Integrin beta 3-overexpressing mesenchymal stromal cells display enhanced homing and can reduce atherosclerotic plaque
2023-10-18HaiJuanHuXueRuXiaoTongLiDeMinLiuXueGengMeiHanWeiCui
Hai-Juan Hu,Xue-Ru Xiao,Tong Li,De-Min Liu,Xue Geng,Mei Han,Wei Cui
Abstract BACKGROUND Umbilical cord (UC) mesenchymal stem cell (MSC) transplantation is a potential therapeutic intervention for atherosclerotic vascular disease.Integrin beta 3(ITGB3) promotes cell migration in several cell types.However,whether ITGBmodified MSCs can migrate to plaque sites in vivo and play an anti-atherosclerotic role remains unclear.AIM To investigate whether ITGB3-overexpressing MSCs (MSCsITGB3) would exhibit improved homing efficacy in atherosclerosis.METHODS UC MSCs were isolated and expanded.Lentiviral vectors encoding ITGB3 or green fluorescent protein (GFP) as control were transfected into MSCs.Sixty male apolipoprotein E-/- mice were acquired from Beijing Vital River Lab Animal Technology Co.,Ltd and fed with a high-fat diet (HFD) for 12 wk to induce the formation of atherosclerotic lesions.These HFD-fed mice were randomly separated into three clusters.GFP-labeled MSCs (MSCsGFP) or MSCsITGB3 were transplanted into the mice intravenously via the tail vein.Immunofluorescence staining,Oil red O staining,histological analyses,western blotting,enzymelinked immunosorbent assay,and quantitative real-time polymerase chain reaction were used for the analyses.RESULTS ITGB3 modified MSCs successfully differentiated into the “osteocyte” and “adipocyte” phenotypes and were characterized by positive expression (> 91.3%) of CD29,CD73,and CD105 and negative expression (< 1.35%) of CD34 and Human Leukocyte Antigen-DR.In a transwell assay,MSCsITGB3 showed significantly faster migration than MSCsGFP.ITGB3 overexpression had no effects on MSC viability,differentiation,and secretion.Immunofluorescence staining revealed that ITGB3 overexpression substantially enhanced the homing of MSCs to plaque sites.Oil red O staining and histological analyses further confirmed the therapeutic effects of MSCsITGB3,significantly reducing the plaque area.Enzyme-linked immunosorbent assay and quantitative real-time polymerase chain reaction revealed that MSCITGB3 transplantation considerably decreased the inflammatory response in pathological tissues by improving the dynamic equilibrium of pro-and anti-inflammatory cytokines.CONCLUSION These results showed that ITGB3 overexpression enhanced the MSC homing ability,providing a potential approach for MSC delivery to plaque sites,thereby optimizing their therapeutic effects.
Key Words: Atherosclerosis;Inflammation;Integrin beta 3;Mesenchymal stem cells;Arg-Gly-Asp structure;Umbilical cord
INTRODUCTION
Atherosclerosis is a serious public health problem and the most commonly diagnosed cardiovascular disease in the general population[1].Implementing early detection of atherosclerosis[2] with systemic pharmacological treatments[3]and percutaneous coronary intervention[4] has contributed to substantial progress in the treatment of atherosclerosisrelated diseases[5,6].Despite medical improvements,the incidence of atherosclerosis-related diseases remains high[7].Atherosclerotic plaques are still associated with high mortality rates in patients with high-risk or visible advanced plaque disease.Therefore,more studies are needed to discover and explore effective molecules and targets for treatments.
Mesenchymal stem cell (MSC) transplantation accomplished improvements in experimental studies of atherosclerosis[8-11].MSCs have the ability to secrete many cytokines that mitigate vascular inflammation and regulate the local microenvironment owing to the effects of their secreted anti-inflammatory factors within the vascular plaque[12-14].However,some limitations preclude the translation of stem cell therapy into clinical applications[15,16],such as stem cells accurately homing to plaques.To determine this,we need to understand the specific mechanisms that underlie the migration and adhesion of MSCs under physiological and pathological conditions.Some studies have attempted to genetically decorate MSCs with specific receptors required for efficient homing.For example,Shahroret al[17] found that overexpression of fibroblast growth factor 21 considerably increased MSC migration to and adhesion in the injured brain tissue.Moreover,overexpression of C-X-C chemokine receptor 5 (CXCR5) increased the migratory capability of MSCs toward CXCL13,which changed most substantially in animal models of contact allergy,accompanied by decreases in inflammatory cellular infiltration and reduced levels of pro-inflammatory cytokine production[18].It is well established that damaged tissues release specific types of cytokines and chemokines.Therefore,elucidating the interrelationships between tissue-specific chemokines and matching receptors on MSCs could offer novel ways of promoting homing and treatment effects of these cells.
The integrin family of receptors,a major family of migration-promoting receptors,plays an important role in the crosstalk between cells and their surroundings[19,20].Arg-Gly-Asp (RGD) site-containing proteins and corresponding integrin receptors constitute the primary recognition system for cell adhesion[21,22].Many inflammatory cytokines,such as intercellular cell adhesion molecule-1 (ICAM-1),osteopontin (OPN),and vascular cell adhesion molecule-1 (VCAM-1),contain an RGD motif in their structure[23,24].The primary sequence of integrin beta 3 (ITGB3),a well-preserved region in all integrin beta subunits,has been called the RGD crosslinking region[25].Based on this information,we genetically modified MSCs usingex vivolentiviral transduction to overexpress ITGB3.In the current study,we first demonstrated that ITGB3-overexpressing MSCs (MSCsITGB3) showed enhanced chemotaxis toward plaque tissuesin vivoand inflammatory cellsin vitro.Compared with green fluorescent protein (GFP)-labeled MSCs (MSCsGFP),MSCsITGB3reduced the formation of atherosclerotic plaques in homozygous apolipoprotein-E (ApoE)-/-mice,suggesting that ITGB3 enhances homing of modified stem cells to plaque tissue,thereby promoting their therapeutic efficacy in the ApoE-/-mouse model of atherosclerosis.
MATERIALS AND METHODS
Isolation of human umbilical cord MSCs
Human umbilical cord (UC) samples were collected from three healthy donors.All the donors provided written informed consent.MSCs were isolated and cultured from the UCs as reported previously[26].Briefly,the UC tissue was collected from healthy pregnant women undergoing labor.After removing the UC tissue's arteries and veins,the remaining Wharton's jelly was cut into small pieces for patch cultures.The tissue fragments were cultured at 37 °C and 5% CO2in mesenchymal stem cell medium (MSC medium;Sciencell,Carlsbad,CA,United States) containing 5% fetal bovine serum(FBS),stem cell growth supplements,penicillin,and streptomycin.After 10-15 d,fibroblast-like MSCs migrated out of the tissue patches.The studies involving pregnant participants were reviewed and approved by The Ethical Committee of The Second Hospital of Hebei Medical University (approval number: 2021-R496).
Raw264.7, vascular smooth muscle cell, and primary microvascular endothelial cell cultures
The murine macrophage cell line Raw264.7 was purchased from the Cell Resource Center of Shanghai Institutes for Biological Sciences,the Chinese Academy of Science (Shanghai,China).Vascular smooth muscle cells were isolated from mouse aortas and cultured in low-glucose Dulbecco’s modified Eagle’s medium containing 10% FBS.Lung primary microvascular endothelial cells were obtained from the lungs of 4-week-old C57 mice through two series of immunoselection with CD31-and CD102-conjugated magnetic beads using a previously described procedure[27] and subsequently cultured in endothelial cell medium containing 5% FBS and endothelial cell growth supplements.
Lentiviral vector construction and transduction
Lentiviral vector (LV) encoding ITGB3 and GFP as control were generated and packaged by Hanbio Biosciences(Shanghai,China).All LVs were used for MSC transfection with an infection multiplicity of 30-40.MSCs were plated at a density of 3-5 × 105cells/cm2in each six-well plate,depending on the subsequent use.When MSCs reached 40%-50%confluence,the transfection was performed in the presence of polybrene (Cyagen Biosciences,Santa Clara,CA,United States).Following transfection for 72 h,the transfection effectiveness of the virus was checked through fluorescent staining.
AS model induction and MSC delivery
ApoE-/-mice (7-8-week-old) were acquired from Beijing Vital River Lab Animal Technology Co.,Ltd (Beijing,China).Sixty male ApoE-/-mice were housed in Hebei Medical University and fed with standard chow and drinking water for 1 wk to adapt to the new surroundings.Then,all animals were fed a high-fat diet (HFD) for 12 wk to induce the formation of atherosclerotic lesions.These HFD-fed mice were randomly separated into three clusters and received the following treatment: (1) HFD mice (n=20) were injected 200 μL of phosphate-buffered saline (PBS) into the caudal vein every week from week 9,four times in total;(2) MSCGFPmice (n=20) receiving 200 μL of PBS containing 1 × 106MSCsGFPintravenously through the tail vein every week from week 9;and (3) MSCITGB3mice (n=20) receiving 200 μL of PBS containing 1 × 106MSCsITGB3intravenously through the tail vein every week from week 9.The animals were euthanized after 12 wk of HFD,the aorta and aortic sinus were collected,and histological samples were processed for subsequent atherosclerosis assessment.At the same time,blood samples were collected to determine lipid levels.All animal procedures complied with the Guide for the Care and Use of Experimental Laboratory Animals and were approved by the Animal Care Committee of The Second Hospital of Hebei Medical University (No.2021-R496).
Echocardiographic assessment
At the end of week 12,10 mice were gas-anesthetizedviaa facemask and kept on a minimal dose of isoflurane (1.0%-2.0%).The mice were placed in a supine position and maintained spontaneous breathing with isoflurane insufflation throughout the echocardiographic assessment.Echocardiographic data were recorded by a VisualSonics Vevo2100 Imaging System (Fujifilm Visual Sonics Inc.,Tokyo,Japan) with an EZ-SA800 Single Animal System (E-Z Systems Inc.,Bethlehem,PA,United States).Ascending aorta functions were evaluated from the parasternal view of the long axis.The diameter of the systolic aorta was measured at the peak anterior movement point of the ascending aorta,and the diameter of the diastolic aorta was measured using a Q-wave (end-diastolic) echocardiogram.The average diameter measurements of five consecutive heart cycles were selected for data analysis.Meanwhile,left ventricle function and heart chamber dimensions were also evaluated,including the left ventricular ejection fraction (EF) and left ventricular fractional shortening (FS),using accompanying software.The data were recorded and analyzed.
Murine aortic tissue culture
Murine aortic rings were cultured as described previously[28].Briefly,after 12 wk of HFD,external organs and tissues were removed.Then,the whole aortic vessel was separated from the adipose and surrounding tissue.The aorta was divided into 2-4 mm rings and cultured in low-glucose Dulbecco’s modified Eagle’s medium supplemented with 5% FBS.Aortic rings were maintained at 37 °C in 5% CO2for 24 h for further experiments.
Hematoxylin and eosin staining, Masson's trichrome staining, and oil red O staining
The aortic arch and aortic valve were harvested after 12 wk of HFD.The animals were euthanized,and the left ventricles were cannulated and injected with PBS containing heparin.Thereafter,the aortic arch and aortic valve were separated,embedded in optimal cutting temperature compound,shortly frozen in liquid nitrogen,and sliced.Subsequently,hematoxylin and eosin (HE) staining was performed to assess plaque size.The cross-sectional areas of the plaque were measured with Image-Pro Plus (IPP) 6.0 software (Media Cybernetics Corp.,Rockville,MD,United States).Collagen depositions were assessed using Masson's trichrome staining.Average values were determined from at least three sections in each sample.
For oil red O (ORO) staining,the fixed whole aorta was incubated with 0.3% ORO solution (Sigma-Aldrich,St Louis,MO,United States) for 20 min and,then,washed with 60% isopropanol.Frozen aortic arch and aortic valve sections were incubated with ORO for 5 min,washed with PBS,and counterstained with hematoxylin.The atherosclerotic lesions were photographed using a light microscope (Carl Zeiss,Jena,Germany) and quantified using IPP 6.0 software.
Cell viability assay
The effects of LV transfection on MSC viability were evaluated using cell counting kit 8 (CCK-8) assays as described previously[29].Briefly,MSCs,MSCsITGB3,and MSCsGFPwere seeded into 96-well plates at a density of 1 × 104cells/well,respectively.After 24 h,CCK-8 was added to each well and incubated at 37 °C for 1 h.The absorbance was measured at 450 nm using a VersaMax (Ocean Springs,MI,United States) microplate scanner.
Chemotaxis assays
Chemotaxis assays were operated in 24-well transmembrane chambers with 8-µm pore filters.To test the ability of stem cells to migrate toward defined chemokines,Raw264.7,vascular smooth muscle cells,and primary microvascular endothelial cells were separately seeded into the lower chamber (1 × 105cells/well).After achieving confluence,serumfree cells were stimulated with tumor necrosis factor-α (TNF-α,20 ng/mL) for 24 h.Then MSCs,MSCsGFP,and MSCsITGB3were respectively plated into the upper chamber at 2 × 104cells/well.After incubation for 24 h,the upper layer of cells was removed,and the lower layer was stained with crystal violet.
For the vascular atherosclerotic plaque,mice were sacrificed after week 12.Aortic rings were separately cultured for 24 h in Dulbecco's modified Eagle's medium with 5% FBS.Next,MSCs,MSCsGFP,and MSCsITGB3were respectively plated into the upper chamber at 2 × 104cells/well.The medium for re-suspending MSCs matched the medium in the lower chamber.After incubation for 24 h,non-migrated cells were removed from the upper surface,and the cells that had migrated across the membrane to the lower surface were fixed with 4% paraformaldehyde and stained with crystal violet.For each membrane,five fields of view were imaged and analyzed.Each experiment was repeated at least three times,and the number of migrated cells was expressed as the mean ± standard error of the mean (SEM) of total cell counts per field.
RNA extraction and quantitative real-time polymerase chain reaction
Total RNA of cells or tissue samples was extracted using TRIzol reagent,according to the manufacturer’s protocol.cDNA was synthesized using the M-MLV First Strand Kit.Quantitative real-time polymerase chain reaction (qRT-PCR) using SYBR Green qPCR SuperMix were performed using an ABI 7500.qRT-PCR analyses were repeated at least three times.The qRT-PCR data were standardized to β-actin expression,using the 2-ΔΔCtmethod.The primers used are listed in Supplementary Table 1.The average threshold cycle for each gene was determined from at least three independent experiments.
Western blot analysis
Cell or tissue lysates were extracted with lysis buffer.Equal amounts of protein were separated by 10% sodium dodecylsulfate polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane.The membranes were blocked with 5% bovine serum albumin for 1 h at room temperature,incubated with specific antibodies against VCAM-1,ICAM-1,OPN,and GAPDH at 4 °C overnight,and,then,incubated with horseradish peroxidase-conjugated anti-mouse or anti-rabbit immunoglobulin G for 1 h at room temperature.Antigen-antibody complexes were assessed using the GE ImageQuant™ LAS 4000 detection system (GE Healthcare Inc.,Chicago,IL,United States).The protein bands of interest were quantified with IPP 6.0 software.
Statistical analyses
Data analysis was performed with GraphPad Prism 8 software (GraphPad Software,Inc.,San Diego,CA,United States).Data are displayed as mean ± SEMs,and each independent experiment was repeated three times.The statistical significance of differences between two groups was determined using the unpaired Student’s t-test,and comparisons of more than two groups were performed using a one-way analysis of variance.For all statistical comparisons,significance was considered atP< 0.05.
RESULTS
Inflammatory factors containing the RGD motif are highly upregulated in atherosclerotic vessels
Atherosclerosis is a chronic inflammatory disease of the arteries with high expression of adherence molecules.We tested the expression of VCAM-1,ICAM-1,and OPN because studies have shown that they contain the RGD motif that can bind to the ITGB3 receptor[19,20,30].Our results demonstrated that the protein expression levels of VCAM-1,ICAM-1,and OPN were six to eight times higher in atherosclerotic than in control blood vessels (Figure 1A).Similarly,qRT-PCR and immunofluorescence staining also confirmed that the three adhesion factors considerably increased at sites of inflammation (Figure 1B-D).
The ITGB3 receptor is scarcely expressed on human UC-derived MSCs, and ITGB3 overexpression by MSCs does not affect other MSC characteristics
Inflammatory factors that are highly expressed at plaque sites and matching receptors expressed on the surface of MSCs play an important role in guiding stem cells.Thus,we analyzed the expression of the ITGB3 receptor using western blot,qRT-PCR,and fluorescence staining methods.First,human MSCs at the third passage had very low expression of the ITGB3 receptor at the protein and mRNA levels (Figure 2A and B).Second,immunofluorescence staining confirmed that MSCs barely expressed the ITGB3 receptor (Figure 2C and D).To further determine whether overexpression of ITGB3 receptor in MSCs can promote their homing capability toward plaque tissues,MSCs were transfected with LVs encoding ITGB3 and GFP (MSCsITGB3for short) or GFP (MSCsGFPfor short).We found that both MSCsITGB3and MSCsGFPhighly expressed GFP,confirming the stable expression of the GFP reporter gene (Figure 2C).In contrast to MSCsGFPand MSCs,MSCsITGB3showed substantially stronger expression of ITGB3 at the protein,mRNA,and fluorescence staining levels(Figure 2A-D).Moreover,ITGB3 overexpression did not affect stem cell viability according to CCK8 analyses (Figure 2E).To further verify whether transfection of ITGB3 affects the differentiation properties of MSCs,we observed several typical stem cell markers using flow cytometry and qRT-PCR analyses.The results showed that in MSCsITGB3,MSC-specific markers (CD73,CD105,and CD29),but not hemopoietic stem cell antigens (CD34 and human leukocyte antigen-DR),were highly expressed (Figure 2F and G).The differentiation of MSCsITGB3into adipocytes and osteoblasts was separately evaluated using ORO and Alizarin red staining (Figure 2H and I).Moreover,marker genes in induced adipocytes and osteoblasts were verified through qRT-PCR (Figure 2J and K).These findings confirmed that transfected MSCs still have stem cell properties.
ITGB3 enhances MSC migration toward inflammatory sites in vitro and in vivo
ITGB3 is vital for cell migration,adhesion,and invasion[20-22,31].Therefore,we next checked whether MSCsITGB3could promote MSC migrationin vitroandin vivo.Thein vitrocell chemotaxis assay showed that in the presence of TNF-α,MSCsITGB3displayed a significantly increased migration toward the bottom chamber,especially if it contained macrophages (Figure 3A and B).However,in the absence of TNF-α,all cells showed similar,very low,nonspecific migration toward the lower,cell-containing chamber (Figure 3A and B).We also performed transwell-based chemotaxis assays using atherosclerotic aorta samplesin vitro(Figure 3C and D).MSCsITGB3had the highest migratory activity,indicating that the atherosclerotic plaque secreted inflammatory factors which were chemoattractants for MSCsITGB3,but not for MSCsGFP(Figure 3C and D).Next,we observed whether MSCs migrate to plaque sitesin vivofollowing intravenous infusion.MSCsITGB3and MSCsGFPwere injected into HFD mice through the tail vein every week from weeks 9 to 12.The aortas were collected from each group at the end of week 12 for in situ fluorescence staining.The results showed that MSCsITGB3were highly aggregated at sites of inflammation,whereas MSCsGFPhad less positive staining at such sites(Figure 3E and F).Meanwhile,the qRT-PCR results also revealed that there was a higher GFP expression in the MSCITGB3group when compared to the GFP expression in the MSCsGFPgroup (Figure 3G).However,there were negligible MSCs in the myocardium,and no difference between the two groups was observed (Supplementary Figure 1).These data suggested that ITGB3 overexpression promoted the targeted migration of MSCs to plaque sitesin vivo,possibly by targeting inflammatory factors containing the RGD structure.
MSCsITGB3 reduce the progression of atherosclerotic plaques in ApoE-/- mice fed an HFD
To explore the effects of MSCsITGB3on the progression of atherosclerosis,the whole aorta,aortic root,and aortic arch were stained with 0.5% ORO,and the stained areas were quantified to determine the atherosclerotic plaque area.The stained area in the aorta was substantially decreased in the MSCITGB3group when compared with that of the HFD and MSCGFPgroups (Figure 4A and B).The stained areas in the aortic root and aortic arch decreased in the MSCITGB3group compared with the corresponding in the HFD and MSCGFPgroups (Figure 4C).HE and Masson's trichrome staining analyses of sections taken from the aortic root and aortic arch demonstrated that MSCsITGB3inhibited aortic plaque formation and collagen deposition more effectively than MSCsGFP(Figure 4D and E).Aortic stiffness is the earliest detectable evidence of changes in arterial wall function.Echocardiographic results showed that both systolic and diastolic diameters were considerably increased while the vessel wall thickness was decreased in the MSCITGB3group (Figure 4F).However,left ventricular EF and FS were not different among the three groups (Supplementary Figure 2).The number of macrophages in atherosclerotic plaques at the aortic arch was determined by F4/80 staining.The F4/80 staining-positive areas were notably reduced in the MSCITGB3group compared with the HFD group (Figure 4G).
MSCsITGB3 do not alter the biochemical parameters of ApoE-/- mice fed an HFD

Figure 1 Expression of inflammatory factors in the vascular atherosclerotic plaque. A: Expression of mouse vascular cell adhesion molecule-1(VCAM-1),intercellular cell adhesion molecule-1 (ICAM-1),and osteopontin (OPN) expression in total tissue lysates of normal and atherosclerotic (AS) aorta analyzed using western blot.GAPDH was used as the internal control.The experiment was repeated thrice with tissues isolated from independent mice;a representative blot is shown;B: Expression levels of various inflammatory factors involved in atherosclerosis analyzed using quantitative real-time polymerase chain reaction of mRNA samples extracted from normal and AS vessels of three independent mice.Data are presented as the mean ± SEM for each group.Fold change represents the expression of each inflammatory factor in AS vessel of a mice fed with high fat diet for 12 wk compared with that in normal blood vessel;C:Representative images of normal and AS vascular sections stained for VCAM1 (red) and ICAM1 (red).The experiment was repeated three times with tissues isolated from independent mice;a representative image is shown.Nuclei were visualized by DAPI staining (blue).Scale bars=100 mm;D: Representative images of normal and AS vascular sections stained for ICAM1 (red).The experiment was repeated three times with tissues isolated from independent mice;a representative image is shown.Nuclei were visualized by DAPI staining (blue).Scale bars=100 μm.bP < 0.001.AS: Atherosclerotic;OPN: Osteopontin;qRT-PCR: Quantitative real-time polymerase chain reaction;SEM: Standard error of the mean;VCAM-1: Vascular cell adhesion molecule-1;ICAM-1: Intercellular cell adhesion molecule-1.
To evaluate the effects of MSCsITGB3on biochemical featuresin vivo,we recorded changes in mouse body weight and measured blood lipid concentrations.The results showed that the body weight did not vary among the three groups during the experiment (Supplementary Figure 3A).Plasma lipid analyses,including those measuring total cholesterol,high-density lipoprotein cholesterol,triglyceride,and low-density lipoprotein cholesterol levels,were performed on terminal blood samples obtained by cardiac puncture but showed no statistical differences among the HFD,MSCGFP,and MSCITGB3groups (Supplementary Figure 3B).These results suggest that intravenous injection of stem cells did not interfere with lipid metabolism in mice receiving an HFD.
MSCsITGB3 modulate cytokine expression in the AS mouse model
The levels of inflammatory factors are positively correlated with the degree of inflammation in atherosclerosis.Cytokines play a pivotal role in chronic inflammatory diseases[31].They affect the expression of adherence molecules,permeability of endothelial cell,as well as proliferation and migration of inherent cells in blood vessels,all of which are associated with atherosclerosis.Interleukin (IL)-1β,an established driver of atherosclerotic disease[32],is involved in the entire process of atherosclerotic lesion progression[33].Advanced atherosclerotic plaques can be alleviated and stabilized through IL-1β inhibition.After observing that MSCITGB3treatment can reduce the plaque area,we further investigated whether MSCITGB3infusion can decrease the levels of pro-inflammatory factors or increase the levels of anti-inflammatory factors in the serum and aorta tissue.Our results showed that pro-inflammatory factors,including TNF-α,IL-1β,and IL-6,showed a significant downward trend in mice who had received MSCITGB3or MSCGFPtreatment.However,the treatment effect of MSCsITGB3was more pronounced than that of MSCsGFP.Likewise,mice receiving MSCsITGB3or MSCsGFPshowed significantly increased levels of anti-inflammatory factors,such as IL-4,IL-10,and tumor necrosis factor-stimulated gene-6 in the serum and aorta tissue (Figure 5).These results indicate that MSCITGB3treatment primarily reduces pathological inflammatory responses by improving the balance between secreted pro-and anti-inflammatory factors.
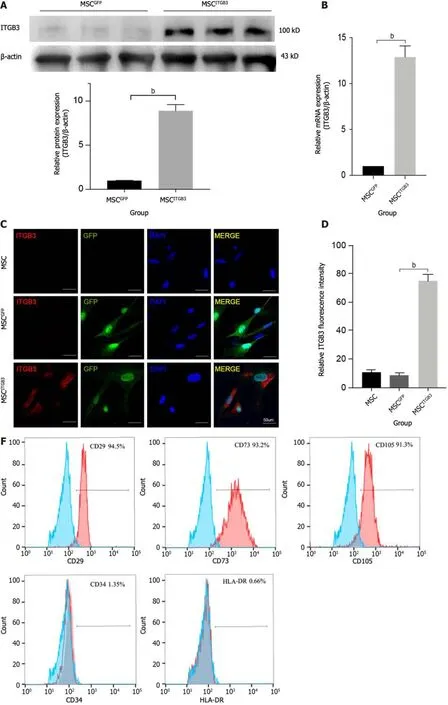
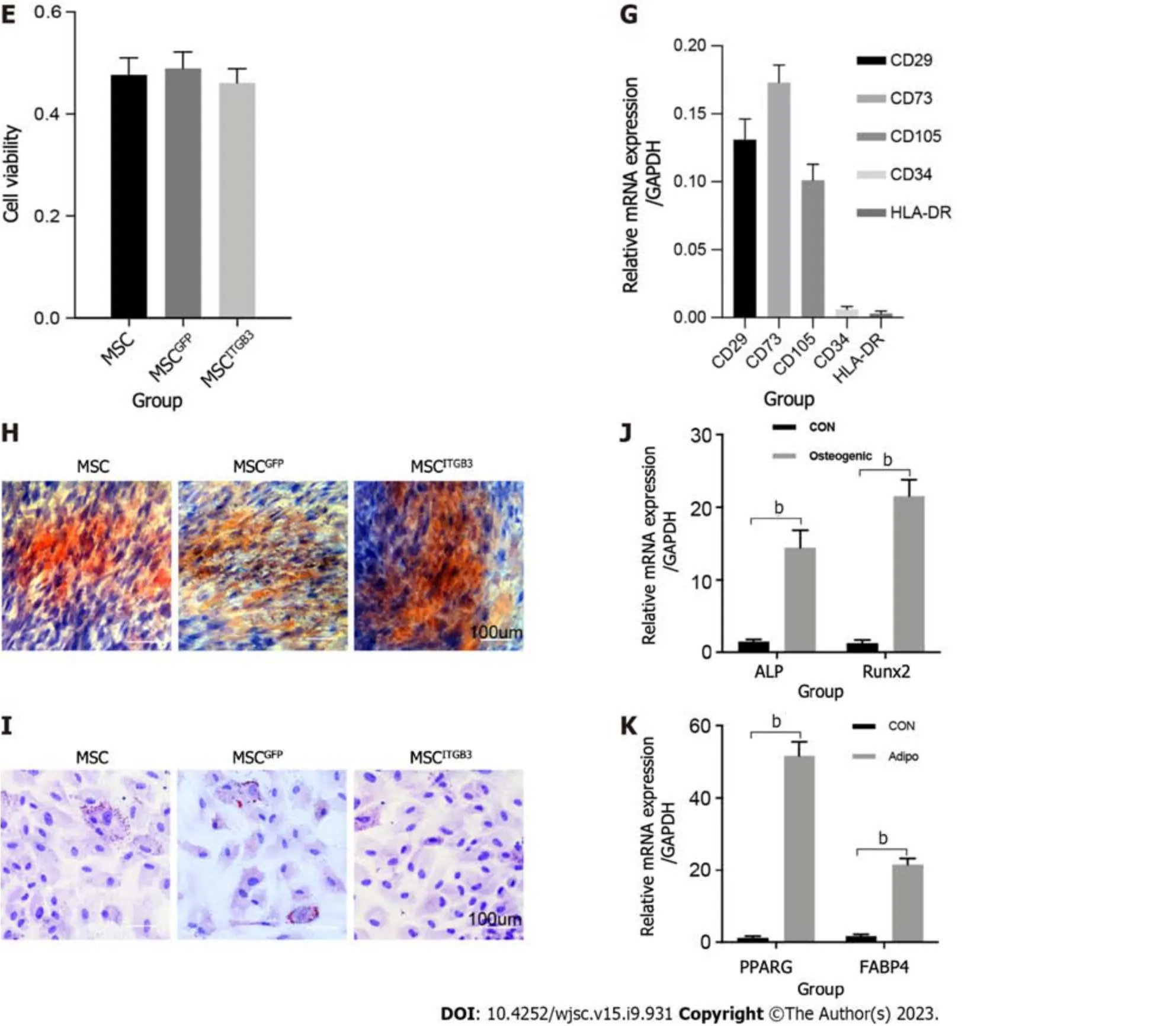
Figure 2 Expression of integrin subunit beta 3 on human umbilical cord-derived mesenchymal stem cells. A: Western blot;B: Quantitative realtime polymerase chain reaction (qRT-PCR) analysis of Integrin subunit beta 3 (ITGB3) expression;C: Representative immunofluorescence image;D: Quantification of ITGB3 and green fluorescence protein staining in untransfected and transfected mesenchymal stem cells (MSCs);E: Cell viability of untransfected and transfected MSCs determined by CCK8 assay.The experiment was repeated three times;F and G: Analysis of cell surface markers on MSCITGB3 using flow cytometry to determine whether they have pluripotent characteristics;H: Osteogenic differentiation of MSCITGB3 identified using Alizarin red staining;I: Oil Red O staining shows differentiation of MSCITGB3 into adipocytes;J: qRT-PCR analysis of marker genes in induced osteoblasts;K: qRT-PCR analysis of marker genes in induced adipocyte.bP < 0.001.GFP: Green fluorescence protein;ITGB3: Integrin subunit beta 3;MSC: Mesenchymal stem cell;ORO: Oil Red O;qRT-PCR: Quantitative real-time polymerase chain reaction.
DISCUSSION
In this study,adhesion molecules containing the RGD motif were highly expressed in an atherosclerosis model,and transfection of ITGB3 into MSCs improved their targeting capability.As expected,intravenous injection of MSCsITGB3substantially reduced atherosclerotic plaque in the ApoE-/-HFD mouse model.
Owing to their adaptive immunomodulatory properties and superior secretory potential,MSCs have attracted extensive attention in the treatment of atherosclerotic diseases[8,14].The route of MSC administration is critical for their therapeutic efficiency,and systemic deliveryviaintravenous infusion is the primary approach for many cell therapies.However,several problems occur with this approach,and one of the major hurdles is insufficient cell engraftment into the damaged tissue[34].It is well known that the therapeutic level of MSCs mainly depends on intercellular interactions and their regulation by the local microenvironment.Therefore,the therapeutic effect of intravenously injected MSCs may be closely related to their localization.It is predicted that the effectiveness of cell therapy can be improved by increasing local recruitment,further promoting tissue repair.However,methods to increase intercellular interactions that promote local engraftment of MSCs remain unclear.

Figure 3 Integrin subunit beta 3 enhances mesenchymal stem cell migration in vitro and in vivo. A: In vitro migration of integrin subunit beta 3(ITGB3)-overexpressing mesenchymal stem cell (MSC) (MSCsITGB3) toward lower Raw264.7,MVSMC and PMVEC stimulated by tumor necrosis factor α.Scale bars=100 μm;B: Quantification of migrated cells.Data are presented as the mean ± SEM;C: Experimental scheme of chemotactic assays with mouse atherosclerotic vascular samples.The blood vessel was divided into three parts.For each of the experiments,each part was cultured for 24 h in medium.To perform the assay,a suspension of 2 × 105 MSCs,2 × 105 MSCsGFP,or 2 × 105 MSCsITGB3 in medium were seeded in the upper chamber.Following incubation for 24 h,non-migrated cells were removed;D: Representative image and quantification of migrated cells stuck in the porous membrane or the lower layer of the transwell stained with 0.1%crystal violet;E: MSCsITGB3 and MSCsGFP,both expressing green fluorescence,were intravenously injected into mice.The expression of green fluorescent protein(GFP) and CD31 was examined by frozen staining.GFP-positive cells were quantified per microscopic field of vascular staining in triplicate mice;F: The expression of GFP and SMA were examined by frozen staining.GFP-positive cells were quantified per microscopic field of vascular staining in triplicate mice.Scale bars=100 μm;G: Expression levels of GFP in atherosclerosis analyzed using quantitative real-time polymerase chain reaction of mRNA samples extracted from MSCGFP and MSCITGB3 vessels of three mice.Data are presented as the mean ± SEM for each group.The data are representative of three independent experiments.aP < 0.05,bP< 0.001.GFP: Green fluorescent protein;ITGB3: Integrin subunit beta 3;MSC: Mesenchymal stem cell;qRT-PCR: Quantitative real-time polymerase chain reaction;SEM: Standard error of the mea;TNF: Tumor necrosis factor.
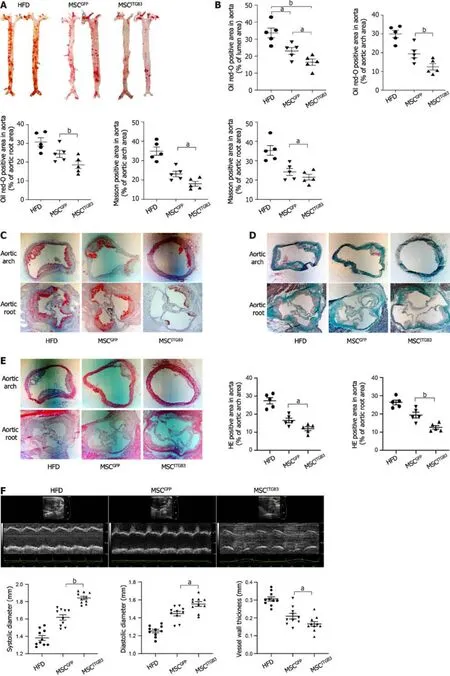
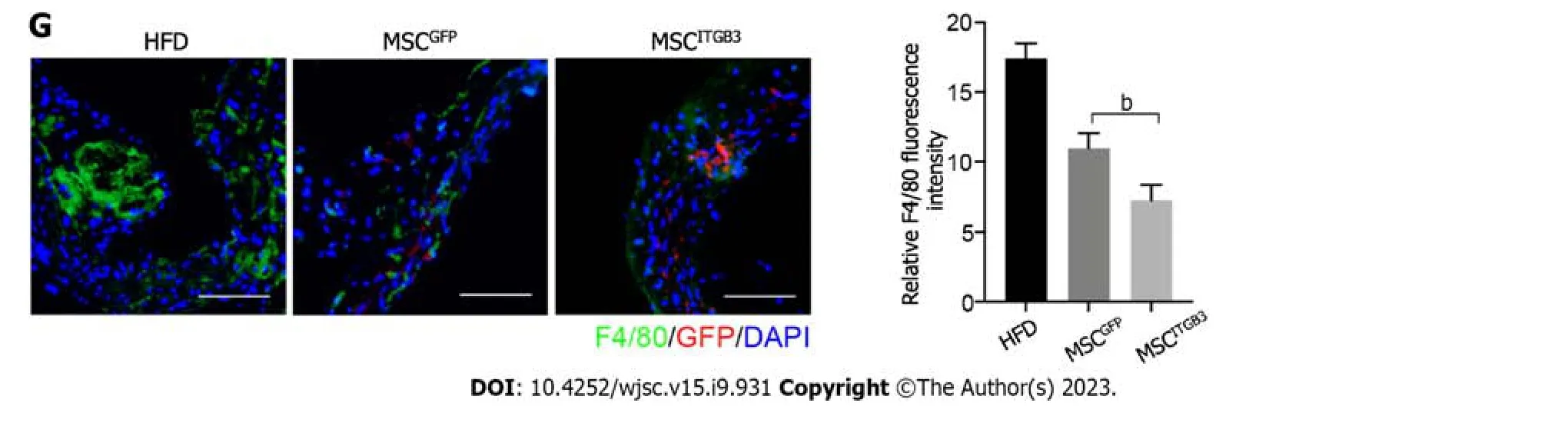
Figure 4 Injection of mesenchymal stem cellITGB3 attenuates atherosclerotic in vivo. A: Representative en face images of mouse aortas stained with Oil Red O (ORO);B: Quantification of the atherosclerotic plaque area as a ratio of the stained area to the total area of the aorta;C: Representative section of mouse aortic arch and aortic root stained with ORO.The ORO-positive area in the aortic root is shown as the ratio of the stained area to the total area of the aortic sinus;D:Representative section of mouse aortic arch and aortic root stained with Masson staining.The Masson staining-positive area in the aortic arch and aortic root is shown as the ratio of the stained area to the total area of the aortic sinus;E: Representative section of mouse aortic arch and aortic root stained with hematoxylin eosin (HE) staining.The HE staining-positive area in the aortic arch and aortic root is shown as the ratio of the stained area to the total area of the aortic sinus;F:Ultrasonography of the aorta in each group,including representative images,systolic diameter,diastolic diameter,and vessel wall thickness;G: Representative images of aortic arch section stained with anti-F4/80 antibody.The F4/80-positive area in the atherosclerotic plaque is shown as the ratio of the stained area to the total area of the atherosclerotic plaque.Scale bar=100 μm.aP < 0.05,bP < 0.001.AS: Atherosclerotic;HE: Hematoxylin eosin;MSC: Mesenchymal stem cell;ORO:Oil Red O.
Integrins,as transmembrane receptors,mediate cell connections by integrating Ig superfamily counterreceptors(ICAM-1 and VCAM-1) on adjacent cells[20].Adhesion molecules and integrin receptors have been identified to facilitate cell migration toward target organs and adhesion in target tissues.Therefore,intravenous injection of MSCsITGB3may provide a new approach to improve stem cell homing.Previous studies have also verified that MSCs with altered chemokine receptors,such as CXCR5[18],integrin α4[35],and CCR5 and CXCR6[36],significantly increased cell motion to lesion sites and enhanced the therapeutic effect of MSCs.MSC homing to a target organ requires the proper integration of interactions of chemokines secreted by injured tissue and adhesion molecules with matching receptors on MSCs.Therefore,determining disease-specific ligand expression profiles will provide the precise signal necessary for MSC homing.In our study,we elucidated that the ITGB3/RGD motif axis is a specific regulator for MSC homing in atherosclerosis.Adhesion molecules containing the RGD motif,such as VCAM-1,ICAM-1,and OPN,are highly expressed in atherosclerotic plaque and are involved in a variety of immune functions,including T cell activation,migration,and extravasation[37].High VCAM-1 and ICAM-1 expression levels have also been reported in the inflamed tissue,including vascular plaque[38].We found that the RGD structure acts as an important feature of the plaque tissue that chemoattracts the migration of MSCsITGB3to lesionsin vivo.Moreover,our findings demonstrated that the ITGB3-RGD motif axis is the atherosclerosis-specific regulator for MSC homing.Factors containing RGD structures can be observed in various inflammatory tissues,including the site of atherosclerotic lesions[39].However,the expression of ITGB3 was almost absent on the surface of MSCs.Thus,MSCsITGB3may serve as a disease-specific therapeutic tool to improve MSC homing when treating atherosclerosis.MSC transplantation and homing appear to improve atherosclerosisviadifferent mechanisms.There is a substantial amount of evidence indicating that transplanted MSCs primarily promote local microenvironmental improvement through their paracrine secretory effects[40-42].The anti-inflammatory cytokines secreted by MSCs can reduce inflammation and modulate immune response while enhancing normal cell survival and differentiation[9,11].It has also been documented that the anti-inflammatory properties of MSCs are primarily mediated by extracellular vesicles(EVs)[8,43].Various disease models,such as those involving endothelial cell senescence[44],autoimmune diseases[45],arterial stiffness and hypertension[46],and lung injury[47],have demonstrated that EVs derived from MSCs can mitigate cell death,prevent apoptosis,and enhance recovery.In this regard,as a result of the continuous release of EVs in the vicinity of the damage site,transplantation of MSCs overexpressing ITGB3 with improved migratory capability could play a more effective role in treating atherosclerotic disease.
A previous study found that ITGB3 stimulated the progression of human pancreatic ductal adenocarcinomaviaactivation of the STAT3 pathway[48].Furthermore,ITGB3 promoted cell senescence and profibrotic changes through p53 signaling activation and secretion of transforming growth factor beta in cultured tubular cells[49].However,these studies have not examined the specific effect of ITGB3 overexpression on MSC migration and homingin vivo.Further research is necessary to elucidate the underlying mechanisms of how overexpression of ITGB3 intensifies homing abilities of MSCs.
This study has certain limitations.First,the MSCsITGB3were generated by lentiviral infection.As lentiviral vectors can randomly integrate into the genome resulting in deleterious mutations,their therapeutic application in humans is limited by an inherent risk of tumorigenesis.Second,other suitable methods are needed to increase the expression of ITGB3.Third,the current experiment did not specifically investigate the survival time of MSCsITGB3in vivo.The next step will be to trace the target location and lifespan of MSCsITGB3throughin vivoimaging technology.
CONCLUSION
Collectively,we genetically modified MSCsin vitroand found that ITGB3 overexpression substantially upregulated MSC migration,aggregation in plaque sites,and immunomodulatory properties of MSCsin vivo.Although the therapeutic potential of modified MSCs in atherosclerosis has not yet been translated into clinical practice,our work improves our understanding of the adhesion molecule-integrin receptor axis,as well as of stem cell features,and may provide novel insights into the rapid and targeted delivery of MSCs to disease sites,thereby optimizing their therapeutic effects.

Figure 5 Mesenchymal stem cellITGB3 modulated cytokine expressions in an atherosclerotic mouse model. A: Serum pro-inflammatory factors tumor necrosis factor (TNF)α,interleukin (IL)-1β,and IL-6 and anti-inflammatory factors IL-4,IL-10,and tumor necrosis factor-stimulated gene-6 (TSG-6) levels measured using ELISA;B: mRNA expression of pro-inflammatory factors TNFα,IL-1β,and IL-6 and anti-inflammatory factors IL-4,IL-10,and TSG-6 in aortic tissue,quantified using quantitative real-time polymerase chain reaction.Expression is shown relative to the expression of housekeeping genes (β-actin).All values are expressed as mean ± SEM.aP < 0.05,bP < 0.001.ELISA: Enzyme-linked immunosorbent assay;IL: Interleukin;MSC: Mesenchymal stem cell;qRT-PCR:Quantitative real-time polymerase chain reaction;SEM: Standard error of the mean;TNF-α: Tumor necrosis factor-a;TSG-6: Tumor necrosis factor-stimulated gene-6.
ARTICLE HIGHLIGHTS
Research background
Umbilical cord mesenchymal stem cell (MSC) transplantation is a potential therapeutic intervention for atherosclerotic vascular disease.Integrin beta 3 (ITGB3) promotes cell migration in several cell types.However,whether ITGB3-modified MSCs can migrate to plaque sitesin vivoand play an anti-atherosclerotic role remains unclear.
Research motivation
Atherosclerosis is a serious public health problem and more treatment options are needed to explore and identify effective molecules and targets.
Research objectives
The objective of our study was to evaluate the chemotaxis ability of ITGB3-overexpressing MSCs toward inflammatory cellsin vitroand plaque tissuesin vivo,promoting their therapeutic efficacy in the atherosclerosis mouse model.
Research methods
Umbilical cord MSCs were isolated and expanded.Lentiviral vectors encoding ITGB3 or green fluorescent protein (GFP)as control were transfected into MSCs.Male apolipoprotein E-/-mice were fed with a high-fat diet (HFD) for 12 wk to induce the formation of atherosclerotic lesions.The HFD-fed mice were randomly separated into three clusters.GFPlabeled MSCs (MSCsGFP) or MSCsITGB3were transplanted into the mice intravenouslyviathe tail vein.Immunofluorescence staining,Oil red O staining,histological analyses,western blotting,enzyme-linked immunosorbent assay,and quantitative real-time polymerase chain reaction were used for the analyses.Statistical evaluation between two groups was determined using the unpaired Student’s t-test,and comparisons of more than two groups were performed using a one-way analysis of variance.
Research results
MSCsITGB3successfully differentiated into the “osteocyte” and “adipocyte” phenotypes and were characterized by positive expression (> 91.3%) of CD29,CD73,and CD105 and negative expression (< 1.35%) of CD34 and human leukocyte antigen-DR.MSCsITGB3showed significantly faster migration than MSCsGFP.ITGB3 overexpression had no effects on MSC viability,differentiation,and secretion.Immunofluorescence staining revealed that ITGB3 overexpression substantially enhanced the homing of MSCs to plaque sites.Oil red O staining and histological analyses further confirmed the therapeutic effects of MSCsITGB3,significantly reducing the plaque area.Enzyme-linked immunosorbent assay and quantitative real-time polymerase chain reaction revealed that MSCITGB3transplantation considerably decreased the inflammatory response in pathological tissues by improving the dynamic equilibrium of pro-and anti-inflammatory cytokines.
Research conclusions
The study demonstrated that ITGB3 overexpression enhanced the MSC homing ability,providing a potential approach for MSC delivery to plaque sites,thereby optimizing their therapeutic effects.
Research perspectives
The ITGB3-modified MSCs can migrate the plaque sites and play an anti-inflammation role,which may be an effective strategy to treat vascular atherosclerotic related diseases.
FOOTNOTES
Author contributions:Cui W and Han M designed and coordinated the study;Hu HJ,Xiao XR,Li T,Liu DM and Geng X performed the experiments and acquired and analyzed data;Cui W interpreted the data;Hu HJ and Liu DM wrote the manuscript;all authors revised the manuscript and approved the final version of the article.
Supported byNational Natural Science Foundation of China,No.82 100301;and Key Science and Technology Research Program of Hebei Provincial Department of Health,No,20221014.
Institutional review board statement:The study was reviewed and approved by the Ethical Committee of the Second Hospital of Hebei Medical University,No.2021-R496.
Institutional animal care and use committee statement:Laboratory Animal Use and Management Committee has been carefully discussed and voted on May 27,2021 for the subject animal-related content,10 voters,10 people suggested a formal experiment,and made the following recommendations.The design is reasonable,in line with the animal requirements.The ethics committee agreed to carry out a formal experiment.
Informed consent statement:All study participants or their legal guardian provided informed written consent about personal and medical data collection prior to study enrolment.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Data sharing statement:No additional data are available.
ARRIVE guidelines statement:The authors have read the ARRIVE guidelines,and the manuscript was prepared and revised according to the ARRIVE guidelines.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORCID number:Hai-Juan Hu 0000-0002-7954-4638;Xue-Ru Xiao 0000-0002-0858-3939;Tong Li 0009-0004-9052-4437;De-Min Liu 0000-0002-8784-1026;Xue Geng 0000-0001-9601-2476;Mei Han 0000-0002-5338-4875;Wei Cui 0000-0002-1214-4146.
S-Editor:Fan JR
L-Editor:A
P-Editor:Zhang XD
杂志排行
World Journal of Stem Cells的其它文章
- Interferon-γ priming enhances the therapeutic effects of menstrual blood-derived stromal cells in a mouse liver ischemia-reperfusion model
- Mechanism of adipose-derived mesenchymal stem cell exosomes in the treatment of heart failure
- Multiomics reveal human umbilical cord mesenchymal stem cells improving acute lung injury via the lung-gut axis
- Enhanced wound healing and hemostasis with exosome-loaded gelatin sponges from human umbilical cord mesenchymal stem cells
