Quantitative evaluation of colorectal tumour vasculature using contrast-enhanced ultrasound: Correlation with angiogenesis and prognostic significance
2023-10-21MingHuiLiWeiWeiLiLingHeJianFangLiSunYanZhang
Ming-Hui Li, Wei-Wei Li, Ling He, Jian-Fang Li, Sun-Yan Zhang
Abstract
Key Words: Contrast-enhanced ultrasound; Colorectal cancer; Tumour angiogenesis; Prognosis; Microvessel density; Vascular endothelial growth factor; Tumour
INTRODUCTION
Colorectal cancer (CRC) is the third most prevalent cancer and the second leading cause of cancer-related fatalities globally, with approximately 1.8 million new cases and nearly 900000 deaths in 2021[1]. The high mortality rate is attributed to late diagnosis, insufficient staging, and ineffective treatment approaches. Therefore, early detection and precise staging are crucial for optimizing treatment plans and improving patient outcomes[2]. In recent decades, advancements in medical imaging techniques have significantly improved diagnostic precision in CRC[3]. Among these imaging methods, contrast-enhanced ultrasound (CEUS) has gained increased interest for its potential in evaluating tumour blood flow and vascularization.
Tumour vasculature plays a critical role in its growth, progression, and metastasis. Rapidly expanding tumours require a substantial blood supply to ensure sufficient delivery of nutrients and oxygen, while simultaneously eliminating waste products[4]. This demand for blood supply is met through angiogenesis, a process of formation of new blood vessels from pre-existing ones. Angiogenesis is regulated by a fine equilibrium between pro-angiogenic and anti-angiogenic factors, with vascular endothelial growth factor (VEGF) being the strongest promoter of angiogenesis[5]. Tumour blood vessel density, also known as microvessel density (MVD), serves as a surrogate marker for angiogenesis and has been associated with tumour aggressiveness and unfavourable prognosis in various cancers, including CRC[6].
CEUS is a relatively novel imaging technique that employs intravenously administered microbubble contrast agents to improve ultrasound images[7]. The echogenicity of microbubbles enhances visualization of blood flow within the vasculature. By utilizing the non-linear behaviour of microbubbles, CEUS can produce real-time, high-resolution images of tissue perfusion and vascular structure with a high signal-to-noise ratio[8,9]. Compared with other imaging techniques, such as ultrasound, computed tomography (CT), magnetic resonance imaging (MRI),etc., CEUS has several advantages for CRC imaging. First, CEUS can provide high-resolution images of tumour vasculature without being affected by bone or gas interference[10]. Second, CEUS can offer real-time dynamic information on tissue perfusion and blood flow velocity with a high temporal resolution[11]. Third, CEUS can provide quantitative parameters for tumour vascularization analysis using time-intensity curve (TIC) analysis[12]. Fourth, CEUS is a non-invasive, safe, and cost-effective method that does not expose patients to ionizing radiation or nephrotoxic contrast agents[13].
Several studies have examined the potential of CEUS in assessing tumour vasculature in CRC[14-17]. Most of these studies have only focused on qualitative or semi-quantitative analyses, such as visual grading of enhancement patterns or evaluating TIC parameters without thoroughly investigating their correlation with angiogenesis markers and prognostic factors. Additionally, a majority of these studies have been limited by small sample sizes and a lack of standardized CEUS examination and analysis techniques.
Considering these limitations, the present study aimed to explore the role of CEUS in quantitatively evaluating colorectal tumour vasculature and its correlation with angiogenesis markers (VEGF and MVD) and prognostic factors. We hypothesized that quantitative CEUS parameters would significantly correlate with angiogenesis markers, and elevated CEUS-derived parameter values would be associated with aggressive tumour features and poor prognosis. To investigate these hypotheses, we conducted a prospective study with a relatively large cohort of patients with histologically confirmed CRC who underwent preoperative CEUS examinations using standardized techniques. Moreover, we used a comprehensive approach to data analysis, including assessment of TIC-derived parameters and their correlation with angiogenesis markers, clinicopathological characteristics, and survival outcomes.
This study aimed to provide valuable insights into the potential of CEUS as a non-invasive tool for evaluating tumour vasculature in CRC and establish its clinical utility in guiding treatment decisions and predicting patient outcomes. The study’s findings may contribute to the growing evidence supporting the use of CEUS in CRC management and pave the way for future large-scale, multicentre trials to validate and expand upon these findings.
MATERIALS AND METHODS
Study population
This study prospectively enrolled 100 patients with histologically confirmed CRC from January 2020 to December 2022. Inclusion criteria were as follows: (1) Age ≥ 18 years; (2) Pathologically confirmed primary colorectal adenocarcinoma; (3) No history of chemotherapy or radiotherapy; and (4) Ability to provide informed consent. Exclusion criteria were as follows: (1) Contraindications to ultrasound contrast agents, such as severe allergic reactions; (2) Pregnancy or lactation; and (3) Severe comorbidities affecting survival outcomes, such as end-stage renal disease, chronic heart failure, or uncontrolled diabetes. This study was approved by the Xinjiang Medical University Affiliated Cancer Hospital institutional review board and adhered to the standards of the Declaration of Helsinki.
CEUS examination
All patients underwent CEUS examinations within two weeks before surgery using a high-end ultrasound system (LOGIQ E9, GE Healthcare, Milwaukee, WI, United States) with a 1-6 MHz convex array transducer (C1-6, GE Healthcare) and dedicated contrast-specific imaging software (Contrast Harmonic Imaging, GE Healthcare). Patients were placed in a supine position, and a baseline grayscale and colour Doppler ultrasound examinations were conducted to assess tumour’s location, size, and morphology.
A 2.4 mL dose of SonoVue (Bracco, Milan, Italy), a microbubble contrast agent containing sulphur hexafluoride gas encapsulated in a phospholipid shell, was administered intravenously as a single bolus injection, followed by a 10 mL saline flush. CEUS examination began immediately after injection and continued for 5 min. Mechanical index was set at a low level (≤ 0.1) to minimize microbubble destruction. Imaging parameters, including gain, time gain compensation, and focal zone, were adjusted to optimize image quality and maintain consistent contrast-enhanced image appearance. Experienced sonographers, blinded to patients’ clinical information, performed all CEUS examinations.
CEUS image analysis
CEUS images were stored in DICOM format and analysed offline using VueBox software (Bracco, Milan, Italy). Regions of interests (ROIs) were manually drawn to encompass the entire enhancing tumour area on CEUS images. The software generated TICs for the selected ROIs, displaying the change in intensity over time. Three quantitative CEUS parameters were derived from the TIC analysis: Peak intensity (PI), time to peak (TTP), and area under the curve (AUC). PI represented the maximum intensity reached within the ROI during the observation period, TTP indicated the time required reaching the PI, and AUC corresponded to the total amount of contrast agent within the ROI during the examination.
Immunohistochemical analysis
During surgery, cancerous tissue samples were collected, preserved in 40 g/L neutral buffered formaldehyde, and embedded in paraffin before being sliced into 4 μm-thick sections. These sections were deparaffinised, rehydrated, and subjected to antigen retrieval using a citrate buffer (pH = 6.0) and microwave heat treatment. Subsequently, they were treated with 3% hydrogen peroxide for 10 min to neutralize endogenous peroxidase activity and incubated overnight at 4 °C with primary antibodies targeting VEGF (1:200, rabbit polyclonal, Abcam, Cambridge, United Kingdom) and CD34 (1:100, mouse monoclonal, Dako, Glostrup, Denmark). After rinsing with phosphate-buffered saline, the sections were treated with secondary antibodies conjugated to horseradish peroxidase for 1 h at room temperature and counterstained with haematoxylin. Immunoreactivity was detected using a 3,3’-diaminobenzidine substrate. The percentage of VEGFpositive tumor cells and CD34-positive microvessels were counted using ImageJ software (National Institutes of Health, Bethesda, MD, United States). Five random fields of view per section were selected and captured at 400 × magnification. The number of positive cells or microvessels and the total number of cells or microvessels were counted manually. The percentage was calculated as the ratio of positive cells or microvessels to total cells or microvessels multiplied by 100.
VEGF expression was evaluated with a semi-quantitative measurement method, which accounted for both the percentage of tumour cells expressing VEGF and the intensity of staining. The percentage of VEGF-positive tumour cells were scored as follows: 0 (0%), 1 (1%-25%), 2 (26%-50%), 3 (51%-75%), and 4 (76%-100%). The intensity of staining was rated using the following categories: 0 (absent), 1 (faint), 2 (moderate), and 3 (vivid). The percentage and intensity scores were multiplied to calculate the final VEGF expression score, ranging from 0-12. High VEGF expression was defined as a score of 6 or higher.
MVD was assessed by enumerating CD34-positive microvessels in the highly vascularized areas of the tumour, termed as ‘hotspots’. These hotspots were located using a low-magnification (100 ×) lens, followed by counting of the microvessels under high magnification (400 ×). Each endothelial cell or cluster, clearly distinguishable from adjacent microvessels, tumour cells, and connective tissues, qualified as a countable microvessel, regardless of lumen presence. MVD was expressed as the mean quantity of microvessels within each high-power field.
Statistical analyses
The patients’ clinicopathological characteristics, CEUS parameters, and immunohistochemical findings are summarized using descriptive statistics. The relationships between CEUS parameters, angiogenesis markers, and clinicopathological factors were examined using either the Spearman’s rank correlation coefficient or Pearson’s correlation coefficient, depending on the situation. The Mann-WhitneyUtest or Kruskal-Wallis test was employed, as necessary, to evaluate differences in CEUS parameters concerning VEGF expression and MVD.
RESULTS
Patient characteristics at baseline
The study enrolled 100 patients, including 57 men and 43 women, with a median age of 63 years (range: 38-84 years). Patients’ clinicopathological traits are shown in Table 1. A majority of the tumours were located in the rectum (n= 52), while others were found in the sigmoid colon (n= 26), ascending colon (n= 12), and descending colon (n= 10). Based on the TNM staging system, 21 patients had stage I tumours, 29 had stage II tumours, 35 had stage III tumours, and 15 had stage IV tumours. Lymph node metastasis was observed in 50 patients, whereas 15 patients displayed distant metastasis.
CEUS parameters were positively correlated with angiogenesis markers
The average values of PI, TTP and AUC were 23.6 ± 7.8 dB, 16.5 ± 5.6 s and 1032.6 ± 361.3 dB × s, respectively. High VEGF expression was detected in 56 patients, and the median MVD was 52.5 (range: 12-125). Table 2 shows the correlations between CEUS parameters and angiogenesis markers. Significant positive correlations were observed between PI and both VEGF expression (r= 0.73,P< 0.001) and MVD (r= 0.75,P< 0.001). TTP demonstrated significant negative correlations with both VEGF expression (r= -0.68,P< 0.001) and MVD (r= -0.72,P< 0.001). Additionally, AUC demonstrated significant positive correlations with both VEGF expression (r= 0.71,P< 0.001) and MVD (r= 0.74,P< 0.001).
CEUS parameters were significantly correlated with aggressive clinicopathological characteristics
Table 3 shows the correlations between CEUS parameters, angiogenesis indicators, and clinicopathological characteristics. As expected, high VEGF expression and MVD were related to advanced tumour stage (P< 0.001), lymph node metastasis (P< 0.001), and distant metastasis (P< 0.001). Additionally, high PI, short TTP, and large AUC values were associated with advanced tumour stage (P< 0.001), lymph node metastasis (P< 0.001), and distant metastasis (P< 0.001). No significant correlations were found between CEUS parameters or angiogenesis markers and age, sex, or tumour location.
CEUS parameters acted as independent prognostic factors for poor OS and DFS
The average follow-up period was 26 mo (range: 3-36 mo). During this period, 28 patients experienced tumour recurrence and 22 patients died. The OS and DFS rates at 3 years were 78% and 72%, respectively. Figures 1 and 2 show the Kaplan-Meier survival curves for OS and DFS based on CEUS parameters, VEGF expression, and MVD, respectively. Poor OS (P< 0.001) and DFS (P< 0.001) were significantly associated with high PI, short TTP, and large AUC values. Additionally, poor OS (P< 0.001) and DFS (P< 0.001) were significantly associated with high VEGF expression and MVD. The multivariate analysis revealed that OS was independently influenced by prognostic factors, including PI [hazard ratio (HR) = 2.55, 95% confidence interval (CI): 1.36-4.78,P= 0.003], TTP (HR = 2.34, 95%CI: 1.24-4.41,P= 0.008), AUC (HR = 2.62, 95%CI: 1.38-4.96,P= 0.003), VEGF expression (HR = 2.47, 95%CI: 1.31-4.65,P= 0.005), and MVD (HR = 2.81, 95%CI: 1.49-5.30,P= 0.001). Similarly, DFS was also independently affected by prognostic factors, including PI (HR = 2.38, 95%CI: 1.28-4.42,P= 0.006), TTP (HR = 2.26, 95%CI: 1.20-4.26,P= 0.011), AUC (HR = 2.54, 95%CI: 1.34-4.81,P= 0.004), VEGF expression (HR = 2.31, 95%CI: 1.24-4.32,P= 0.008), and MVD (HR = 2.67, 95%CI: 1.42-5.03,P= 0.002). Table 4 summarizes the results of the multivariate analysis.
DISCUSSION
Our study demonstrated significant correlations between quantitative CEUS parameters (PI, TTP, and AUC) and angiogenesis markers (VEGF expression and MVD) in CRC. High PI, short TTP, and large AUC values were significantly associated with aggressive tumour features and unfavourable prognosis, independent of other clinicopathological factors. These results suggest that CEUS may be a useful non-invasive imaging modality for assessing tumour vasculature in CRC and could potentially help in guiding treatment planning and predicting patient outcomes.

Table 2 Correlations between contrast-enhanced ultrasound parameters and angiogenesis markers

Table 3 Associations between contrast-enhanced ultrasound parameters, angiogenesis markers, and clinicopathological characteristics

Table 4 Multivariate analysis of prognostic factors for overall survival and disease-free survival

Figure 1 Kaplan-Meier survival curves for overall survival according to contrast-enhanced ultrasound parameters, vascular endothelial growth factor expression, and microvessel density. A: Kaplan-Meier curve for peak intensity; B: Kaplan-Meier curve for time to peak; C: Kaplan-Meier curve for area under the curve; D: Kaplan-Meier curve for vascular endothelial growth factor; E: Kaplan-Meier curve for microvessel density. OS: Overall survival;VEGF: Vascular endothelial growth factor; PI: Peak intensity; TTP: Time to peak; MVD: Microvessel density; AUC: Area under the curve.
The significant correlations between CEUS parameters and angiogenesis markers observed in our study align with the findings of previous literature[18-20]. The positive correlations between PI and VEGF expression and MVD can be explained by the generally greater density of blood vessels in tumours with high VEGF expression and MVD, which leads to increased blood flow and higher PI values on CEUS. Similarly, the negative correlations between TTP and VEGF expression and MVD can be attributed to the faster blood flow in tumours with high VEGF expression and MVD, resulting in a shorter time required to reach PI on CEUS.
The associations between CEUS parameters and clinicopathological characteristics observed in our study are also consistent with the findings of previous research. High PI, short TTP, and large AUC values have been found to correlate with advanced stage, lymph node metastasis, and distant metastasis in various cancers, including CRC[18,21,22]. These associations can be explained by the fact that aggressive tumours typically exhibit higher angiogenesis, leading to increased blood flow and more pronounced contrast enhancement on CEUS. Our survival analysis revealed that high PI, short TTP, and large AUC values were significantly associated with poor OS and DFS, independent of other clinicopathological factors. These results suggest that CEUS parameters could serve as potential prognostic biomarkers in CRC. Furthermore, the significant associations between high VEGF expression, MVD, and poor survival outcomes observed in our study are consistent with those in previous studies, further emphasizing the importance of tumour vasculature in CRC progression and prognosis[23,24].
日本大学从民间获取的资金主要包括共同研究费、受托研究费、临床实验费、知识产权转让费。2011—2016年日本大学从民间获取的这4项经费的数量逐年增加,2016年达到约848亿日元(见表3)。虽然大学从民间获取的经费有所增加,但从国际比较来看,日本大学从民间获得经费的比例还比较低:2010年时,经济合作与发展组织(OECD)国家大学研发经费中民间经费的平均占比为5%,而日本2013年时的这一占比只有2.6%,比经济合作与发展组织国家中较高的德国(14%)、韩国(12.3%)、加拿大(7.2%)、西班牙(6.6%)等低很多,也不及低于平均水平的美国(4.8%)、英国(4.1%)。
CEUS provides multiple benefits compared with other imaging techniques, such as CT and MRI, in evaluating tumour vasculature. It is a real-time, non-invasive, and radiation-free imaging modality that provides high spatial and temporal resolution, allowing for a detailed assessment of tumour blood flow and microvasculature[25,26]. Moreover, microbubble contrast agents used in CEUS are purely intravascular, which enables a more accurate evaluation of blood vessel density and perfusion characteristics compared to the contrast agents used in CT and MRI, which have a tendency to extravasate into the interstitial space[27]. Furthermore, CEUS is generally less expensive than CT and MRI, making it a more costeffective option for patients and healthcare systems[28].
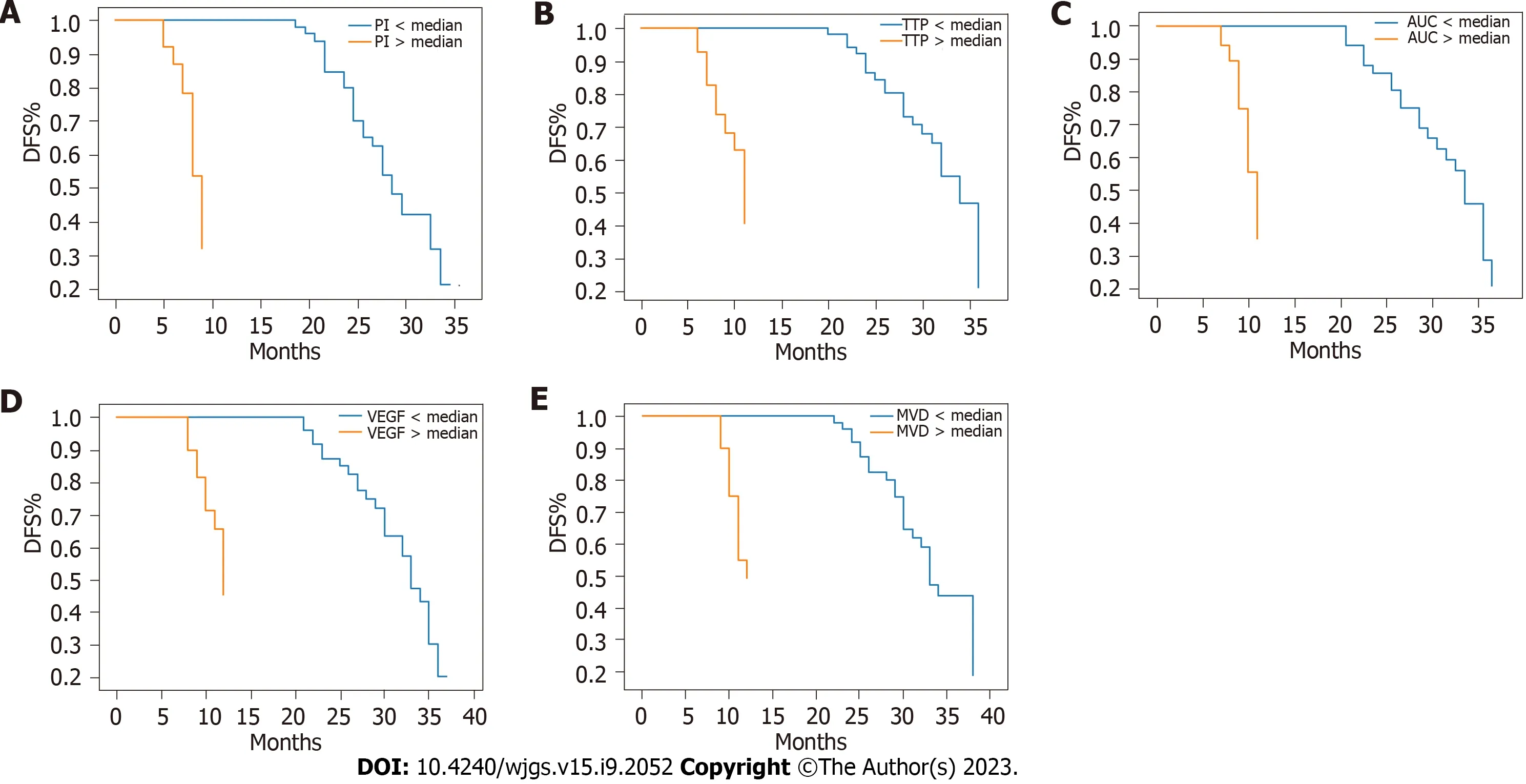
Figure 2 Kaplan-Meier survival curves for disease-free survival according to contrast-enhanced ultrasound parameters, vascular endothelial growth factor expression, and microvessel density. A: Kaplan-Meier curve for peak intensity; B: Kaplan-Meier curve for time to peak; C:Kaplan-Meier curve for area under the curve; D: Kaplan-Meier curve for vascular endothelial growth factor; E: Kaplan-Meier curve for microvessel density. DFS:Disease-free survival; VEGF: Vascular endothelial growth factor; MVD: Microvessel density; TTP: Time to peak; AUC: Area under the curve; PI: Peak intensity.
In addition to assessing tumour vasculature, CEUS has also been investigated for other clinical applications in CRC, including detecting and characterizing primary tumours, lymph node staging, and assessing treatment response[29,30]. It has shown to improve the accuracy of primary tumour detection in CRC compared to that of conventional ultrasound, particularly in early-stage tumours, where the sensitivity and specificity of CEUS are reported to be high[31]. Furthermore, CEUS has been investigated for differentiating benign and malignant colorectal lesions based on enhancement patterns and kinetics, with some studies reporting promising results in terms of diagnostic accuracy[32].
CEUS has demonstrated potential utility in identifying metastatic lymph nodes in CRC patients[33]. Several studies have reported that evaluation of lymph node vascularization using CEUS can help differentiate metastatic from nonmetastatic lymph nodes, with higher accuracy than conventional ultrasound or CT[34-36]. However, further research is warranted to establish standardized criteria for assessing lymph node involvement using CEUS and to compare its performance with that of other imaging modalities, such as MRI or positron emission tomography.
Monitoring treatment response is another potential clinical application for CEUS in CRC. In recent years, neoadjuvant chemoradiotherapy has become a standard treatment approach for locally advanced rectal cancer, with the goal of downstaging the tumour and improving the likelihood of complete surgical resection[37]. Monitoring the response to neoadjuvant therapy is crucial for determining the optimal timing of surgery and predicting patient outcomes. CEUS has been investigated as a non-invasive method for monitoring changes in tumour vasculature during neoadjuvant treatment, with some studies suggesting that early changes in CEUS parameters can predict treatment response and long-term outcomes[38]. Further research is warranted to establish the role of CEUS in the assessment of treatment response in CRC and to determine the optimal timing and criteria for CEUS evaluation.
Although our study yielded encouraging results, it had certain limitations. First, a relatively small sample size could have affected the statistical power of our analysis. Future research with larger sample size is essential to validate and broaden the implications of our findings. Second, the retrospective design of our study may have led to selection bias; therefore, prospective studies are required to confirm the prognostic significance of CEUS parameters in CRC. Third, our study primarily focused on the correlations between CEUS parameters and angiogenesis markers; however, the underlying biological mechanisms remain to be elucidated. Therefore, further investigations, such as lab-based and animal studies, are required to explore the molecular pathways linking CEUS parameters to angiogenesis and tumour progression in CRC.
CONCLUSION
Our study demonstrated that quantitative CEUS parameters were significantly associated with angiogenesis markers and prognostic factors in CRC. These findings suggest that CEUS could be a valuable non-invasive tool for assessing tumour vasculature in CRC and may have potential clinical utility in guiding treatment decisions and predicting patient outcomes. Future studies should focus on validating and expanding upon our findings in large cohorts and exploring the underlying biological mechanisms linking CEUS parameters to angiogenesis and tumour progression in CRC. Moreover, the potential applications of CEUS in other aspects of CRC management, such as primary tumour detection, lymph node staging, and treatment response assessment, should be further investigated to fully understand the clinical potential of this imaging modality.
ARTICLE HIGHLIGHTS

ACKNOWLEDGEMENTS
Thank you to all those who have contributed to this manuscript.
FOOTNOTES
Author contributions:Li MH and Li JF designed and coordinated this study; Li WW and He L conducted experiments to obtain and analyze data; Zhang SY, Li MH, Li WW, and Zhang SY explained the data; Li JF, He L, Li WW, Li MH, and Zhang SY wrote the manuscript; and all authors have approved the final version of this article.
Institutional review board statement:The study was reviewed and approved by the Institutional Review Board at Tumor Hospital Affiliated to Xinjiang Medical University.
Clinical trial registration statement:The research registration number is 8823.
Informed consent statement:All participants provided written informed consent.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Data sharing statement:No additional data are available.
CONSORT 2010 statement:The authors have read the CONSORT 2010 statement, and the manuscript was prepared and revised according to the CONSORT 2010 statement.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORCID number:Ming-Hui Li 0009-0001-3514-5643; Sun-Yan Zhang 0009-0003-6411-1973.
S-Editor:Wang JJ
L-Editor:A
P-Editor:Wang JJ
猜你喜欢
杂志排行
World Journal of Gastrointestinal Surgery的其它文章
- Risk factors for myocardial injury during living donor liver transplantation in pediatric patients with biliary atresia
- Value of enhanced computed tomography in differentiating small mesenchymal tumours of the gastrointestinal from smooth muscle tumours
- Multifactor analysis of the technique in total laparoscopic gastric cancer
- Clinical significance of serum oxidative stress and serum uric acid levels before surgery for hepatitis Brelated liver cancer
- Prediction model of stress ulcer after laparoscopic surgery for colorectal cancer established by machine learning algorithm
- Short- and long-term results of open vs laparoscopic multisegmental resection and anastomosis for synchronous colorectal cancer located in separate segments
