHA from SMA LADG Roux-en-Y D2 Usual side 446 3 14 Sagawa et al[41], 2022 64, M LGA from LHA TLDG (total robotic)Billroth ⅠD1+Usual side 286 44 7 Katano et al[42], 2022 62, M Lack of CHA, PHA from SMA, ALHA from LGA, 2 branches from LGV, 3 branches from LGA TLPG (roboticassisted) lower esophagectomy Esophagogastrostomy D2 (including lower mediastinal LND)Opposite 296 NA 11 Lamture et al[43], 2022 48, F NA ODG Billroth Ⅱ anastomosis of a part of the transverse colon NA Opposite NA NA NA Doden et al[44], 2023 74, M None LADG Billroth ⅠD1+Opposite 220 100 10 Huang et al[45], 2023 58, M None LADG (3D) Billroth ⅡD2 Opposite 220 10 8 Lee et al[46], 2023 79, F NA TLTG (single-port)Roux-en-Y D1+Opposite 269 NA 14 Comorbidity: Present case 1 55, M None LATG Roux-en-Y D2 Opposite 168 50 10

POD: Postoperative day; LND: Lymphadenectomy; M: Male; F: Female; NA: Not available; ALHA: Accessory left hepatic artery; CA: Celiac artery; CHA: Common hepatic artery; SMA: Superior mesenteric artery; LGA: Left gastric artery; LHA: Left hepatic artery; AO: Aorta; RHA: Right hepatic artery; GDA: Gastroduodenal artery; RGEA: Right gastroepiploic artery; RGEV: Right gastroepiploic vein; PHA: Proper hepatic artery; LGV: Left gastric vein; ODG: Open distal gastrectomy; OPG: Open proximal gastrectomy; OTG: Open total gastrectomy; LADG: Laparoscopy-assisted distal gastrectomy; LATG: Laparoscopy-assisted total gastrectomy; LAPG: Laparoscopy-assisted proximal gastrectomy; TLDG: Total laparoscopy distal gastrectomy; TLPG: Total laparoscopy proximal gastrectomy; TLTG: Total laparoscopy gastrectomy.
Surgery modality
Surgical modality selection is a significant concern when treating GC in patients with SIT. Minimally invasive surgery is usually preferred due to its less invasive nature and shorter associated recovery time. However, in 8 of the 33 cases collected from PubMed, the patients underwent open gastrectomy. Surgeons performing open surgery claimed that they took the patient's high body fat and anatomical difficulties of total visceral inversion into consideration comprehensively. Furthermore, laparotomy could provide not only a broader field of vision but also a pathway to insert an applicator for intraoperative radiotherapy[16]. In contrast, laparoscopic surgery is helpful for visualizing vessels and nerves due to the magnified operating field[17]. The application of radical laparoscopic surgery in patients with SIT may have several benefits (such as reduced trauma, milder postoperative pain, and shorter recovery times) compared to traditional open surgical procedures. From our perspectives, identifying anatomical structures may prove problematic; however, an experienced surgeon may overcome this challenge to a certain extent. So, we opted for laparoscopic surgery in our two cases. Nonetheless, laparoscopic gastrectomy requires much expertise, and each patient should be evaluated on an individual basis. The choice of surgical modality should depend on the doctor's technique and the patient's physical condition.
Robotic surgery has been a promising approach for GC patients with SIT. The first case of a GC patient with SIT treated by robotic-assisted distal gastrectomy was reported in 2012[18], and ten cases have been reported so far. Robotic surgery can reduce the errors caused by the cooperation of multiple operators in laparoscopic surgery[19]. Yoshimotoet al[20] concluded that compared to conventional laparoscopic surgery, robotic surgery has two main advantages: (1) It circumvents the need to consider the standing position; and (2) The operator can handle the devices with the nondominant hand with almost the same accuracy as the dominant hand. Although robotic surgery can sew faster and remove deep lymph nodes more easily, it lacks tactility and takes longer[21]. Long-term and short-term comparative studies have shown that robotic gastrectomy is as acceptable as laparoscopic gastrectomy in surgical and oncologic outcomes for normal GC patients[22,23]. Further evidence is needed to fully evaluate the efficacy of robotic surgery in GC patients with SIT.
Extent of LND
Regional LND is an essential requirement for radical gastrectomy, with D2 surgery being widely recognized as the standard surgical procedure for advanced GC. D2 LND is performed under an extrasac that originates from the anterior transverse mesenteric lobe to the pancreatic capsule. We performed complete resection of the anterior transverse mesangial lobe, greater omentum, pancreatic capsule, and hepatogastric ligaments, ligation of the blood vessels involved at the root, and thorough removal of the corresponding second station lymph nodes according to the tumor site. Therefore, it is crucial to differentiate the relevant important blood vessels (including hepatoduodenal ligaments, abdominal trunk, common hepatic artery (CHA), splenic artery initiation, superior mesenteric vein,etc.). However, in the case of SIT, the occurrence of vascular anomalies is not common. Out of the 33 cases that are reviewed in the literature, 10 involving vascular anomalies have been described. The most common anomaly observed was that the CHA exited from the superior mesenteric artery, with three cases reported. The solution proposed by Fujitaet al[24] re-confirmed the anatomical landmarks of LND in the suprapancreatic area as the upper borders of the pancreas, portal vein, and left gastric artery from the celiac axis. Therefore, during the preoperative evaluation period, sufficient imaging examination is necessary to detect the normal anatomy and variations in the branching pattern of the celiac trunk, which could significantly reduce the duration of surgery and the intraoperative blood loss. With the advent of imaging technology, the three-dimensional reconstruction image of CT angiography (3DCTA) has been widely used to confirm surgical anatomy. Several studies have reported that 3DCTA adequately demonstrates vascular anomalies. In our cases, a contrastenhanced CT scan was performed, and no abnormal course of vascularity was found in this process.
Position of the surgeon
The appropriate surgical positioning for surgeons during GC surgery in patients with SIT has been a topic of ongoing debate. To this day, there is still no definitive conclusion on the optimal standing position of the surgeon during this type of surgery. The surgical positioning of the surgeon may be related to the surgical modality. In open surgeries, the surgeon’s position was reported in five out of eight cases, with four of them being performed on the patient's left side (the usual side). These surgical procedures were carried out according to the principle of precise manipulation of local lymph nodes and smooth transition of the cleaned area. The optimal positioning for the surgeon during laparoscopic gastrectomy in patients with SIT is still controversial. It is natural to think that it is better for the surgeon to stand in the reverse position due to the left-right reversal of the positions of organs. Out of 15 counted laparoscopic gastrectomy cases, 11 were performed on the patient's right side (opposite to the usual side). However, a few surgeons hold a different view, as they believe that standing on the opposite side from where they normally stand would require them to dissect with their nondominant hand, leading to inevitable difficulties. Nevertheless, we still chose to stand on the opposite side. Thanks to our sophisticated skills and well-coordinated team, the procedure was smooth and without complications.
Robotic surgery provides a new solution to this obstacle. In robotic gastrectomy, the robotic arm performs most of the operation, enabling the surgeon to perform the operation without changing their position or experiencing any confusion resulting from the patient's reversed anatomy. This technology may overcome the difficulties encountered in laparoscopic gastrectomy for patients with SIT, and it could be considered a safe and effective surgical approach.
Furthermore, we summarize the clinical features, estimated blood loss, surgery time, and prognoses of 31 reported cases of GC with SIT. The majority of cases occurred in middle-aged and elderly men, and these cases were reported in Japan, China, and Korea (15, 10, and 6 cases, respectively). However, there is a paucity of studies on the correlation and regional differences between GC and SIT. The main treatment methods were surgical resection, reconstruction, and chemotherapy, which effectively improved patients’ survival. Combining the experiences of others and ours, our suggestion is that, first of all, a sufficient preoperative imaging evaluation is recommended as it can help predict abnormal anatomical positions and vascular directions. Contrast-enhanced abdominal CT can help the surgeon initially predict the abnormal anatomical positions and vascular directions, and this helps to clarify the problem and avoid accidental injury during surgery. Second, the operation should be performed patiently and carefully. Careful confirmation of the patient’s anatomy and vascular location can mitigate intraoperative complications. After postoperative discussions, we all agreed on the fact that the main difficulties facing surgery are the patient’s vascular anatomy and precision of manipulation. Finally, it is obvious that an experienced surgical team is a necessity. A sophisticated surgeon and a well-coordinated team can alleviate the awkwardness caused by abnormal anatomical positions, enabling the operation to be completed smoothly.
CONCLUSION
In conclusion, laparoscopic gastrectomy with D2 LND should be considered an accessible, safe, and curative procedure for advanced GC with SIT.
FOOTNOTES
Author contributions:Liu HB and Peng CW contributed to manuscript writing and editing, and data collection; Cai XP and Lu Z contributed to data analysis; Xiong B and Peng CW contributed to conceptualization and supervision; all authors have read and approved the final manuscript.
Supported byNational Natural Science Foundation of China, No. 81401515; Zhongnan Hospital of Wuhan University Science Technology and Innovation Seed Fund, No. znpy2018030; “351Talent Project (Luojia Young Scholars)” of Wuhan University.
Informed consent statement:Informed written consent was obtained from both patients for the publication of this case report.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
CARE Checklist (2016) statement:The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORCID number:Hong-Bo Liu 0000-0002-4356-7555; Zhao Lu 0000-0002-1489-205x; Chun-Wei Peng 0000-0001-6349-1618.
S-Editor:Lin C
L-Editor:Wang TQ
P-Editor:Wu RR
杂志排行
World Journal of Gastrointestinal Surgery的其它文章
- Preoperative and postoperative complications as risk factors for delayed gastric emptying following pancreaticoduodenectomy: A single-center retrospective study
- Comparative detection of syndecan-2 methylation in preoperative and postoperative stool DNA in patients with colorectal cancer
- Preoperative prediction of microvascular invasion in hepatocellular carcinoma using ultrasound features including elasticity
- Surgical management of gallstone ileus after one anastomosis gastric bypass: A case report
- Hepatic ischemia-reperfusion syndrome and its effect on the cardiovascular system: The role of treprostinil, a synthetic prostacyclin analog
- Advances and challenges of gastrostomy insertion in children
