Progress in the research of cuproptosis and possible targets for cancer therapy
2023-10-18JiangWangLanZhuLuoDaoMiaoLiangChaoGuoZhiHongHuangGuoYingSunJieWen
Jiang Wang,Lan-Zhu Luo,Dao-Miao Liang,Chao Guo,Zhi-Hong Huang,Guo-Ying Sun,Jie Wen
Abstract Developing novel cancer therapies that exploit programmed cell death pathways holds promise for advancing cancer treatment.According to a recently published study in Science,copper death (cuproptosis) occurs when intracellular copper is overloaded,triggering aggregation of lipidated mitochondrial proteins and Fe-S cluster proteins.This intriguing phenomenon is triggered by the instability of copper ions.Understanding the molecular mechanisms behind cuproptosis and its associated genes,as identified by Tsvetkov,including ferredoxin 1,lipoic acid synthase,lipoyltransferase 1,dihydrolipid amide dehydrogenase,dihydrolipoamide transacetylase,pyruvate dehydrogenase α1,pyruvate dehydrogenase β,metallothionein,glutaminase,and cyclin-dependent kinase inhibitor 2A,may open new avenues for cancer therapy.Here,we provide a new understanding of the role of copper death and related genes in cancer.
Key Words: Cuproptosis;Cuproptosis-related genes;Cancer;Targeted therapy
INTRODUCTION
Tsvetkovetal[1] have proposed an intriguing new form of programmed cell death related to the mitochondrial tricarboxylic acid (TCA) cycle,resulting in proteotoxic stress and copper-induced death,referred to as cuproptosis.These forms of oxidative-stress-induced cell death are characterized by mitochondrial stress,including the accumulation of fatty acylated mitochondrial enzymes and the loss of Fe-S cluster proteins[1].The dysregulation of copper homeostasis promotes cancer growth and causes irreversible cellular damage.A variety of mechanisms have been suggested for the ability of copper to induce cell death,such as oxidative stress,proteasome inhibition,and antiangiogenesis[2].
The exact molecular mechanism underlying cuproptosis remains unclear,but recent studies have shed light on potential contributors.For instance,knockout of the ferredoxin (FDX) 1 gene attenuates copper ionophore-induced cell death.Additionally,genes associated with the loss of lipidated mitochondrial enzymes and Fe-S cluster proteins loss,such as lipoic acid synthase (LIAS),lipoyltransferase (LIPT) 1,and dihydrolipoamide transacetylase (DLAT),may contribute to cuproptosis[1,3].
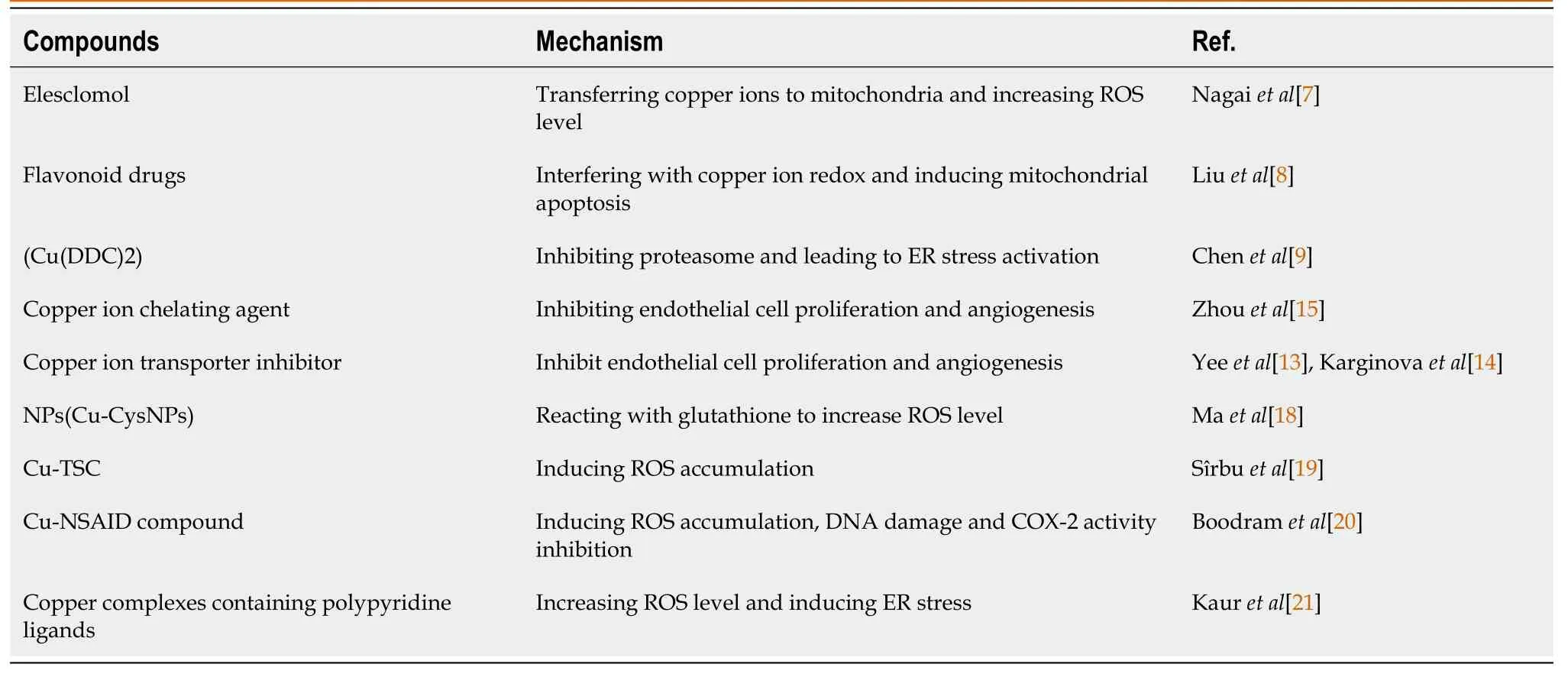
Table 1 Copper-related compounds and their antitumor mechanism
Although the precise correlation between cuproptosis and cancer is yet to be fully understood,imbalances in copper homeostasis have been implicated in cancer growth and cause irreversible cellular damage.Copper metabolisminvivoand cancer therapy has been extensively studied[4,5].Certain genes involved in the cuproptosis pathway,such as FDX1,may also play a role in cancer development,serving as a key regulator of proptosis and associated with poor prognoses in specific cancer types[6].Here,we review the progress of copper ions in cancer therapy,the function of cuproptosisrelated genes in cancer,and the possible target in cuproptosis.

Table 2 Functions of cuproptosis-related genes in cancer
COPPER IONS AND CANCER THERAPY
Recent studies have revealed three distinct mechanisms through which copper ions may induce cancer cell death.(1)Oxidative stress induction: Anticancer drug elesclomol has been found to exert its therapeutic effects through the transfer of copper ions to mitochondria,leading to oxidative stress[7].Liuetal[8] demonstrated that flavonoids can induce mitochondrial apoptosis through modification of the redox cycle of copper ions;(2) inhibition of proteasomes: Chenetal[9] synthesized copper diethyldithiocarbamate [Cu(DDC)(2)] nanoparticles (NPs) that improved the resistance of prostate cancer to treatment.Copper-ion-mediated endoplasmic reticulum (ER) stress is induced by proteasome inhibition and accumulation of ubiquitinated proteins.Proteasome inhibitors like bortezomib and carfilzomib have been explored for their potential as cancer treatment options in the form of various complexes,such as clioquinol and dithiocarbamates[10];and (3) reduce angiogenesis: Copper ions play a significant role in endothelial cell migration,proliferation,and fibronectin synthesis,crucial steps in angiogenesis[11,12].However,copper depletion can act as an antiangiogenic switch,blocking the growth of endothelial cells and preventing their proliferation.By inhibiting copper transporters or chaperones like human antioxidant protein 1 and consolidation tumor ratio-1,in addition to direct capture of intracellular copper,copper imbalance can be induced,leading to antiangiogenic effects[13,14].Combining this approach with vascular targeting techniques,such as immunotherapy,can enhance the cancer-killing effects[15].The tumor microenvironment (TME) is a complex ecosystem where various immune cells interact and influence tumor growth and progression[16,17].In the early stage of tumor growth,neutrophils promote inflammation and tumor cell apoptosis by releasing cytokines.However,in the middle and late stages of tumor formation,neutrophils contribute to angiogenesis,accelerating tumor progression and local infiltration.Different T cell populations are involved in TME,among which CD8+T cells can target and destroy tumor cells,secrete interferon,and inhibit angiogenesis.CD4+T cells coordinate immune responses,with Th1 cells promoting cancer and T regulatory cells promoting tumor formation and survival,by secreting auxin and cytokines,which then interacts with fibroblasts and epithelial cells.Although less prevalent than T cells,tumor-infiltrating B cells have antitumor effects,including antigen presentation to T cells,production of antitumor antibodies,and secretion of cytokines that promote cytotoxic immune responses.Regulatory B cells,in contrast,promote tumors by producing cytokines that promote the immunosuppressive phenotype in macrophages,neutrophils,and cytotoxic T cells.Tumor-associated macrophages (TAMs) are the predominant immune cells in the TME.They are involved in coordinating cancer-related inflammation and can release macrophage colony-stimulating factor to recruit TAMs,which have been implicated in cancer development.Moreover,TAMs can release epidermal growth factor,modify cancer cells,and accelerate cell migration and metastasis.Medullary suppressive cells promote tumor invasion by weakening innate and adaptive antitumor responses.
In light of the mechanisms described above for copper ions in cancer treatment,copper complexes have been extensively studied for their potential in anticancer therapy (Figure 1).For instance,copper-amino acid sulfhydryl NPs can reduce Cu2+to Cu+when reacting with localized glutathione.The generated Cu+then reacts with hydrogen peroxide,resulting in an increase in reactive oxygen species (ROS) levels.Excessive ROS can induce apoptosis of cancer cells[18].A copper-containing complex known as Cu-tuberous sclerosis complex (TSC) is another widely used complex to enhance cytotoxicity of TSC and ROS production[19].Chronic inflammation in the body can induce carcinogenesis and facilitate cancer spread.Copper complexes containing nonsteroidal anti-inflammatory drugs (NSAIDs) are used to treat inflammation and prevent cancer development (Table 1).In breast cancer stem-cell-like cells,Boodrametal[20] demonstrated that Cu-NSAID complexes could induce ROS accumulation,DNA damage,and cyclooxygenase-2 inhibition.Copper complexes with subcellular targeting properties can deliver more precise attacks on cancer cells.Kauretal[21] reported that copper complexes containing polypyridine ligands could enter the ER in situ,leading to increased ROS levels and ER-stress-induced immunogenic cell death in cancer cells[22].Although copper-complex-related therapies hold promise as a new anticancer strategy,their biocompatibility and application safety are critical challenges.Researchers have shown that copper complexes are cancer-killing,but long-term stability and biosafety tests remain to be conducted before these therapies can be translated into clinical applications.
THE ROLE OF CUPROPTOSIS-RELATED GENES IN CANCER
Cuproptosis remains an area of active exploration in its relationship with cancer.However,significant research has been conducted to understand the mechanisms through which cuproptosis-related gene molecules contribute to cancer development (Table 2).Figure 2 illustrates how these genes induce cuproptosis.
FDX1
FDX1 is a FDX protein primarily found in mitochondria,with diverse physiological functions,including the conversion of cytochromes during steroid hormone synthesis and vitamin D metabolism[23].Shietal[24] demonstrated that FDX1 is critical for Fe-S cluster biogenesis.Recent research has identified FDX1 as a key gene in the regulation of cuproptosis[25].Zhangetal[26] study found that FDX1 expression did not significantly differ across clinical stages in most cancers.Although the reduction in FDX1 expression may not directly impact the growth,apoptosis,or cell cycle distribution of LUAD cells,it could affect their metabolism,as FDX1 knockout has been shown to promote glycolysis and fatty acid oxidation.Further investigations into the mechanisms of FDX1 in cancer pathogenesis revealed significant positive correlations between FDX1 expression and immune cells in most cancers.FDX1 has been associated with major histocompatibility complex,immune activation,immune suppression,chemokines,and chemotaxis[27].Additionally,the products of factor receptors were positively coexpressed with FDX1,except for 1-aminocyclopropane-1-carboxylic acid and tetrahydrocannabinolic acid.This indicates that FDX1 expression is closely related to the immune response of cancer cells,which has implications for prognosis and represents a potential target for immunosuppressants[28,29].Given the crucial role of copper ions in cuproptosis,the significance of FDX1 as a key gene in this process makes it an intriguing target for cancer therapy.Studies exploring its role may offer valuable insights as it directly influences the protein fatty acylation cycle,leading to the aggregation of these proteins and interference with respiratory chain iron-sulfur cluster proteins.
LIAS
LIAS encodes a protein belonging to the biotin and LIAS families.Located in the mitochondria,this Fe-S enzyme contributes to lipoic acid biosynthesis,serving as the final step in the process.Diseases like diabetes,atherosclerosis,and neonatal epilepsy are associated with a lack of LIAS expression.Current studies on the association between the LIAS gene and cancer have predominantly focused on lung cancer[29].
Using in situ hybridization and real-time quantitative PCR,Mabetaetal[30] investigated the differential expression of the LIAS gene in normal lung tissue and lung cancer samples.Their findings suggest that alteration in LIAS expression levels can promote lung cancer development,making LIAS an attractive target for novel therapies[29].However further studies are warranted to confirm its therapeutic effectiveness.
LIPT1
As a member of the fatty acyltransferase family,LIPT1 encodes an enzyme that catalyzes the transfer of fatty acyl groups from fatty acyl-AMPs to specific lysine residues in fatty-acid-dependent enzymes.LIPT1-related disorders include fatty acyltransferase 1 deficiency and leukodystrophy[31].While there have been relatively few studies on LIPT1 in cancer,Chenetal[32] conducted a systematic investigation of genes related to prognosis in bladder cancer using the pathologicalatlas of the Cancer Genome Atlas.Their findings revealed a correlation between LIPT1 expression and bladder cancer prognosis[32].However,further research is needed to elucidate the role of LIPT1 in other cancer types.
DIHYDROLIPOAMIDE DEHYDROGENASE (DLD)

Figure 1 Effects of excess copper and copper deficiency in cancer. Four copper-related pathways with cancer inhibition effects are described.Elesclomol mediates the entry of Cu2+ into the mitochondria and causes reactive oxygen species accumulation.Flavonoids interfere with copper ion oxidation and reduction,inducing mitochondrial apoptosis pathway activation.Copper diethyldithiocarbamate can inhibit proteasome and result in endoplasmic reticulum stress.Copper deficiency can suppress the proliferation and migration of endothelial cells and the formation of connexin,bridling tumor angiogenesis.TCA: Tricarboxylic acid;ROS: Reactive oxygen species;FDX1: Ferredoxin 1;MTF1: Metallothionein;CTR1: Consolidation tumor ratio-1.
DLD,encoded by the DLD gene,is an essential enzyme that significantly impacts cell metabolism,particularly pyruvate metabolism and the TCA cycle[33].There is evidence that DLD could be used as a cancer-targeted therapy.In head and neck squamous cell carcinoma,DLD has been shown to be closely related to cystine deprivation and glutaminolysis.The biological function of DLD enhances mitochondrial KDH,MMP,and glutaminase activity.Increasing mitochondrial iron levels can facilitate mitochondrial lipid peroxidation,or silencing DLD,which effectively reduces the proportion of cells undergoing death from cystine deprivation and reduces ROS levels in cystine-deprived cells.These processes have been closely related to cancer-programmed death[34].Patients with endometrial cancer have exhibited abnormal levels of IgA and non-DLD IgG autoantibodies in their sera,indicating a correlation with mitochondrial DLD protein[35].Comparing DLD protein expression levels between breast cancer and normal tissues revealed significant differences,highlighting the potential of DLD as a diagnostic and therapeutic target in breast cancer[36].Using DLDH-based exogenous ROS to target skin cancer cells,Avrahametal[37] developed a method for targeting cancer cells,which could be a potential approach for melanoma treatment in the future.
DLAT
DLAT is an essential component of the pyruvate dehydrogenase complex,along with DLD and pyruvate dehydrogenase.This enzyme complex plays a crucial role in the synthesis of pyruvate acetyl-CoA.As the sole enzyme capable of converting citric acid into acetyl-CoA,DLAT can control the citric acid cycle-oxidative phosphorylation pathway,thus affecting the energy supply of cancer cells[38].In gastric cancer cells,DLAT expression was significantly upregulated[39],making it a potential therapeutic target.DLAT promotes the growth of cancer cells by activating the pentose phosphate pathway[40].Alternol,a compound that binds to multiple Krebs cycle enzymes,inhibits mitochondrial respiration and ATP production.This discovery offers a novel therapeutic strategy for treating prostate cancer[41].

Figure 2 General molecular biological process of cuproptosis. Copper can be transported into cells through the action of consolidation tumor ratio-1 and elesclomol encapsulation.When Cu2+ encapsulated by elesclomol enter the mitochondria,it gains an electron from ferrodoxin 1 (FDX1) (FDX1 expression can be promoted by metallothionein) and converts into Cu+.Concurrently,proteins responsible for dehydrogenation and acyl transfer (dihydrolipoamide transacetylase,dihydrolipoamide S-succinyltransferase,dihydrolipoamide dehydrogenase,pyruvate dehydrogenase α1,and pyruvate dehydrogenase β) undergo electron loss and are liporated by lipoic acid synthase.Subsequently,Cu+ promotes the oligomerization of liporated proteins.This cascade of events leads to a series of phenomena,including reactive oxygen species accumulation,mitochondrial dysfunction,and tricarboxylic acid inhibition,ultimately culminating in cuproptosis.CTR1:Consolidation tumor ratio-1;(Cu (DDC)2): Copper diethyldithiocarbamate;FDX1: Ferrodoxin 1.
PYRUVATE DEHYDROGENASE α1 (PDHA1) AND PYRUVATE DEHYDROGENASE β (PDHB)
PDHA1 and PDHB encode subunits of the pyruvate dehydrogenase complex,an essential enzyme complex within the mitochondria responsible for catalyzing pyruvate oxidation to acetyl-CoA,connecting glycolysis and the TCA cycle.
PDHA1 inhibition can increase proliferation,glycolysis,and Warburg effect in certain cancer cells.Gastric cancer has been shown to downregulate PDHA1,and elevated expression of PDHA1 correlates with poor prognosis[42].Downregulation of PDHA1 promotes the growth of gastric cancer.Exosomal miR-21-5p suppresses PDHA1 expression,thereby promoting glycolysis and cell proliferation in gastric cancer cells.PDHA1 expression in gastric cancer samples is negatively correlated with miR-21-5p levels[42].Additionally,miR-21-5p/PDHA may influence ovarian cancer drug resistance through exosomal miR-21-5p-mediated regulation of PDHA1 expression[43].The knockout strains had increased glycolysis,glucose intake,and glutamine consumption,while oxidative phosphorylation was inhibited,indicating enhanced Warburg effect and PDHA1.The proliferative capacity,angiogenic capacity,and drug resistance of the knockout esophageal cancer cells were significantly improved[44].PDHA1 is closely associated with prostate cancer growth,where it is involved in mitochondrial lipid synthesis.Therefore,PDHA1 may be useful as a therapeutic target for prostate cancer[45].
Frank s voice dropped a bit. When the weather was bad he would drive me to school. He had this old truck that he used in his fishing business. That truck was older than he was. It would wheeze2() and rattle3 down the road. You could hear it coming for blocks. As he would drive toward the school,I would shrink down into the seat hoping to disappear. Half the time, he would slam to a stop and the old truck would belch4 a cloud of smoke. He would pull right up in front, and it seemed like everybody would be standing5 around and watching. Then he would lean over and give me a big kiss on the cheek and tell me to be a good boy. It was so embarrassing for me. Here, I was twelve years old, and my Dad would lean over and kiss me goodbye!
PDHB also acts as a cancer suppressor gene.PDHB overexpression inhibits colon cancer cell proliferation,invasiveness,and glycolysis as it targets miR-146b-5p at the 3'-UTR end of the gene,promoting cancer cell growth[46].Gastric cancer cells overexpressing PDHB exhibit reduced proliferation and migration[47].PDHB inhibitors have also been shown to suppress cancer growth in various studies.For instance,reduced PDHB expression in non-small cell lung cancer indicates poor prognosis for patients[48],while PDHB may serve as a biomarker for breast cancer[49].Thus,the progress made in the research on PDHA1 and PDHB in cancer highlights the broad potential applications of therapeutic drugs targeting these molecular targets.
METALLOTHIONEIN (MTF1)
MTF1 plays a crucial role in the treatment resistance of malignant cancers[50].Cells stimulated with heavy metals,such as copper,trigger the production of products encoded by MTF1,leading to the induction of metal sulfur production.During tumor biogenesis and progression,coexpression of proteins and other genes involved in metal homeostasis is implicated.Notably,MTF1 is highly expressed in ovarian cancer tissues,and its high expression is associated with poor patient survival and disease recurrence[51].MTF1 knockout can inhibit the epithelial-mesenchymal transition process of ovarian cancer cells,thereby suppressing their proliferation,migration,and invasion,indicating that MTF1 may serve as a novel biomarker and therapeutic target for ovarian cancer[50].Given the multiple aspects of MTF-1 activities,monitoring changes in its expression and activity during cellular stress and cancer may prove valuable for cancer screening and prognosis studies.
GLUTAMINASE (GLS)
GLS encodes mitochondrial glutaminase K,which is dysregulated in many cancers.GLS can modulate promoter methylation modification and influence the clinical prognosis.In both in vitro and in vivo studies,GLS-targeted therapy has demonstrated its potential to inhibit cancer growth[52,53].Similarly,GLS has been detected in clinical samples from breast cancer,esophageal cancer,head and neck cancer,and leukemia.The expression of GLS is associated with poor prognosis in statistical analysis.Therefore,GLS can be considered a prognostic biomarker for certain types of cancer[54].However,its use as a prognostic biomarker remains controversial and further research is necessary to clarify its role and potential clinical applications[55].
CYCLIN-DEPENDENT KINASE INHIBITOR 2A (CDKN2A)
During cancer development,aberrant gene silencing is highly associated with cell cycle regulation.Dysregulation of CDKN2A,which encodes the p16INK4a protein,has been causally linked to the pathogenesis of various cancer types,contributing to cancer recurrence,poor prognosis,cancer genesis,and metastasis[56].CDKN2A mutations are responsible for 20%-40% of familial cancers and 2%-3% of sporadic melanomas[57].Nonsynonymous mutations of CDKN2A were found in approximately 16% (9/56) of cutaneous melanoma metastases[58].Activation of CDKN2A has been reported in 95% of pancreatic adenocarcinoma cases due to promoter hypermethylation[59].In lung cancer,CDKN2A inactivation has been observed in 75% of cases (30/40),including 16 homozygous deletions,10 methylations,and four mutations[60].CDKN2A gene mutations and abnormal methylation have also been reported in ovarian,gastric,and colorectal cancers,among others[56].Reactivating CDKN2A genetically and epigenetically could offer promising approaches for cancer prevention and treatment.
DISCUSSION
Copper ion concentration in the human body is tightly regulated by a homeostatic mechanism to maintain trace levels,as excess copper becomes toxic and leads to cell death.However,the mechanism underlying copper-induced cytotoxicity is still unclear [61,62].Recently,a novel form of cell death,cuproptosis,was discovered,which operates independently of known cell death mechanisms[1].Cuproptosis-related genes were identified using CRISPR-Cas9 loss-of-function screens,which revealed seven positively regulated and three negatively regulated genes.
So far,the identified copper-ionophore-induced death genes include DLD,fatty acylated protein targets PDH complex including DLAT,PDHA,and PDHB.While studies on these genes in cancer have been more extensive[3],other components of the lipoic acid pathway,such as fatty acyl synthase LIAS and FDX1,remain relatively understudied in cancer,and further experiments are needed to verify their roles in different cancer types[1,3].High cuproptosis activity status has been found to be a good prognostic indicator.
While some progress has been made in utilizing other types of programmed cell death for cancer treatment,there are still limitations in their application.Cuproptosis,being a novel form of programmed cell death,offers new perspectives on the correlation between its related genes and cancer prognosis.The combination of cuproptosis-targeted molecular drugs with existing therapies might open up new avenues for cancer treatment.
Currently,cuproptosis research is still in its infancy,and the existence of other signaling pathways for cell cuproptosis is not yet clear.Additionally,existing copper agents have poor targeting specificity and can cause serious side effects in patients undergoing treatment.These limitations and deficiencies impede the development and clinical implementation of cancer treatment strategies based on cuproptosis mechanisms.
In the future,researchers should focus on improving our understanding of the mechanism of cuproptosis in cancer cells and conducting thorough investigations into relevant mechanisms.Additionally,efforts should be directed towards developing copper-related formulations with high targeting and specificity (such as targeted nano-drug delivery systems)to maximize the targeting of cancer treatment while reducing toxic side effects.Lastly,it is necessary to develop and improve copper treatment plans in clinical practice in order to conduct relevant clinical trials and treatments for patients with cancer.
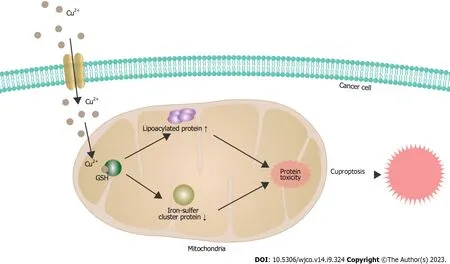
Figure 3 The mechanisms underlying cuproptosis in cancer cells. GSH: Glutathione.
CONCLUSION
Cuproptosis is triggered by the direct interaction of copper ions with the fatty acylated components in the citric acid cycle of mitochondrial respiration.This interaction results in the aggregation of fatty acylated proteins and subsequent down regulation of Fe-S cluster proteins,leading to protein toxic stress and,ultimately leading to cell death (Figure 3).The elucidation of this mechanism provides a clear understanding of how previous copper ion drugs exert their antitumor effects.This provides potential possibilities for the clinical application of these drugs in antitumor therapy and also broadens the path for the development of new drugs targeting copper in the future.
FOOTNOTES
Author contributions:Wang J and Luo LZ contributed equally to this study,and share joint first authorship;Wang J wrote the paper;Luo LZ and Liang DM did the literature review;Guo C and Huang ZH did the data analysis;Luo LZ conceived and coordinated the study;Sun GY and Wen J contributed equally to this study,and are joint corresponding authors;All authors reviewed the results and approved the final version of the manuscript.
Supported byScientific Research Project of Hunan Education Department,No.21A0054.
Conflict-of-interest statement:There is no conflict of interest associated with any of the senior author or other coauthors contributed their efforts in this manuscript.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORCID number:Jiang Wang 0000-0002-2036-1263;Jie Wen 0000-0002-5734-4678.
S-Editor:Qu XL
L-Editor:Kerr C
P-Editor:Zhang XD
