Efficacy of ileus tube combined with meglumine diatrizoate in treating postoperative inflammatory bowel obstruction after surgery
2023-10-21WenYangJingPu
Wen Yang, Jing Pu
Abstract
Key Words: Ⅰleus tube; Meglumine diatrizoate; Colorectal cancer; Ⅰnflammatory bowel obstruction; Early postoperative inflammatory small bowel obstruction
INTRODUCTION
Early postoperative inflammatory small bowel obstruction (EPISBO) is a common postoperative complication following treatment for colorectal cancer. EPISBO is an adhesive intestinal obstruction caused by intestinal wall edema and inflammatory exudation caused by abdominal surgery, intestinal tube injury, and leakage of contents. In patients with colorectal cancer undergoing radical surgery, the intestinal canal is exposed for a long time, and abdominal bleeding and foreign bodies can lead to inflammation. Many inflammatory cells accumulate, eventually leading to inflammation and adhesion[1,2].
If not treated in time, this enhanced inflammatory state can lead to short bowel syndrome, intestinal fistula, infection, and other serious complications. Several studies have shown that EPISBO mainly occurs within two weeks after surgery, and the main clinical manifestations include abdominal distention, cessation of anal exhaust, and defecation.
Clinical-based EPISBO therapy remains conservative, including fasting/water restriction, parenteral nutrition support, and reoperation that can damage the intestine. Traditional nasogastric tube decompression can only remove gastric juice. Additionally, it is difficult to drain the contents of the small intestine, resulting in a long conservative treatment time for early postoperative inflammatory ileus. While semi-effective, some patients have a poor curative effect.
The transnasal ileus tube could drain fluid in the small intestine, reducing edema and intestinal pressure. Meglumine diatrizoate has the characteristics of hypertonicity and has been shown to induce no apparent adverse reactions. After decompression through the intestinal obstruction catheter, angiography can significantly reduce the dilution of contrast medium by intestinal effusion and improve the effectiveness of diagnosis and treatment[3-5]. In addition to being used as a contrast agent, oral administration of meglumine diatrizoate can reduce intestinal wall edema, dilate the small intestine at the distal end of obstruction, stimulate gastrointestinal peristalsis, and relieve intestinal obstruction. This study aimed to probe the therapeutic efficacy of ileus tubes and meglumine diatrizoate for treating EPISBO after surgery for colorectal cancer.
MATERIALS AND METHODS
Ethics
This study was approved by the Ethics Committee of Lanzhou Second People’s Hospital. Due to the retrospective design, patient consent was not required.
General information
Data from 60 patients with colorectal cancer and intestinal obstruction admitted to the Proctology Department of our hospital from April 2018 to May 2022 were collected and analyzed. The patients were divided into three cohorts, namely A (n= 20), B (n= 20), and C (n= 20), according to different treatment regimens. Cohort A comprised 14 males and 6 females, with a mean age of 57.95 ± 3.10 years (50-64 years). Within this cohort, these patients presented with the following obstruction locations: Four cases of obstruction in the rectum, six in the descending colon, and 10 in the sigmoid colon. Regarding TNM staging, 10 cases were identified as stage II and 10 as stage III. Cohort B comprised 12 males and 8 females, with a mean age of 59.10 ± 3.46 years (53-65 years). Within this cohort, the patients presented with the following obstruction locations: Two cases of obstruction in the rectum, seven cases in the descending colon, and 11 cases in the sigmoid colon. Regarding TNM staging: Seven cases were identified as stage II and 13 as stage III. Lastly, cohort C comprised 16 males and four females, with a mean age of 60.20 ± 4.29 years (51-68 years). Within this cohort, the patients presented with the following obstruction locations: Six rectum cases, five descending colon cases, and nine sigmoid colon cases. Regarding TNM staging: 11 cases were identified as stage II and nine as stage III.
Inclusion and exclusion criteria
Patients with complete case data that presented with symptoms including abdominal distension, abdominal pain, vomiting, stop of exhaustion, and defecation, had a palpable mass in the right lower abdomen, had no signs of peritonitis, and weakened or absent bowel sounds were included. Furthermore, only patients whose X-ray examination showed intestinal effusion, abdominal computed tomography examination showed intestinal wall edema, thickening, and extensive exudation were included.
Patients with intestinal obstruction caused by intestinal hernia or intussusception, intestinal obstruction or cancerous obstruction caused by mesenteric disease or intestinal paralysis, or patients with hematological diseases, severe infections, and immune diseases were excluded. Furthermore, pregnant and nursing women, patients with neurological diseases, and patients allergic to drugs used in this study were excluded.
Treatment
Cohort A was administered a transnasal ileus tube combined with meglumine diatrizoate, cohort B was administered a transnasal ileus tube combined with liquid paraffin, and cohort C was administered oral meglumine diatrizoate. All three cohorts were given primary treatment, which consisted of fasting and gastrointestinal decompression, early deep vein nutrition treatment to maintain water, electrolyte, and acid-base balance, the correction of hypoalbuminemia and anemia, administration of omeprazole and octreotide to inhibit the secretion of digestive juices. Lastly, a broad-spectrum antibiotic was administered as an anti-infection treatment.
Cohort A: The ileus tube was placed and connected to an external negative pressure suction device, and 100-150 mL of 76% meglumine was injected into the lesion through the ileus tube for intestinal angiography. The ileus tube was retained for continuous negative pressure suction for patients with extensive weakened intestinal peristalsis and apparent pleural effusion. For patients with segmental intestinal peristalsis caused by local adhesion, and if distal intestinal peristalsis was expected, the tube provided enteral nutrition through the obstruction site, and negative pressure drainage was performed through the lateral hole. Parenteral nutrition support was given during treatment.
Cohort B: The transnasal ileus tube was inserted into the stomach under gastroscopy and delivered to the distal descending part of the duodenum with the help of a guide wire or foreign body forceps. Approximately 15 mL of sterilized water was injected into the front balloon and relaxed the tube; the external nasal edge was not fixed. The tube was connected to a negative pressure suction device, and 50-80 mL of liquid paraffin was injected through the negative pressure suction port of the tube. The patient was told to move around more, turn over on the bed, and the tube was sent to the obstruction position through intestinal peristalsis.
Treatment outcomes to be assessed
Baseline data, clinical efficacy, the time of first exhaust/defecation, length of hospital stays, gastrointestinal decompression time, abdominal pain relief time, abdominal distension relief time, laboratory indicators, nutritional indicators, and the occurrence of adverse reactions were collected and assessed. The clinical efficacy, the time of first exhaust/defecation, length of hospital stays, gastrointestinal decompression time, abdominal pain relief time, and abdominal distension relief time were compared among the three cohorts. The levels of C-reactive protein (CRP), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), monocyte chemotactic protein-1 (MCP-1), serum albumin, and transferrin were compared among the three cohorts before and after treatment and the occurrence of adverse reactions in the three cohorts was compared.
Efficacy evaluation criteria
Cases where the clinical symptoms and signs of the patient disappeared, and the abdominal vertical position plain film showed normal were defined as “cured” following treatment. Cases where the clinical symptoms and signs disappeared, and the abdominal vertical position plain film showed that the intestinal tube was slightly inflated or had a small amount of liquid level were defined as receiving “effective” treatment. Finally, cases whose clinical symptoms, signs, and abdominal vertical position plain film did not meet the above criteria were defined as responding “ineffectively” to treatment. Totally effective treatment = cure + effective[6].
Statistical methods
SPSS 20.0 was employed for analyzing/processing datasets, with measurement data reflecting mean ± SD. The independent samplet-test was used for comparisons across cohorts, and the pairedt-test was used for comparison pre-/post-therapy within cohorts. Counting datasets reflected frequency/composition ratio. The comparison of disordered classification data used the chi2test or Fisher’s exact probability method, and the rank sum test compared rank data. APvalue < 0.05 was deemed to confer statistical significance.
RESULTS
Comparative analyses for clinical efficacy among all cohorts
The effectiveness rate of cohort A was significantly elevated compared to cohort C. The effective rates across cohorts “A and B” and “B and C” were similar (Table 1).
Comparative analyses for prognosis among all cohorts
There were statistically significant differences in the time of first exhaust/defecation, length of hospital stays, gastrointestinal decompression time, relief time of abdominal pain, and relief time of abdominal distention among all cohorts (Table 2). Compared to cohort C, the time of first exhaust/defecation, length of hospital stays, gastrointestinal decompression time, relief time of abdominal pain, and relief time of abdominal distention in cohort A were significantly reduced (Table 2).
Comparative analyses for inflammatory factor expression pre-/ post-therapy in all cohorts
Pre-therapy, all cohorts had a similar secretion of serum biomarkers, including CRP, TNF-α, IL-6, and MCP-1 expression. Post-therapy, serum CRP, TNF-α, IL-6, and MCP-1 expression in all cohorts were increased, and the indexes in cohort A were significantly elevated compared to cohort B and C, while cohort B expression profiles were significantly upregulated compared to cohort A (Table 3).
Comparative analyses for nutritional status of all cohorts pre-/ post-therapy
Pre-therapy, serum albumin, and serum transferrin levels were similar among all cohorts. However, post-therapy, serum albumin and serum transferrin levels in all cohorts were increased. Specifically, the serum albumin level in cohort A was significantly elevated compared to cohort C, and the serum transferrin level in cohort A was significantly elevated compared to cohort B and C (Table 4).
Comparative analyses for the incidence of adverse reactions among all cohorts
The widespread occurrence of adverse events within cohorts A and B was significantly elevated compared to cohort C. Additionally, the occurrence of adverse events between cohorts A and B was similar (Table 5).
DISCUSSION
EPISBO pathogenesis after colorectal cancer surgery is mainly related to neuroinhibitory effects, hormones, hypoalbuminemia, inflammatory response, and anesthesia. Intestinal wall tissue damage during surgery can lead to infiltration of a high quantity of macrophages/neutrophils, combined with the release of increased levels of IL-6 and CRP, forming aseptic inflammation. Such inflammatory substances inhibit the inhibition of gastrointestinal vagal nerve and gastrointestinal peristalsis disorder[7-9]. Additionally, inflammatory factors can excite gastrointestinal sympathetic nerves, leading to intestinal wall congestion and mechanical obstruction[10-12]. The rise of intestinal canal pressure can result in intestinal blood circulation disorder, eventually leading to intestinal perforation, necrosis, and abdominal infection. Reoperation can further damage the intestinal canal, leading to postoperative infection and bleeding. Therefore, conservative therapy is often used in clinical therapy of the disease.
Conservative EPISBO therapy includes fasting, gastrointestinal decompression, spasmolysis and analgesia, and correction of water, electrolyte, and acid-base balance disorders. A traditional nasogastric tube decompression can only aspirate gastric juice but cannot drain the contents of the small intestine, the therapeutic cycle is long, and the therapeutic effect is poor. A transnasal ileus tube can be delivered into the duodenum under the guidance of a gastroscope. Peristalsis and water sac can promote the tube to move to the distal part of the small intestine and reach the proximal part of the obstruction site for decompression. The transnasal ileus tube can quickly play the role of intestinal hypotension, relieve intestinal edema, and promote gastrointestinal function recovery. Water injection by the posterior airbag and water pumping by the anterior airbag can ensure the unidirectional movement of the contrast agent, promote further determination of obstruction location and nature, and promote intestinal decompression. Meglumine diatrizoate was initially used as a contrast agent and, recently, was employed within therapy for intestinal obstruction in several studies with sound therapeutic effects[13-15]. The hypertonic 76% meglumine diatrizoate solution assists in transferring interstitial fluid to the intestinal lumen, relieving intestinal wall edema. In addition, meglumine diatrizoate helps determine the size and shape of intestinal filling. According to relevant studies, meglumine diatrizoate can improve local microcirculation, protect intestinal mucosal barrier function, and relieve inflammation. Furthermore, the body can quickly metabolize an appropriate amount of meglumine diatrizoate in a short period with reasonable safety, leading to high clinical tolerance.

Table 1 Comparative analyses for clinical efficacy among all cohorts [cases (%)]
Table 2 Comparative analyses for prognosis among all cohorts ( ± s)
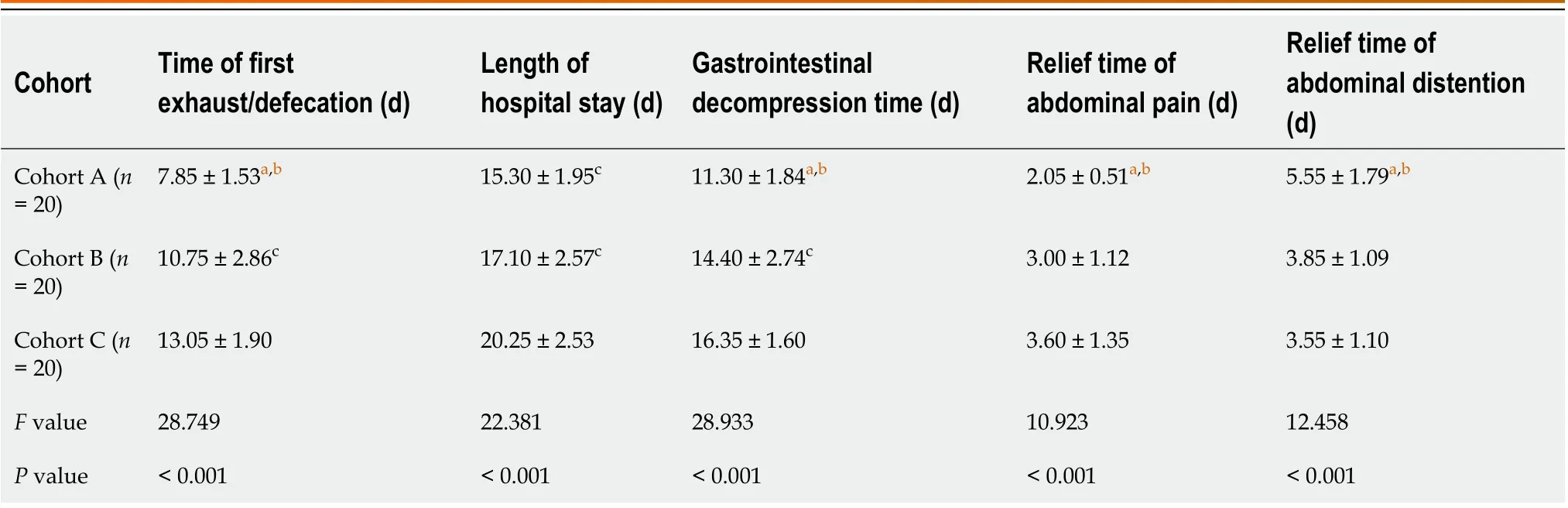
Table 2 Comparative analyses for prognosis among all cohorts ( ± s)
aIndicated P < 0.05 when compared to cohort B.bIndicated P < 0.05 when compared to cohort C.
Cohort Time of first exhaust/defecation (d)Length of hospital stay (d)Gastrointestinal decompression time (d)Relief time of abdominal pain (d)Relief time of abdominal distention(d)Cohort A (n = 20)7.85 ± 1.53a,b 15.30 ± 1.95c 11.30 ± 1.84a,b 2.05 ± 0.51a,b 5.55 ± 1.79a,b Cohort B (n = 20)10.75 ± 2.86c 17.10 ± 2.57c 14.40 ± 2.74c 3.00 ± 1.12 3.85 ± 1.09 Cohort C (n = 20)13.05 ± 1.90 20.25 ± 2.53 16.35 ± 1.60 3.60 ± 1.35 3.55 ± 1.10 F value 28.749 22.381 28.933 10.923 12.458 P value < 0.001< 0.001< 0.001< 0.001< 0.001
Table 3 Comparative analyses for inflammatory factors levels pre-/post-therapy in all cohorts ( ± s)
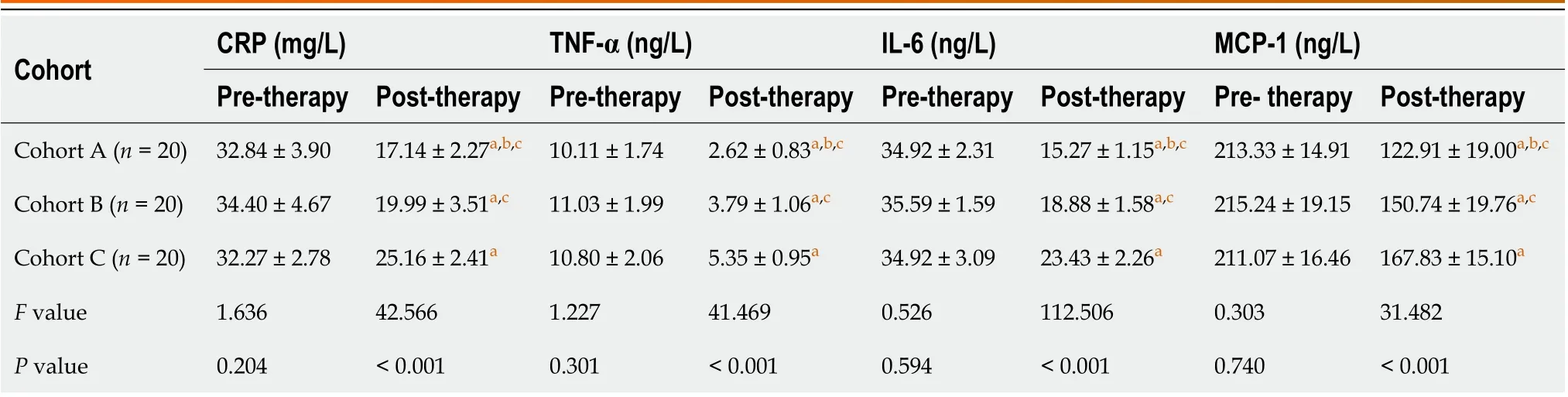
Table 3 Comparative analyses for inflammatory factors levels pre-/post-therapy in all cohorts ( ± s)
aIndicated P < 0.05 when compared with the same cohort pre-therapy.bIndicated P < 0.05 when compared with cohort B.cIndicated P < 0.05 when compared with cohort C.CRP: C-reactive protein; TNF-α: Tumor necrosis factor-alpha; IL-6: Interleukin-6; MCP-1: Monocyte chemoattractant protein-1.
Cohort CRP (mg/L)TNF-α (ng/L)IL-6 (ng/L)MCP-1 (ng/L)Pre-therapy Post-therapy Pre-therapy Post-therapy Pre-therapy Post-therapy Pre- therapy Post-therapy Cohort A (n = 20)32.84 ± 3.90 17.14 ± 2.27a,b,c 10.11 ± 1.74 2.62 ± 0.83a,b,c 34.92 ± 2.31 15.27 ± 1.15a,b,c 213.33 ± 14.91 122.91 ± 19.00a,b,c Cohort B (n = 20)34.40 ± 4.67 19.99 ± 3.51a,c 11.03 ± 1.99 3.79 ± 1.06a,c 35.59 ± 1.59 18.88 ± 1.58a,c 215.24 ± 19.15 150.74 ± 19.76a,c Cohort C (n = 20)32.27 ± 2.78 25.16 ± 2.41a 10.80 ± 2.06 5.35 ± 0.95a 34.92 ± 3.09 23.43 ± 2.26a 211.07 ± 16.46 167.83 ± 15.10a F value 1.636 42.566 1.227 41.469 0.526 112.506 0.303 31.482 P value 0.204< 0.001 0.301< 0.001 0.594< 0.001 0.740< 0.001
This investigation’s dataset outcomes demonstrated that cohort A’s effective rate was significantly elevated compared to cohort C. Furthermore, the effective rate across cohorts “A and B” and “B and C” were similar. Compared to cohort C, the time of first exhaust/defecation, length of hospital stays, gastrointestinal decompression time, relief time of abdominal pain, and relief time of abdominal distension in cohort A were significantly reduced. Together, these results indicate that combined therapy has a better effect on EPISBO after colorectal cancer surgery and can more effectively promote the recovery of gastrointestinal function and shorten the therapy time.
Table 4 Comparative analyses for nutritional status of all cohorts pre- and post-therapy (± s)
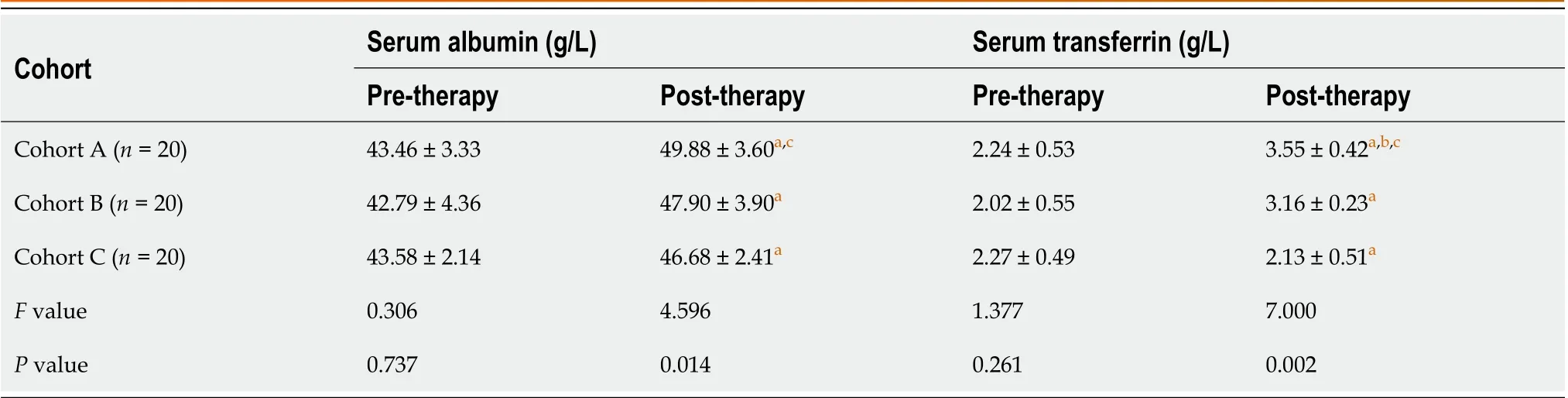
Table 4 Comparative analyses for nutritional status of all cohorts pre- and post-therapy (± s)
aIndicated P < 0.05 when compared with the same cohort pre-therapy.bIndicated P < 0.05 when compared with cohort B.cIndicated P < 0.05 when compared with cohort C.
Cohort Serum albumin (g/L)Serum transferrin (g/L)Pre-therapy Post-therapy Pre-therapy Post-therapy Cohort A (n = 20)43.46 ± 3.33 49.88 ± 3.60a,c 2.24 ± 0.53 3.55 ± 0.42a,b,c Cohort B (n = 20)42.79 ± 4.36 47.90 ± 3.90a 2.02 ± 0.55 3.16 ± 0.23a Cohort C (n = 20)43.58 ± 2.14 46.68 ± 2.41a 2.27 ± 0.49 2.13 ± 0.51a F value 0.306 4.596 1.377 7.000 P value 0.737 0.014 0.261 0.002

Table 5 Comparative analyses for adverse event occurrence among all cohorts [cases (%)]
The therapy plan of the ileus tube combined with meglumine diatrizoate injection combines the therapeutic advantages of the ileus tube and meglumine diatrizoate. Using an ileus tube, meglumine diatrizoate can quickly reach the site of intestinal obstruction, dilute intestinal obstruction contents, relieve intestinal stenosis, recover gastrointestinal function, and avoid further aggravation of intestinal obstruction. Thus, this method can effectively shorten the hospital stay and reduce clinical manifestations in patients. Cohort B was administered an ileus tube combined with liquid paraffin, which also took advantage of the dual advantages of an ileus tube and liquid paraffin. Therefore, cohorts A and B’s clinical efficacy and prognosis were better than cohort C, who were administered oral meglumine diatrizoate alone.
CRP is synthesized by stem cells, and its expression level can be abnormally elevated when the body is subjected to inflammatory stimulation or stress response[16,17]. Lymphocytes and fibroblasts produce TNF-α, and endothelial cells, which can enhance the chemotaxis of neutrophils, release inflammatory factors, aggravate the body’s inflammatory response, and exacerbate tumor cellular proliferative rate, leading to patient condition deterioration. IL-6 is an inflammatory cytokine produced by endothelial cells, lymphoid cells, and mononuclear macrophages, which can regulate inflammatory response and induce stem cells to synthesize CRP. MCP-1 can reduce the speed of gastrointestinal motility through inhibitory adrenergic nerve pathway activity and is abnormally expressed in various inflammatory responses, affecting gastrointestinal neuromuscular movement. Additionally, several studies have shown that MCP-1 expression level is intimately linked with the severity of intestinal obstruction[18-20]. This investigation revealed that serum CRP, TNF-α, IL-6, and MCP-1 levels in all cohorts were significantly increased post-therapy. In contrast, the levels of each index in cohort A were elevated compared to cohort B and C, and the levels of each index in cohort B were significantly elevated compared to cohort A. These data indicate that an ileus tube combined with meglumine diatrizoate for treating EPISBO after colorectal cancer surgery could effectively relieve the inflammatory response of patients and that the effect is better than instances where an ileus tube combined with liquid paraffin therapy and meglumine diatrizoate is used alone. This observation may be because, compared with liquid paraffin, meglumine diatrizoate can play a particular therapeutic effect in addition to the contrast effect in the therapy of EPISBO; thus, the combination of ileus tube and meglumine diatrizoate has a better therapeutic effect. We hypothesize that the mechanism underlying this effect may be because the ileus tube combined with meglumine diatrizoate relieves the body’s inflammatory response, improving clinical symptoms.
EPISBO patients, after colorectal cancer surgery, are prone to malnutrition. Parenteral nutrition can provide adequate nutritional support to patients and reduce the incidence of complications. However, long-term enteral nutrition can damage the intestinal microbial barrier function, cause entheogenic infection, and affect the postoperative recovery of patients. Therefore, enteral nutrition is generally given to patients with intestinal obstruction to improve their nutritional status and promote the recovery of gastrointestinal function. In this study, serum albumin and serum transferrin levels in all cohorts increased post-therapy. Specifically, the serum albumin level in cohort A was significantly elevated compared to cohort C and the serum transferrin level in cohort A was significantly elevated compared to cohort B and C. These results suggest that an ileus tube combined with meglumine diatrizoate in the therapy of EPISBO after colorectal cancer surgery can effectively improve the nutritional status of patients. Because the transnasal ileus tube can effectively shorten the recovery time of the gastrointestinal function and provide enteral nutrition as soon as possible, the nutritional status of patients in cohort A was better than in cohort B and C. The incidence of total adverse reactions in cohorts A and B was significantly elevated compared to cohort C. The incidence of adverse reactions was similar across cohort A and cohort B. The higher incidence of adverse reactions observed in cohorts A and B could be attributed to using the ileus tube in these groups.
This study has several limitations. First, this is a retrospective study with a small sample size; unintentional biases may have been introduced. Further large-scale, multi-center prospective studies are expected to explore the effect of ileus tubes combined with meglumine diatrizoate in the therapy of EPISBO after colorectal cancer surgery and provide references for clinical treatment.
CONCLUSION
In conclusion, the use of an ileus tube combined with meglumine diatrizoate in the therapy of EPISBO after colorectal cancer surgery can effectively shorten the length of hospital stay, promote the recovery of gastrointestinal function, and relieve the inflammatory response of the body, with good therapeutic effect and clinical application value.
ARTICLE HIGHLIGHTS

FOOTNOTES
Author contributions:Yang W initiated the project, designed the experiment, performed postoperative follow-up and recorded data, and wrote the original manuscript; Pu J conducted collated data, assisted with the statistical analysis, and revised the paper; all authors reviewed and approved the paper; and all authors have read and approved the final manuscript.
Institutional review board statement:This study was approved by the Ethics Committee of Lanzhou Second People’s Hospital.
Informed consent statement:Due to the retrospective design, patient consent was not required.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Data sharing statement:All data generated or analyzed during this study are included in this published article.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORCID number:Jing Pu 0000-0001-8925-3060.
S-Editor:Wang JJ
L-Editor:A
P-Editor:Wang JJ
2Haumann A, Ongaro S, Detry O, Meunier P, Meurisse M. Acute pelvic inflammatory disease as a rare cause of acute small bowel obstruction.Acta Chir Belg2019; 119: 328-330 [PMⅠD: 29560794 DOⅠ: 10.1080/00015458.2018.1453438]
杂志排行
World Journal of Gastrointestinal Surgery的其它文章
- Preoperative and postoperative complications as risk factors for delayed gastric emptying following pancreaticoduodenectomy: A single-center retrospective study
- Comparative detection of syndecan-2 methylation in preoperative and postoperative stool DNA in patients with colorectal cancer
- Preoperative prediction of microvascular invasion in hepatocellular carcinoma using ultrasound features including elasticity
- Surgical management of gallstone ileus after one anastomosis gastric bypass: A case report
- Hepatic ischemia-reperfusion syndrome and its effect on the cardiovascular system: The role of treprostinil, a synthetic prostacyclin analog
- Advances and challenges of gastrostomy insertion in children
