Coupled effects of pH and kaolinite colloids on antibiotic transport in porous media
2023-10-16ShuhuaJIXiaowenLIUXiuMENGShaohuiXUandQingLIN
Shuhua JI,Xiaowen LIU,Xiu MENG,Shaohui XU and Qing LIN
Department of Environmental Science and Engineering,Qingdao University,Qingdao266071(China)
ABSTRACT Antibiotics can interact with natural colloids and the surrounding media upon entry into soil and groundwater systems,which significantly alters their dynamic behavior and complicates our understanding of antibiotic fate and transport in porous media.In this study,co-transport of antibiotics and kaolinite colloids was systematically investigated using combined column experiments and numerical simulation under different pH conditions.Sulfadiazine(SDZ)transport was enhanced by kaolinite colloids under neutral and alkaline conditions,which was attributed to the higher mobility of colloids as SDZ carriers,as well as competitive sorption.However,most injected SDZ was transported in a dissolved form owing to the low sorption capacity of SDZ to kaolinite colloids and quartz sand.The colloid-facilitated transport model provided a good description of total SDZ transport,but underestimated colloidal SDZ transport using parameters from kinetic sorption experiments.Kaolinite colloids significantly promoted ciprofloxacin(CIP)transport at pH 4.0,but inhibited it at pH 7.0 and 9.0.Interestingly,enhanced CIP transport was due to the decreased number of effective sorption sites on quartz sand and the increased desorption of CIP from kaolinite colloids.Under neutral and alkaline conditions,deposited colloids provided additional sorption sites for CIP,which contributed to CIP retention.Moreover,CIP significantly inhibited the transport of kaolinite colloids owing to the increases in colloidal aggregate size and zeta potential.Overall,our results highlighted the different effects of mobile and immobile colloids on antibiotic transport,in addition to the implications of antibiotic speciation and clay colloids when predicting the transport behavior of these compounds.
Key Words: advection-dispersion equation,ciprofloxacin,colloid-facilitated transport model,numerical simulation,sulfadiazine,two-site model
INTRODUCTION
Antibiotics are commonly used to protect humans and animals against microbial infection.Due to their extensive use,low bioavailability,and high resistance to traditional treatment strategies for their disposal,large amounts of antibiotics inevitably end up in the environment(Michaelet al.,2013;Zhanget al.,2015;Guanet al.,2017;Xuet al.,2021).Indeed,the widespread occurrence of antibiotics in aquatic and terrestrial environments has been described in numerous studies encompassing different matrices,such as soil,sediments,and underground water(Battet al.,2006;Kimet al.,2011;Blairet al.,2013;Srinivasan and Sarmah,2014).Liet al.(2014)found that the highest mean concentration of ciprofloxacin(CIP)in soil was 104.4 μg kg-1.Antibiotic contamination is expected to alter soil microbial populations,thus interfering with their role in chemical cycles,such as nitrification(Verlicchi and Zambello,2015).Furthermore,antibiotics can be transported from soil to plants(Pan and Chu,2017),ultimately posing a great threat of severe health problems in humans further up the food chain.Therefore,the fate of antibiotics in soil and groundwater systems remains a major concern.A comprehensive understanding of the mechanisms controlling antibiotic transport in porous media is of major importance for the prevention of potential collateral interactions of antibiotics with humans and other living organisms.
Antibiotic transport in the subsurface soil depends on several factors such as antibiotic chemical properties,pH,ionic strength,soil charge density,and contact time(Chenet al.,2013;Zhanget al.,2014;Donget al.,2016;Park and Huwe,2016).Antibiotics can undergo pH-dependent speciation and form cations,anions,or neutral molecules,which show different sorption mechanisms and affinities,such as cation exchange,surface complexation,cation bridging,and electrostatic attraction,thus affecting their transport and fate.Antibiotics and other pollutants(such as heavy metals)can be transported in a dissolved form or attached to mobile colloidal particles(Yinet al.,2010;Qiet al.,2014).Clay colloids are ubiquitous in soil and groundwater and play a critical role in antibiotic transport because of their large specific surface area and numerous sorption sites(Sen and Khilar,2006;Chenet al.,2015;Molnaret al.,2015).Furthermore,as pollutant carriers,colloids can critically change the mobility of antibiotics and modulate their transport forms,thereby influencing associated environmental risks.Previous studies have shown that colloidal particles may affect antibiotic bioavailability and increase the risk of antibiotic resistance(Aguzziet al.,2007).
To clarify the impact of colloids on pollutant transport,a deeper understanding of colloidal particle transport and retention in different subsurface environments is required.Attachment to solid-water interfaces and mechanical straining(i.e.,the situation in which pore throats are too small for colloids to pass through)are two mechanisms that dominate colloid transport in saturated porous media.While surface attachment is controlled by the balance between DLVO and hydrodynamic forces,colloidal straining is mainly determined by the ratio of the mean particle size to the collector diameter (dp/dc) (Derjaguin and Landau,1993;Bradfordet al.,2006;Xuet al.,2006).Numerous studies have shown that straining can be significant fordp/dc>0.001(Bradfordet al.,2002;Xuet al.,2006;Duet al.,2013).pH is an important chemical factor that affects the potential and charge density of colloid and collector surfaces,consequently affecting colloid stability and sorption on porous media(Zhouet al.,2011;Lüet al.,2014).Moreover,changes in pH modulate the antibiotic form and can thereby crucially impact the co-transport properties of colloids and antibiotics.
Although colloid-associated transport of antibiotics has received considerable attention(Peiet al.,2010;Jalilet al.,2015;Septianet al.,2019),very limited efforts have been devoted to understanding the effects of antibiotics on colloid transport or how antibiotic transport forms(dissolved and/or colloidal)are affected by colloids.Fenget al.(2017)and Zhanget al.(2020)found that sulfamethazine(SMZ)and CIP inhibited clay colloid transport.Yanget al.(2020)reported that SMZ inhibited biochar colloid transport in sand columns under acidic and neutral conditions,but enhanced their mobility under alkaline condition,because SMZ sorption changed the surface properties of biochar colloids.Despite the aforementioned progress,the mechanisms of colloid-antibiotic co-transport and retention remain poorly understood and quantified,particularly with regard to the antibiotic transport state.
In this study,we explored the co-transport properties of antibiotics and kaolinite colloids under different pH conditions in saturated quartz sand media using laboratory column experiments.Sulfadiazine (SDZ) and CIP have different sorption capacities and mobilities and are widely used and observed in both soil and groundwater.Therefore,SDZ and CIP were selected as representative sulfonamide and fluoroquinolone antibiotics,respectively(Srinivasanet al.,2014;Zhanget al.,2017).Kaolinite is an important clay mineral that is ubiquitous in soil.To further reveal the influence of kaolinite colloids on antibiotic transport,both dissolved and colloidal forms of antibiotics were examined during the experiments in order to comprehensively characterize antibiotic transport behavior.Our specific objectives were to:i)determine kaolinite colloid effect on SDZ and CIP transport in saturated porous media,ii)characterize the effects of SDZ and CIP on kaolinite colloid deposition and transport,iii)investigate the impact of pH on the co-transport of antibiotics and kaolinite colloids,and iv)simulate antibiotic transport as affected by kaolinite colloids.
MATERIALS AND METHODS
Antibiotics and kaolinite colloids
Antibiotics,SDZ and CIP (purity ≥98%),were purchased from Bailingwei Technology Co.(China)and Tokyo Chemical Industry (Japan),respectively.Both antibiotics were amphoteric compounds(Figs.S1 and S2,see Supplementary Material for Figs.S1 and S2),and their stability was tested under the same conditions as those used in the column experiments.Briefly,10 mg L-1SDZ or CIP solution was added to conical flasks,and their concentration was measured at intervals.Negligible degradation was detected after 48 h,indicating that both SDZ and CIP were stable under the experimental conditions used.
Kaolinite is a 1:1 layered aluminosilicate consisting of a tetrahedral sheet ([Si2O5]2-) and an octahedral sheet([Al2O2(OH)4]2-) along thecdirection.Colloidal suspensions of kaolinite were obtained by ultrasonication of kaolinite grains in distilled water for 1 h(Wikiniyadhaneeet al.,2015).The detailed procedure is described in Text S1(See Supplementary Material for Text S1).The colloidal particle hydrodynamic diameter and zeta potential were measured using a zeta potential analyzer(ZetaPALS,Zetasizer Nano ZSE,Malvern Panalytical Ltd.,England) to understand colloid deposition behavior.Measurements showed that colloidal particles were monodispersed at different pH values.
Column experiments
To clearly understand the mechanisms controlling the co-transport behavior of antibiotics and kaolinite colloids,duplicate column experiments were conducted under saturated flow conditions using the column setup packed with quartz sand ranging from 425 to 600 μm described by Zhanget al.(2021).To remove surficial impurities,quartz sand was washed sequentially with 0.01 mol L-1NaOH,0.01 mol L-1HCl,and deionized water,and then oven-dried at 105°C.The zeta potential of the quartz sand was measured using a zeta potential analyzer.Briefly,a few quartz sand grains were crushed into a fine powder and mixed with an appropriate solution to form a sufficiently stable suspension for zeta potential measurements(Mitropoulouet al.,2013).Porosity was calculated gravimetrically to be 0.44.
The column was slowly injected with deionized water from the bottom until it approached saturation.Subsequently,the inflow direction was changed,and the background solution(deionized water with pH of 4.0,7.0 or 9.0)was input at a constant rate of 0.116 cm min-1until hydrochemical equilibration was reached after approximately 40 h.Then,3 pore volumes (PVs) (C0) of mixed solutions of the antibiotic (SDZ or CIP,10 mg L-1) and kaolinite colloids(200 mg L-1)were introduced at a constant rate of 0.116 cm min-1,followed by the background solution at the same rate,until neither antibiotics nor kaolinite colloids could be detected.Input solutions were adjusted to the appropriate pH using HCl and KOH.Experiments were also performed using antibiotics or kaolinite colloids alone to determine their individual transport behavior.Hydraulic characteristics of the column were evaluated by a tracer experiment using a conservative tracer(Br-).In other words,a NaBr solution(0.05 mol L-1)was introduced into the column and subsequently eluted with deionized water.Effluents were collected from the column outlet every 20 min using an automated fraction collector.The measured concentrations of antibiotics and kaolinite colloids in the effluent(C)were then used to develop breakthrough curves.Mass recovery in the effluent was qualified using the integral method reported by Parket al.(2008).Throughout the experiment,colloidal suspensions were stirred,and their concentrations were measured at predetermined time intervals to ensure that no colloid sedimentation occurred.
Batch experiments
Kinetic experiments on the sorption of SDZ onto kaolinite colloids were conducted to estimate the kinetics of SDZ sorption.Sulfadiazine(10 mg L-1)was added to 200 mg L-1colloidal solution in centrifuge tubes.The mixed solution was gently stirred continuously for 24 h,and the suspension was sampled at selected time intervals.Immediately thereafter,the suspension was filtered,and the SDZ concentration in the aqueous phase(Caq,mg L-1)was measured.Data were analyzed using the following equation(Zhanget al.,2017):
whereCaq0is the initial SDZ concentration in the aqueous phase (mg L-1) andkamcandkdmc(min-1) are the rate coefficients for SDZ sorption to and desorption from the suspended colloids,respectively.
Analytical methods
Antibiotic concentrations were measured using ultraviolet-visible (UV/Vis) spectrophotometer (UV9100,LabTech,China),at wavelengths of 254 and 278 nm for SDZ and CIP,respectively(Fig.S3,see Supplementary Material for Fig.S3)(Wanget al.,2010).Similarly,colloidal concentrations were determined using UV/Vis spectrophotometer at 400 nm(Fig.S4,see Supplementary Material for Fig.S4)at which the antibiotic absorbance was 0(Fig.S5 and Text S2,see Supplementary Material for Fig.S5 and Text S2).Each effluent sample containing colloids and antibiotics was separated into two subsamples to measure dissolved and total antibiotic concentrations.One aliquot was filtered using a 0.22-μm membrane filter to determine the dissolved antibiotic concentration,and another aliquot without filtration was used to determine the total antibiotic concentration using a dual-wavelength method(Fenget al.,2017).Thus,the kaolinite colloid absorbance at 254 nm (or 278 nm)was calculated from the colloid concentration measured at 400 nm using a standard curve,and the absorbance of SDZ at 254 nm(or CIP at 278 nm)was obtained by subtracting the absorbance of the kaolinite colloids from the total absorbance of each effluent at the corresponding wavelength.Total SDZ and CIP concentrations were determined using standard curves.This method was validated(Figs.S6 and S7,Text S3,see Supplementary Material for Figs.S6 and S7 and Text S3).Colloidal SDZ and CIP concentrations were determined by subtracting the dissolved mass from the total concentrations.Finally,Br-concentration in the effluent was measured using a Br--selective electrode.
Numerical modeling
Additionally,we performed simulations of the transport and co-transport properties of SDZ,CIP,and kaolinite colloids.Colloid transport was simulated by the advectiondispersion equation coupled with a two-site model.The two kinetic retention sites described the mass transfer of colloidal particles between the aqueous and solid phases(Bradfordet al.,2003).The first kinetic site(Site 1)assumed reversible retention using the first-order attachment(k1a)and detachment(k1d)coefficients,whereas the second kinetic site(Site 2) accounted for irreversible (straining) retention using a first-order retention coefficient(k2a).Correspondingly,Sc1andSc2are the deposited colloid concentrations at Sites 1 and 2,respectively.Details of the colloid transport model are shown in Text S4(see Supplementary Material for Text S4).
The transport behavior of the two antibiotics(SDZ and CIP) has also been previously described using a two-site model(Zou and Zheng,2013;Linet al.,2022).In this model,the first site(Site 1′)assumes equilibrium sorption described by a first-order linear sorption equation,whereas the second site (Site 2′) assumes time-dependent sorption following a first-order dynamic equation.Correspondingly,S1′andS2′are the antibiotic concentrations adsorbed on quartz sand at Sites 1′and 2′,respectively.At Site 1′,the fraction of equilibrium sorption sites (f) and partition coefficient(kd)were used to account for the instantaneous sorption of antibiotics on the quartz sand surfaces.At Site 2′,the firstorder mass transfer rate(ω)was employed for elucidating antibiotic time-dependent sorption.The antibiotic transport model was detailed in Text S5(see Supplementary Material for Text S5).
In the presence of colloids,the combined transport of dissolved and colloidal antibiotics is described by a colloidfacilitated transport model(Pang and Šimůnek,2006;Zhanget al.,2017).It is assumed that contaminants can be adsorbed onto the surfaces of both the deposited and mobile colloids.Concentrations of antibiotics adsorbed on the mobile and immobile colloids are represented bySmcandSic,respectively.The first-order coefficients,kamcandkdmc,describe the rates of antibiotic sorption to and desorption from mobile colloids,respectively,and the first-order coefficients,kaicandkdic,describe the rates of antibiotic sorption to and desorption from immobile colloids,respectively.We assumed that the sorption rate coefficients did not change with colloid concentration.The main equations involved in colloid-facilitated contaminant transport are listed in Text S6(See Supplementary Material for Text S6).
The model parameters were determined by calculation or model fitting.Simulations were performed using the HYDRUS_1D software and its C-Ride module (Šimůneket al.,2013,2015).Coefficient of determination(R2)was used to evaluate model performance.
RESULTS AND DISCUSSION
Surface charge and particle size ofkaolinite colloids under different pH conditions
As expected,the absolute values of zeta potential of kaolinite colloids increased with increasing pH due to the deprotonation of surface hydroxyl groups,which induced an increase in repulsive force,thereby enhancing colloid stability(Table SI,see Supplementary Material for Table SI).Ciprofloxacin significantly increased kaolinite colloid zeta potential,whereas SDZ reduced it,similar to the observed phenomenon of SMT sorption on colloids(Yanget al.,2020).Our observations can be explained by the fact that more CIP than SDZ is adsorbed onto the surface of negatively charged kaolinite colloids,thereby inducing charge heterogeneity on the colloid surface and leading to a less negative overall zeta potential of kaolinite colloids.Particle size of kaolinite colloids varied slightly from 469.2 to 440.9 nm as pH increased from 4.0 to 9.0,while SDZ had a minor effect on colloidal particle size due to the weak interaction between SDZ and kaolinite colloids.In contrast,CIP significantly increased colloidal particle size from 469.2 to 1 733.2 nm at pH 4.0 and from 458.9 to 1 949.7 nm at pH 7.0,indicating kaolinite colloid aggregation in the presence of CIP.
Transport ofSDZ and CIP without colloids under different pH conditions
Column packing uniformity was evaluated by measuring the behavior of water flow,as described by conservative Br-breakthrough curves(BTCs).Reasonable symmetry of BTCs was observed,with no tailing,which indicated that the columns were evenly filled without macropores or preferential flow(Fig.S7,see Supplementary Material for Fig.S7).Additionally,the hydraulic parameters dispersion coefficient(D)and volumetric water content(θ)were obtained by fitting the Br-curves(Table SII,see Supplementary Material for Table SII).Specifically,SDZ and CIP transport was examined at pH values of 4.0,7.0,and 9.0.The effluent pH showed little variation during the experiment,indicating no change in the amount of different antibiotic forms or in the characteristics of kaolinite colloids and quartz sand.The corresponding breakthrough curves for SDZ and CIP are shown in Fig.1,where the relative effluent concentration(C/C0)is plotted as a function of PV.
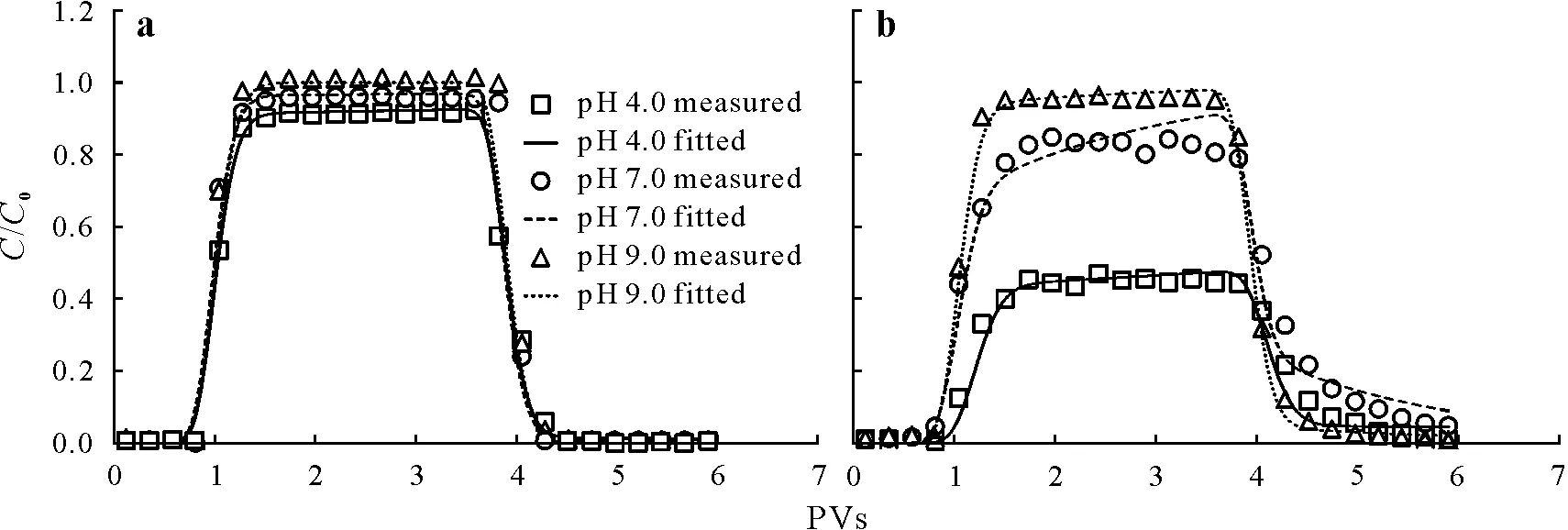
Fig.1 Breakthrough curves plotted using the relative effluent concentration(C/C0)against solution volume(pore volumes,PVs)for the transport of sulfadiazine(a)and ciprofloxacin(b)under different pH conditions.C and C0=effluent and influent antibiotic concentrations in the column experiments,respectively.
The pH sensitivity ofC/C0for SDZ and CIP differed considerably(Fig.1).The characteristics of SDZ transport under different pH conditions were roughly similar,with all breakthroughs occurring atca.1 PV and all curves reaching a steady-state plateau(Fig.1a).The relative effluent concentration increased significantly(P <0.05)with increasing pH,withC/C0greater than 0.9 over the tested pH range.Our results not only confirmed the high SDZ mobility in saturated porous media,but also suggested a marginal effect of pH on SDZ retention and transport under the tested conditions.As pH increased,the number of SDZ in the neutral form(SDZ0)decreased,whereas that in the ionic form(SDZ-)increased(Fig.S1),resulting in a reduction in the hydrophobic force and a boost in the repulsive force between SDZ and quartz sand,which promoted SDZ transport.Notably,the variation was not significant because of the small sorption capacity of quartz sand for SDZ.Previous studies have shown that a minor amount of zwitterionic species of SDZ(SDZ±)is in the tautomeric equilibrium with SDZ0and can be adsorbed onto negatively charged silica surfaces(i.e.,≡SiO-functional groups)viathe protonated nitrogen in the piperazinyl group(Gao and Pedersen,2005).
Meanwhile,C/C0of CIP increased from 0.46 to 0.96,indicating that pH played a key role in controlling CIP retention and transport in the saturated porous media(Fig.1b).This is because CIP exists mainly in the cationic form(CIP+,100%)at pH 4.0;hence,it can be adsorbed by the negatively charged surfaces of quartz sand through electrostatic attraction.As pH increased,the amount of CIP+decreased,while the amouts of the neutral(CIP0)and anionic(CIP-)forms increased,resulting in decreased electrostatic attraction and increased electrostatic repulsion.Conversely,CIP mainly exists as neutral molecules at pH 7.0,enabling sorption on quartz sand surfacesviahydrogen bonding and hydrophobic forces(Septianet al.,2019).Nevertheless,the resulting CIP sorption capacity was rather small.Importantly,the roughness and chemical heterogeneity of the quartz sand surface decreased at pH 9.0(Donget al.,2016),which contributed to the lowering of CIP sorption to the quartz sand grains.Thus,the impact of pH on the mobility of SDZ and CIP in porous media differed significantly,suggesting that the fate and transport of antibiotics were governed by the specific pH-dependent speciation of each antibiotic compound.
The sorption and transport behaviors of SDZ/CIP were simulated using a two-site sorption model.In this model,the hydraulic parameters(Dandθ)were obtained from the tracer experiment.As shown in Fig.1,the measurements showed a very close fit,withR2>0.970 and root mean square error (RMSE)<0.04 (Table I).Further,the increasingfvalue suggests enhanced equilibrium sorption and weakened kinetic sorption with increasing pH,which is fully consistent with the sorption mechanism under the different conditions studied.Specifically,thefvalue was 1 for SDZ at pH 9.0.Moreover,the first-order linear sorption isotherm provided an accurate description of SDZ and CIP transport in quartz sand,suggesting that antibiotics did not saturate the available sorption sites.Thekdvalue decreased with higher pH,which is consistent with the column experiment results,and further indicates that increasing pH enhances SDZ and CIP transport.Additionally,kdvalues for CIP were greater than those for SDZ,especially at pH 4.0,indicating that SDZ showed higher mobility than CIP.Finally,the lowωvalue suggests that a portion of SDZ and CIP was retained in the columnviacovalent bonding or physical occlusion,which was confirmed by the results of our tracer experiment.Of the applied Br-,95.6%—96.1%was recovered in the effluent,indicating marginal trapping in dead pores.Moreover,because theωvalue of CIP is an order of magnitude greater than that of SDZ,we can infer that more rate-limited sorption occurred on the quartz sand surfaces(further supported by the tailing of the breakthrough curves).
Transport ofkaolinite colloids with and without SDZ/CIP under different pH conditions
Although significant differences in zeta potential and particle size of kaolinite colloids were found under different pH conditions(Table SI),the BTCs for colloids alone were roughly similar(Fig.2a).The input colloids generally broke through simultaneously,andC/C0approached a plateausoon after 1.5 PVs,with recoveries of 93.64%,94.96%,and 95.11%at pH 4.0,7.0,and 9.0,respectively.The two-site model for colloid transport fitted well with BTCs obtained for kaolinite colloids(R2>0.98).Because kaolinite colloids had no pore-structure effects in our experiments,Dvalue of kaolinite colloids was assumed to be the same as Brin our simulations.Clearly,pH had almost no influence on kaolinite colloid transport,as confirmed by thek1aandk2avalues,with no significant difference over the pH range of 4.0—9.0 (Table II).This can be attributed to the repulsive force between the colloidal particles and the porous medium,given that both surfaces are negatively charged,which results in unfavorable conditions for colloid deposition.Meanwhile,the average particle size of kaolinite colloids was essentially the same (440.9—469.2 nm) withdp/dc<0.001,indicating no straining(Bradfordet al.,2002,2007;Shenet al.,2008).Therefore,we hypothesized that surface roughness might be the main factor causing colloidal retention in the column,which is in agreement with the results of Shenet al.(2011)and Bradford and Torkzaban(2013).Retention occurs primarily at the reversible sites with highk1aand lowk2avalues.Moreover,reversible retention facilitates the maintenance of the height of the BTCs at approximately 1.Because kaolinite colloids reached the column outlet without acceleration compared to the tracer Br-(Fig.S7),pore size exclusion was not observed in our experiments.
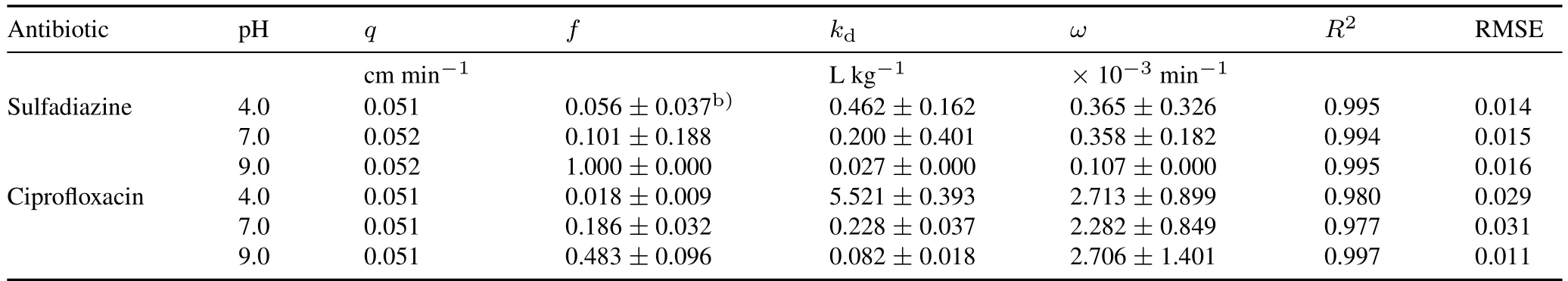
TABLE ICalculated and fitted two-site model parametersa) for antibiotic transport under different pH conditions

Fig.2 Breakthrough curves plotted using the relative effluent concentration(C/C0)against solution volume(pore volumes,PVs)for the transport of kaolinite colloids alone(a)and in the presence of sulfadiazine(b)and ciprofloxacin(c)under different pH conditions.In c,the simulated data are not given because the two-site model did not accurately fit the curve obtained.C and C0=effluent and influent kaolinite colloid concentrations in the column experiments,respectively.
Several studies have shown that antibiotics can affect the properties of colloid aggregation (Fenget al.,2017;Fanget al.,2018;Zhanget al.,2020),thus affecting colloid transport in porous media.As shown in Fig.2b,SDZ had different effects on kaolinite colloid transport under different pH conditions.At pH 4.0,it inhibited colloid transport,exhibiting a plateau of the colloidal breakthrough curve that decreased from 0.94(colloid alone)to 0.80(in the presence of SDZ),with mass recovery declining from 93.64% to 76.82%.These results are consistent with those of Yanget al.(2020),who showed that sulfamethazine could reduce colloid mobility in a quartz sand column under acidic conditions.However,SDZ barely affected the transport of kaolinite colloids at pH 7.0 and 9.0.The reason may be that SDZ was adsorbed to a greater extent onto quartz sand at pH 4.0(SDZ0and SDZ±),increasing surface roughness and chemical heterogeneity of the quartz sand(Bradford and Torkzaban,2013;Srinivasanet al.,2014;Huet al.,2019),which in turn enabled higher colloid deposition on the quartz sand surface.Furthermore,the reduction ink1aand the increase ink2asuggest a higher extent of irreversible deposition of kaolinite colloids in the presence of SDZ.Ciprofloxacin significantly reduced the mobility of kaolinite colloids,and almost all injected colloids were retained in quartz sand,except at pH 9.0(Fig.2c).There are two possible explanations for such inhibitory effect.One explanation was that CIP considerably increased colloidal particle size at pH 4.0 and 7.0(Table SI),with values ofdp/dcbeing 0.003 5 and 0.003 9,respectively,which were higher than the critical value(0.002)obtained by Bradfordet al.(2002).Therefore,most kaolinite colloids may have been trapped across the sand column through straining.The other explanation was that CIP sorption on colloid surfaces severely increased the colloid zeta potential(Table SI),resulting in lower electrostatic and steric repulsion between kaolinite colloids and the sand surface.However,the particle size barely changed in the presence of CIP at pH 9.0,and the zeta potential was more negative than that at pH 4.0 or 7.0,yet less negative than that of kaolinite colloids alone or with SDZ at pH 9.0.TheC/C0reachedca.60%and then decreased toca.4%within 3 PVs during the colloid injection phase,presumably because of colloid deposition onto colloids deposited earlier on the quartz sand surface(Liet al.,2008;Fanget al.,2018).The two-site model did not accurately fit the curve obtained(Fig.2c).

TABLE IIFitted parametersa) of the two-site model for the transport of kaolinite colloids alone and in the presence of sulfadiazine(SDZ)under different pH conditions
Previous studies have shown that colloidal particles can be successively retained by a solid matrix,resulting in alterations of the pore space geometry and,in turn,a reduction in permeability(Alemet al.,2013;Yousifet al.,2017;Zhaoet al.,2020).In our study,the experiment duration was relatively short,and the rolling of particles over the grain surface prevented clogging under unfavorable deposition conditions(Samari-Kermaniet al.,2021).Therefore,particle deposition or straining did not significantly alter the flow or transport properties of the porous media.
Transport ofSDZ influenced bykaolinite colloids under di-fferent pH conditions
Facilitated SDZ transport in quartz sand was observed in the presence of kaolinite colloids(Fig.3).Specifically,we simulated SDZ breakthrough curves using the C-Ride module of the HYDRUS-1D software.The parameters for the interactions between SDZ and quartz sand(f,kd,andω)were obtained by fitting the transport curves for SDZ alone.The parameters for the interactions between kaolinite colloids and quartz sand(k1a,k1d,andk2a)were obtained by fitting the curves of colloid co-transport with SDZ.The parameters for the interactions between SDZ and mobile (kamcandkdmc)or immobile(kaimandkdim)colloids were obtained by fitting the SDZ kinetic sorption curves(Table III,Fig.S8,see Supplementary Material for Fig.S8).We assumed that the SDZ sorption and desorption rates were identical for the mobile and immobile colloids.
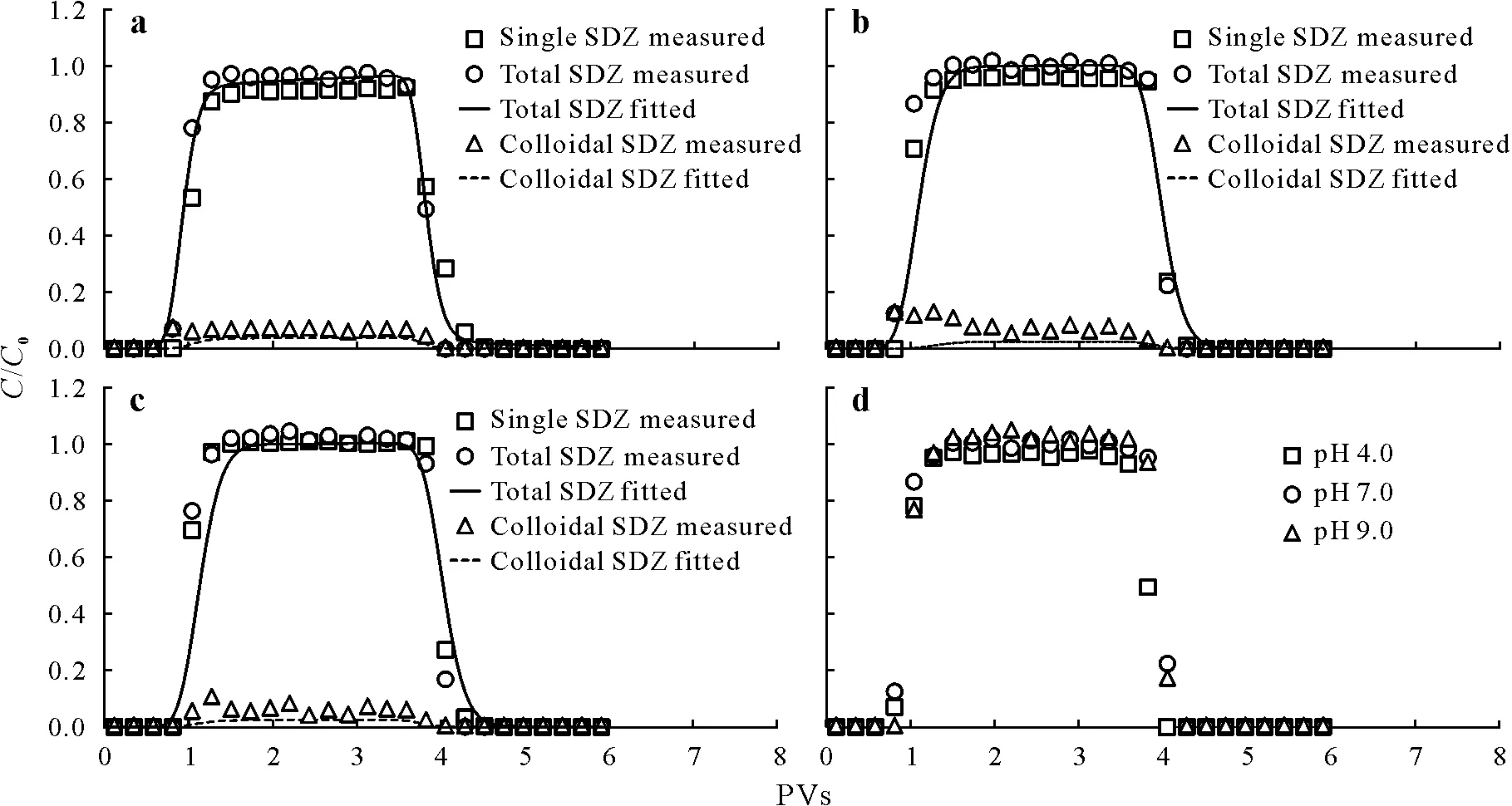
Fig.3 Breakthrough curves plotted using the relative effluent concentration(C/C0)against solution volume(pore volumes,PVs)for the transport of sulfadiazine(SDZ)alone and in the presence of kaolinite colloids at pH 4.0(a),7.0(b),and 9.0(c)and total SDZ transport under different pH conditions(d).C and C0=effluent and influent SDZ concentrations in the column experiments,respectively.
In the presence of kaolinite colloids,the breakthrough plateau for total SDZ increased significantly (P <0.05)from 0.91 and 0.96 to 0.97 and 1.0 at pH 4.0 and 7.0,respectively,which demonstrated that kaolinite colloids promoted SDZ transport(Fig.3).Enhanced transport occurs because SDZ0can be adsorbed onto kaolinite colloid surfaces through hydrogen bonding,hydrophobic,and van der Waals interactions(Kasteelet al.,2010;Srinivasanet al.,2014;Chenet al.,2017;Huet al.,2019),to be subsequently transported by colloidal particles.Zeta potentials of kaolinite colloids with SDZ were -23.5,-34.6,and -39.2 mV at pH 4.0,7.0,and 9.0,respectively,which were more negative than those measured in the absence of SDZ(Table SI).These measurements indirectly confirmed that SDZ was adsorbed onto kaolinite colloid surfaces.In addition,kaolinite colloids competed with SDZ for sorption sites on the surface of quartz sand(withca.5%of colloids retained in the column,Fig.2)and promoted SDZ transport.At pH 9.0,the breakthrough plateau and mass recovery of SDZ in the absence of kaolinite colloids reached values of 1 and 100%,respectively,owing to the electrostatic repulsion between SDZ-and quartz sand with negative charges.In this case,the presence of kaolinite colloids barely altered the total effluent concentration of SDZ.However,it changed the transport form,i.e.,some SDZ was transported through colloidal particles.
The breakthrough curves of colloidal SDZ at different pH roughly coincided with the breakthrough plateau observed atC/C0values<0.2 and the mass recovery values<8%(Fig.3),indicating that SDZ was mainly transported in a dissolved state despite the presence of kaolinite colloids.This behavior is fundamentally related to the structural properties of both kaolinite colloids and SDZ.Kaolinite colloids have a small specific surface area(Liet al.,2011;Zhanget al.,2020),and compound sorption occurs at the surface of clay minerals,resulting in a weak sorption capacity for sulfonamides(Essingtonet al.,2010).Meanwhile,in the pH range of 4.0—9.0,SDZ mainly exists as neutral and anionic species,resulting in weak sorption and strong electrostatic repulsion between SDZ and kaolinite colloids.The weak sorption of SDZ on kaolinite colloids was also confirmed by the results of batch experiments(Fig.S8),with less than 20% SDZ adsorbed on colloids after 24 h.Although the sorption capacity was slightly greater at pH 4.0,owing to the lower colloid effluent concentration,colloidal SDZ in the effluent was invariant,with recoveries of 6.30%,7.11%,and 5.07%,at pH 4.0,7.0,and 9.0,respectively.The low sorption capacity and recoveries suggest minor interactions of SDZ with kaolinite colloid surfaces exhibiting variable charges over this pH range.
Unfortunately,the parameters obtained from the SDZ kinetic sorption experiments underestimated the colloidal SDZ concentration in the effluent.However,the model fitted the curves of total SDZ reasonably well(R2>0.82)(Table III).Owing to the small amount of kaolinite colloids retained in the quartz sand column,colloidal particles would not cover enough of the quartz sand surfaces to significantly alter their sorption properties.Pang and Šimůnek(2006)found that the Cd sorption rate in aquifer media and the fraction of kinetic sites were not affected by colloids.The underestimation may be due to the overestimatedkdmcparameters obtained from kinetic sorption resulting from the different conditions between the column and batch experiments,such as the hydraulic condition.The desorption rates (kdmc) were significantly greater than the sorption rates (kamc) due to partial SDZadsorbed on nano-sized particles(<0.22 μm)entering the aqueous phase during the experiments.Additionally,kamcwas greater at pH 4.0 than at pH 7.0 and 9.0,whereas the opposite was true forkdmc.The H+ions in the solution could form a double electric layer on the quartz sand surface,hindering SDZ desorption from kaolinite colloids.However,when pH was 7.0 and 9.0,SDZ mainly existed as ions with strong hydrophilicity and was easily released into solution.
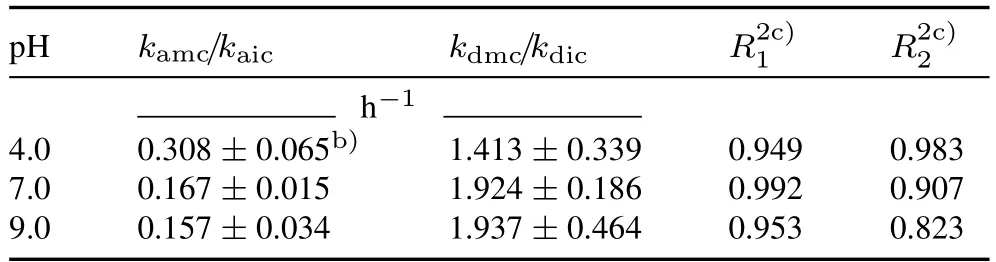
TABLE IIIRate coefficientsa) of sulfadiazine(SDZ)sorption to vs.desorption from colloids under different pH conditions derived from the kinetic batch experiments and performance of the colloid-facilitated contaminant transport model
The retention of SDZ in the presence of kaolinite colloids was higher at pH 4.0 than at pH 7.0 and 9.0 (P <0.05,Fig.3),which was consistent with the transport behavior of SDZ alone (Fig.1).However,there was no significant difference between our observations at pH 7.0 and 9.0(Fig.3).Generally,we found that pH had a minor impact on SDZ cotransport with kaolinite colloids,because of the low sorption capacity of SDZ over the tested pH range,suggesting that the variable charge edge-sites of kaolinite colloids do not substantially contribute to overall sorption.
Transport ofCIP influenced bykaolinite colloids under di-fferent pH conditions
Regarding the co-transport of CIP and kaolinite colloids,no colloids were detected in the effluent at pH 4.0 or 7.0.Therefore,only the total concentration of CIP in the dissolved form(Fig.4)was simulated by the two-site sorption model.
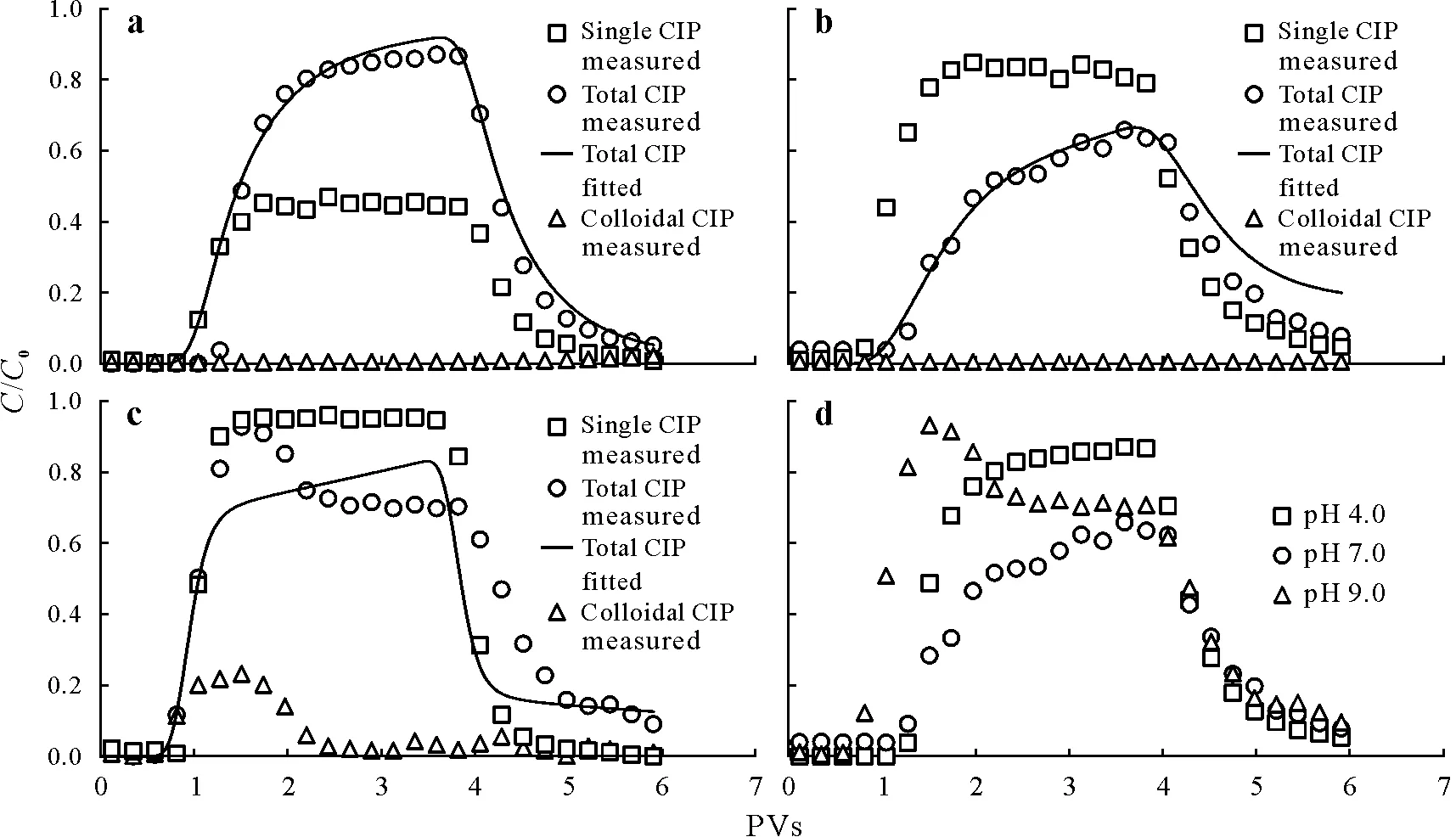
Fig.4 Breakthrough curves plotted using the relative effluent concentration(C/C0)against solution volume(pore volumes,PVs)for the transport of ciprofloxacin(CIP)alone and in the presence of kaolinite colloids at pH 4.0(a),7.0(b),and 9.0(c)and total CIP transport under different pH conditions(d).C and C0=effluent and influent CIP concentrations in the column experiments,respectively.
A distinct,pH dependent colloidal net effect on CIP transport was observed.Ciprofloxacin transport was enhanced at pH 4.0,with the breakthrough plateau increasing from 0.46 to 0.87.However,it was inhibited at pH 7.0 and 9.0,with the breakthrough plateau decreasing from 0.91 and 0.96 to 0.65 and 0.73,respectively.At pH 4.0,mobile kaolinite colloids did not contribute to the increasedC/C0of CIP,because all colloids were retained in the quartz sand.Ciprofloxacin showed a higher affinity for kaolinite colloids than quartz sand.However,the total mass of quartz sand was much greater than that of kaolinite colloids within the column,resulting in a small amount of CIP adsorbed on the kaolinite colloids.Notably,colloidal particles attached to the quartz sand surface not only contributed to reducing the effective sorption surface,but also sterically blocked the diffusion of CIP onto the quartz sand surface,which comprehensively reduced the sorption of CIP on quartz sand and consequently promoted CIP transport through quartz sand.Conversely,kaolinite colloids enhanced CIP desorption,leading to a higher fraction of equilibrium sorption sites(f)(Table IV).In this case,thekdvalue(0.526 cm3g-1)was much smaller than that of single CIP transport(5.521 cm3g-1).
At pH 7.0 and 9.0,quartz sand has a lower sorption capacity for CIP (Fig.1),resulting in a negligible impact of competitive sorption between kaolinite colloids and CIP.The observed inhibitory effect was most likely attributed to immobile colloids,providing additional sorption sites for CIP and resulting in greaterkdvalues(1.339 cm3g-1at pH 7.0 and 0.324 cm3g-1at pH 9.0,Table IV),compared to those of CIP without kaolinite colloids(0.228 and 0.082 cm3g-1at pH 7.0 and 9.0,respectively,Table I).Moreover,the lowerωvalue indicates that the kinetic processes for CIP sorption/desorption at Site 2′were suppressed,suggesting that CIP molecules were less likely to diffuse to quartz sand surfaces.At pH 9.0,a portion of CIP was transported in the colloidal form(viamobile colloids),resulting in a higher effluent concentration at the early stage of breakthrough.
As shown in Fig.4d,CIP exhibited a greater sensitivity to pH variation that can be attributed to its higher sorption capacity and pH-dependent species distribution.The mobility of CIP under acidic and alkaline conditions was greater than that under neutral condition,which was inconsistent with CIP transport alone(i.e.,CIP mobility increased at a higher pH,Fig.1b).Anionic ciprofloxacin accounted for 60%of total CIP at pH 9.0,which induced electrostatic repulsion between CIP and both quartz sand(-45.3 mV)and kaolinite colloids(-18.6 mV),thereby enhancing CIP transport(Table SI).Owing to the electrostatic repulsion and colloid carrying effect,the CIP breakthrough time occurred earlier,andC/C0was higher at the beginning of the breakthrough.However,unexpectedly,CIP mobility was greater at pH 4.0,despite being in a cationic form (100%),and was adsorbed on the negatively charged surface of quartz sand and retained kaolinite colloidsviaelectrostatic attraction.A higher zeta potential(-6.17 mV)and greater particle size(1 733.2 nm)of kaolinite colloids under such pH conditions result in weak electrostatic sorption and small specific sorption sites(Tolls,2001;Zhanget al.,2020).Moreover,kaolinite colloid deposition increased CIP equilibrium sorption,as discussed above,thus enhancing CIP transport.
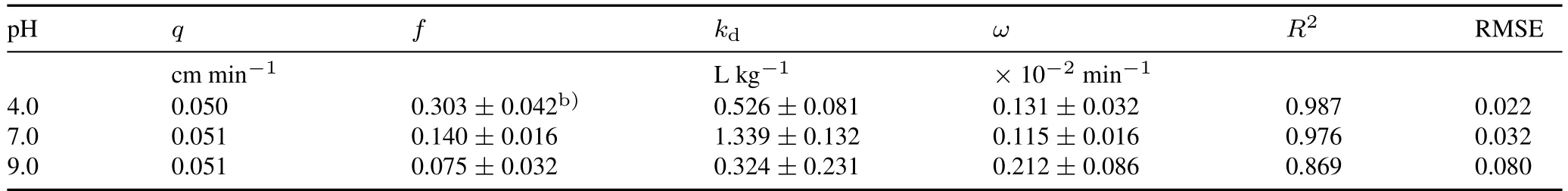
TABLE IVCalculated and fitted parametersa) of the two-site model for the co-transport of ciprofloxacin and kaolinite colloids under different pH conditions
CONCLUSIONS AND PROSPECTS
This study addressed the impacts of kaolinite colloids on SDZ and CIP transport in quartz sand media under different pH conditions,providing new insights into antibiotic transport.Specifically,the mobility of SDZ increased with pH because of its pH-dependent speciation.Moreover,SDZ transport was enhanced by kaolinite colloids under acidic and neutral conditions,with a minor amount of SDZ being transported in the colloidal form.Generally,pH had a marginal effect on SDZ transport with or without kaolinite colloids,owing to the low SDZ sorption capacity of quartz sand and kaolinite colloids over the tested pH range.The colloidfacilitated transport model provided a good description of total SDZ transport,although it underestimated colloidal SDZ transport when using parameters from kinetic sorption experiments.Conversely,CIP transport displayed a greater sensitivity to variation in pH.Ciprofloxacin mobility increased significantly with pH in the absence of kaolinite colloids,confirming that electrostatic interactions played a crucial role in controlling CIP transport in porous media.Moreover,the presence of kaolinite colloids enhanced CIP transport under acidic conditions owing to the deposition of kaolinite colloids,consequently reducing the number of effective sorption sites of CIP on the quartz sand surface,while concomitantly,the presence of colloids increased the desorption of CIP,which also enhanced CIP mobility.However,kaolinite colloids inhibited CIP(CIP0and CIP-)transport under neutral and alkaline conditions because the deposited colloids provided additional CIP sorption sites that assisted CIP retention.Concurrently,kaolinite colloid transport was significantly inhibited by CIP because of the straining effect and reduced electrostatic repulsion.
Overall,colloids can promote antibiotic transport not only through their carrying effect,but also because of their competitive sorption,which shielded the sorption sites on the solid matrix.In contrast,colloids retained in porous media can inhibit the transport of antibiotics by providing additional sorption sites.Determining the concentrations of different antibiotic forms is extremely challenging,and whether the dual-wavelength method is applicable to other antibiotics or colloids remains to be explored in future research.Moreover,soil solution is not a pure solution in nature,but contains various solutes or pollutants,which may have a synergistic impact on antibiotic transport and require further investigation.For example,metal cations(Na+and Ca2+)are widespread in soil and groundwater and can induce alterations in molecular speciation,colloid aggregation,and environmental behavior of antibiotics.
ACKNOWLEDGEMENT
This study was supported by the National Natural Science Foundation of China(No.41807010).
SUPPLEMENTARY MATERIAL
Supplementary material for this article can be found in the online version.
杂志排行
Pedosphere的其它文章
- Biochar for future and futuristic biochar
- Career opportunities in Institute of Soil Science,CAS,Nanjing,China
- Main environmental drivers of abundance,diversity and community structure of comammox Nitrospira in paddy soils
- Stoichiometry of base cations and silicon during weathering of a deep soil profile derived from granite
- Five-year warming does not change soil organic carbon stock but alters its chemical composition in an alpine peatland
- Effects of nanofertilizer and nano-plant hormone on soil chemical properties and microbial community in two different soil types
