Effect of taxifolin on cold-shock damages in spermatozoa in rabbits
2023-10-12rfanlmazGaffarirkAslhanakCihangiroluTutkuCanAcsubrahimHalilngzdeArkalEdanurlerEkmen
İrfan Yılmaz ,Gaffari Türk ,Aslıhan Çakır Cihangiroğlu ,Tutku Can Acısu ,İbrahim Halil Güngör✉ ,Gözde Arkalı ,Edanur Güler Ekmen
1Fırat University,Faculty of Veterinary Medicine,Department of Reproduction and Artificial Insemination,Elazığ,Türkiye
2Fırat University,Faculty of Veterinary Medicine,Department of Physiology,Elazığ,Türkiye
ABSTRACT Objective:To investigate the effect of taxifolin added to rabbit semen on freezing-induced cold-shock damages in spermatozoa.Methods:Semen was collected from six adult New Zealand rabbits once a week by artificial vagina.The collected semen was pooled at 38 ℃ and divided into four equal volumes.They were diluted with 0,50,100 and 200 µM taxifolin-containing Tris+egg yolk extender at 38 ℃ and their temperatures were lowered to 4 ℃.Following equilibration,semen drawn into 0.25 mL straws were frozen in an automatic semen freezing device and stored in liquid nitrogen container at -196 ℃.Samples were thawed in 38 ℃ water for 25 s and the analyses of motility,kinematic parameters,morphological deformities,changes in membrane integrity,mitochondrial membrane potential,dead-live ratio,acrosomal damages and as well as oxidative stress analyses were performed in semen.Results:Addition of 50 µM taxifolin significantly improved motility(total,progressive,rapid and static),high mitochondrial membrane potential and the ratios of spermatozoa with acrosomal damage compared to the control group.Compared to the control group,malondialdehyde (MDA) levels in the 50 and 100 µM taxifolin groups were significantly lower,while the MDA level was high and viable spermatozoa ratio was low in the 200 µM taxifolin group.There were no significant differences between the groups in terms of kinematic parameters,morphological deformities,membrane integrity and antioxidant levels.Conclusions:The low dose of taxifolin (50 µM) has a positive effect and the high dose (200 µM) has a negative effect.Therefore,it is concluded that the addition of low-dose (50 µM) taxifolin to the extenders would be a useful additive in reducing cold-shock damage that occurs during freezing of rabbit semen.
KEYWORDS: Taxifolin;Semen freezing;Cold-shock;Oxidative stress;Rabbit
1.Introduction
Approximately 300 million rabbits are used as a source of meat,hair and fur every year worldwide.This shows that rabbit breeding is an economic source of income.In addition,the fact that rabbits are more used in biomedical studies and that they are in danger of extinction compared to farm animals is scientific aspect of rabbit breeding.For this reason,it is important to ensure the continuity of rabbit strains and breeding.Artificial insemination is one of the most important methods of conserving rabbit strains in terms of continuity[1].From the beginning of artificial insemination until today,many techniques have been tried to obtain strong breeders with high genetic productivity.During the last century,studies on artificial insemination has started to gain momentum especially in animals with high economic income such as horse,cattle,sheep and goat.Artificial insemination has been given more importance with the starting of semen or embryo freezing studies in animals that are in danger of extinction.However,some issues arise during the short or long-term storage of semen obtained from various animal species.Although these issues are closely related to the spermatozoon structure of the species,and ram,goat spermatozoa are more sensitive to freeze process than the spermatozoa of humans,dogs and rabbits.In general,semen freezing in all species causes serious decreases in semen quality[2].Rabbits are one of the animal species used in scientific research and biological testing.The most important feature that distinguishes rabbits from other laboratory animals in semen handling studies is that it is much easier to collect semen by artificial vagina without euthanasia[3].
Semen can be exposed to cold-shock when it is stored outside for a short-or long-time.In exposure to cold-shock,which has been the subject of research in many studies,irreversible damages are observed in motility,viability,DNA structure,membrane integrity and fertilization ability of spermatozoa[4].In order to reduce these damages,numerous studies have been conducted in which semen is stored by adding many substances to the extender during the storage of semen in different animal species[5,6].The fact that oxidative stress is one of the causes of cold-shock damage in spermatozoa has enabled the studies carried out by adding antioxidant substances to come to the fore and still maintain their topicality[7].In this context,being very limited information about the effectiveness of taxifolin,which is one of the highly effective antioxidant substances,to prevent damage during storage of semen and also being fewer studies on freezing of rabbit semen compared to domestic animal species allowed the investigation of the efficacy of taxifolin in order to minimize cold-shock damage during freezing of rabbit semen.
Taxifolin [(2R,3R)-2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-2,3-dihydro chromen-4-on;C15H12O7],which is in the class of flavonoids,is a dihydroflavonol.It is also known as dihydroquercetin and abundant in citrus and onion.Flavonoids show their antioxidant activities by inhibiting the formation of lipid peroxidation (LPO) and free radicals[8].Many studies have been carried out by utilizing the antioxidant properties of taxifolin.In a study,taxifolin was reported to have strong anti-inflammatory activity on the exudative and proliferative phases of inflammation[9].It has been suggested that the extract obtained from milk thistle containing taxifolin in its chemical composition has an effective role in preventing heat stress inducedtesticular damage,-increase in LPO,-decrease in antioxidant enzymes and spermatological cells in quail[10].Similarly,it has been reported that milk thistle seed extract improves semen quality and testosterone level of male rabbits[11].Although it has been reported that taxifolin has beneficial effects on short-term storage of semen in rabbits[12] and on freezing in rams[13],no information has been found about its effect on the quality of frozen-thawed rabbit semen.Therefore,in this study,it was aimed to investigate the effect of taxifolin added to rabbit semen on cold-shock damages to spermatozoa induced by freezing.
2.Materials and methods
2.1.Animal housing
Six adult healthy New Zealand White male rabbits at 1-year-old and weighing 4.2-4.5 kg procured from Erciyes University Experimental Research Application and Research Center (Kayseri,Türkiye) were used in the study.After one-week adaptation period,the animals were housed in the Fırat University Experimental Research Center under routine care and feeding conditions (ad libitum water and feed)with 12 h of light/12 h of darkness,22 ℃-24 ℃ temperature and 55%-60% relative humidity.
2.2.Semen collection and pre-dilution process
Semen was collected by artificial vagina once a week between 9:00 and 10:30 a.m.for six weeks.Thus,six repetitions were performed.The gel was separated from the liquid part by forceps.The semen of each animal was diluted with pre-dilution extender including Tris-buffer solution [3.80 g (313.79 mM) Tris (hydroxymethyl)aminomethane,1.92 g (103.07 mM) citric acid,0.59 g (33.30 mM)glucose and bi-distilled water up to 100 mL] and 16% centrifuged egg yolk and then brought to the laboratory in a beaker containing hot water at 38 ℃.Initial motility and concentration were determined by computer assisted sperm analyzer (CASA) (ISASv1,Proiser,Spain).Semen samples with initial motility of 70% and above,and concentration of 150 million/mL and above were used for further processing.
2.3.Preparation of semen freezing extender and taxifolin stock solution
Tris-buffer solution containing 6% dimethyl sulfoxide (DMSO),16% non-centrifuged egg yolk,100.000 IU/mL penicillin-G potassium (Penicillin G Potassium 1.200.000 IU,İ.E.Ulagay Co.,İstanbul,Türkiye) and 100 mg/mL streptomycin sulfate(Streptomycine-ie 1 g,İ.E.Ulagay Co.,İstanbul,Türkiye) was prepared for semen freezing extender.To prepare taxifolin(C15H12O7H2O,Sigma-Aldrich Chemical Co.,NY,USA) stock solution,30 mg of taxifolin was dissolved in 30 mL of DMSO to obtain a total stock solution of 3 334 µM and subsequently divided into three equal volumes of 10 mL.Since taxifolin was added to the semen freezing extender (not pre-dilution extender),in order to adjust the taxifolin rates in total extender (pre-dilution+semen freezing) to 200,100 and 50 µM,each volume of stock solution was extra diluted with DMSO as 400,200 and 100 µM,respectively,and stored at -20 ℃ until being used.
2.4.Formation of groups,and freezing and thawing of semen
Collected and pre-diluted each semen sample was mixed at 38 ℃(pooling) and divided into four different test tubes in equal volumes to create the control,50,100 and 200 µM taxifolin groups.Semen freezing extender containing 0,50,100 and 200 µM taxifolin was then added to the control,taxifolin-50,taxifolin-100 and taxifolin-200 tubes,respectively.The spermatozoa number per each tube was approximately 120-130 million/mL.Within 2 h,the temperature was lowered from 38 ℃ to 4 ℃ in refrigerator and allowed to equilibrate at this temperature for 3 h.Following the equilibration process,the semen samples in each group were drawn into 0.25 mL straws at 4 ℃,and open ends of the straws were covered with polyvinyl alcohol powder.Straws placed on the racks were left to the automatic freezing device (Micro-Digitcool,IMV Technologies,Paris,France).Freezing process was carried out by liquid nitrogen vapor at -140 ℃in the device of which temperature transitions were automatically adjusted by the manufacturer for rabbit semen (3 ℃/min from 4 ℃to -6 ℃,47 ℃/min from -6 ℃ to -100 ℃,20 ℃/min from -100 ℃to -140 ℃).Frozen semen straws were stored in a liquid nitrogen container (-196 ℃) until being analyzed.After at least 24 h storage,the straws were thawed in hot water at 38 ℃ for 25 s.
2.5.Analyses in frozen-thawed semen
2.5.1.Motility and kinematic analyses
CASA was used for the analyses.Semen samples were diluted with Tris-buffer solution at 38 ℃ in an Eppendorf tube to reduce the number of spermatozoa in each image area of CASA.Afterwards,3.5 µL of diluted samples was put on a special slide (SpermTrack 20,Proiser,Spain) and motility (total,progressive,rapid,medium,slow and static) and kinematic parameters [curvilinear velocity(VCL),straight line velocity (VSL),average path velocity (VAP),linearity (LIN),straightness (STR),wobble (WOB),amplitude of lateral head displacement (ALH) and beat cross frequency (BCF)]were automatically evaluated[14].Progressive motility was defined as the rate of spermatozoa moving straight forward or moving in large circles.The speed range was static < 10 µm/s < slow < 45 µm/s <medium < 75 µm/s < rapid.
2.5.2.Plasma membrane integrity-hypoosmotic swelling(HOS) test
Semen samples were diluted with Tris-buffer solution and 25 µL of diluted sample was mixed with 250 µL of hypotonic solution (0.49 g citric acid,0.9 g fructose,100 mL distilled water).It was kept at 38 ℃ for 60 min,and 30 µL of the sample was taken and dropped on a heated slide and covered with coverslip.Twohundred spermatozoa were examined at 400× magnification of the phase contrast microscope (Nikon,Tokyo,Japan) and spermatozoa with curled tails that are intact were expressed as percentage[14].
2.5.3.Morphological analysis of spermatozoa
Smear was prepared by using 50 µL semen samples,which diluted with Tris-buffer for HOS test,and it was then air-dried within 5 min.Diff-Quick staining kit (Gündüz Kimya,stanbul,Türkiye)was used for staining.Then,it was washed for 1 min with distilled water and air-dried.The smears were examined under phase contrast microscope using 600× magnification.A total of 200 spermatozoa were examined for each smear,and the proportion of abnormally shaped (head,tail and total abnormal) spermatozoa was expressed as percentage[14].
2.5.4.Flow-cytometric analyses
2.5.4.1.Dead and live rates
First,860 µL of phosphate buffer solution (PBS) was added into an Eppendorf tube kept in a dark environment at 38 ℃.Frozenthawed semen (30 µL) and fluorescent dyes [5 µL SYBR-14 and 5 µLpropidium iodide (PI)] were added to it.Thus,the number of spermatozoa became approximately 1 million/mL.Then,the mixture was vortexed and incubated at 38 ℃ for 30 min.The reading process was performed in the flow-cytometer (Cytoflex,Beckman-Coulter,CA,USA).Dead and live spermatozoa rates were expressed as percentage[15].
2.5.4.2.Acrosomal damage
First,862 µL of PBS solution was added into an Eppendorf tube kept in a dark environment at 38 ℃.Frozen-thawed semen (30µL) and fluorescent dyes [5 µL peanut agglutinin (PNA) and 3 µL PI] were added to it.Thus,the number of spermatozoa became approximately 1 million/mL.Then,the mixture was vortexed and incubated at 38 ℃ for 30 min.The reading process was performed in the flow-cytometer.PNA+regions in the dot blot image area of flow cytometry were considered as damaged acrosomes.Acrosomal damage rate was expressed as percentage[16].
2.5.4.3.Mitochondrial membrane potential
First,867.5 µL of PBS solution was added into an Eppendorf tube kept in a dark environment at 38 ℃.Frozen-thawed semen (30 µL) and fluorescent dye (2.5 µL JC-1) were added to it.Thus,the number of spermatozoa became approximately 1 million/mL.Then,the mixture was vortexed and incubated at 38 ℃ for 30 min.The reading process was performed in the flowcytometer.The rates of spermatozoa with high and low mitochondrial membrane potentials were expressed as percentage[16].
2.5.5.Oxidative stress analyses
2.5.5.1.LPO
One volume of semen sample was mixed with two volumes of stock solution [15% trichloroacetic acid (TCA) in 0.25 N hydrochloric acid(HCl) and 0.375% thiobarbituric acid in 0.25 N HCl] in a centrifuge tube.The mixture was left to incubate in boiling water at 100 ℃ for 15 min.After cooling,the resulting precipitate was removed after centrifugation at 100×g for 10 min.The absorbance was read with an ultraviolet spectrophotometer (Shimadzu,2R/UV,Tokyo,Japan) at a wavelength of 532 nm.The level of malondialdehyde (MDA),an index of LPO,was expressed as nmol/mL[17].
2.5.5.2.Glutathione (GSH)
Semen samples were precipitated with 50% trichloroacetic acid(TCA) and centrifuged at 45×g for 5 min to remove the precipitate.0.5 mL of the supernatant was taken,and the reaction mixture was formed by adding 2 mL Tris-EDTA buffer (0.2 mol/L,pH: 8.9) and 0.1 mL 0.01 mol/L 5.5’-Dithio-bis-2-nitrobenzoic acid on it.After the mixture was left for 5 min at room temperature,the absorbance was read with an ultraviolet spectrophotometer at a wavelength of 412 nm.GSH level was expressed as nmol/mL[18].
2.5.5.3.Glutathione-peroxidase (GSH-Px)
For the determination of GSH-Px activity,semen samples were precipitated with 50% TCA and centrifuged at 45×g for 5 min to remove the precipitate.0.1 mL of the supernatant was taken,and 0.8 mL of reaction mixture [50 mmol/L PBS(pH: 7.0),1 mmol/L EDTA,1 mmol/L sodium azid,0.2 mmol/L NADPH,1 IU/mL oxidized glutathione reductase,1 mmol/L GSH and 0.25 mmol/L hydrogen peroxide (H2O2)] was added on it.It was incubated at 25℃ for 5 min before reacting with 0.1 mL of peroxide solution.The absorbance was read in an ultraviolet spectrophotometer at 340 nm for 5 min.GSHPx activity was expressed as IU/g protein[19].
2.5.5.4.Catalase
To determine catalase activity,0.2 mL semen sample was left to incubate in 1 mL substrate (50 mmol/L PBS with 65 µmol/L H2O2,pH: 7.0) at 38 ℃ for 60 s.The enzymatic reaction was terminated with 1 mL of 32.4 mmol/L ammonium molybdate.The color change resulting from this reaction was read against the blank with an ultraviolet spectrophotometer at a wavelength of 405 nm.Catalase activity in the samples was measured according to the decrease in the level of H2O2and expressed as ku/g protein[20].
2.6.Statistical analysis
All analyses were done by using IBM SPSS (Statistical Package for the Social Sciences,Version 26.0.IBM Corp.Armonk,NY).Using the Shapiro-Wilk test of normality,it was analyzed whether the data showed normal distribution or not.While one-way analysis of variance (ANOVA) and post hoc Tukey-HSD tests were used to compare parametric data,Kruskal-Wallis analysis of variance and Mann-Whitney-U tests were used to compare non-parametric data.Data are presented as mean±standard deviation (mean±SD)or median (interquartile range).P<0.05 value was considered to be significant.
2.7.Ethics statement
This study was approved by Fırat University Animal Experiments Local Ethics Committee (Elazığ,Türkiye) (protocol No: 25/11/2020-2020/14).
3.Results
3.1.Motility and kinematic values
Motility and kinematic values of frozen-thawed rabbit spermatozoa supplemented with different doses of taxifolin are given in Table 1.Although total (P<0.001),progressive (P<0.001) and rapid (P<0.05)motility rates of the 50 µM taxifolin group were found significantly higher than the control and other taxifolin-treated groups,static spermatozoon rate of the 50 µM taxifolin group was significantly lower (P<0.001).However,the addition of 50,100 and 200 µM taxifolin caused no statistically significant change in terms of medium and slow motility rates and as well as kinematic parameters compared to the control group.
3.2.HOS test and morphologic abnormality values
HOS test and morphologic abnormality rates of frozen-thawed rabbit spermatozoa supplemented with different doses of taxifolin are given in Table 1.There was no statistical difference between the control and experimental groups and also between the experimental groups themselves in terms of head,tail and total abnormal spermatozoon rate and HOS test rate.
3.3.Flow-cytometric values
Flow cytometric dot blot and boxplot images for dead/live spermatozoa are presented in Figures 1 and 2,respectively.Addition of 200 µM taxifolin significantly increased the rate of dead spermatozoa compared to the 50 µM taxifolin group (P<0.01)(Figure 2A),while it caused a significant decrease in the rate of live spermatozoa compared to the control and 50 µM taxifolin groups(P<0.05) (Figure 2B).
Flow cytometric dot blot and boxplot images for damaged acrosome are shown in Figures 3 and 4,respectively.Addition of 50 µM taxifolin significantly decreased the rate of spermatozoa with acrosomal damage compared to the control group (P<0.01),while the addition of 200 µM taxifolin significantly increased it compared to the 50 µM taxifolin group (P<0.01) (Figure 4).
Flow cytometric dot blot and boxplot images for mitochondrial membrane potential are presented in Figures 5 and 6,respectively.High mitochondrial membrane potential (HMMP) of the 50 µM taxifolin group was significantly higher than the other groups(P<0.01) (Figure 6A).However,in terms of low mitochondrial membrane potential (LMMP),the increase detected in the 200 µMtaxifolin group was significantly higher than the 50 µM taxifolin group (P<0.01) (Figure 6B).HOS:

Table 2.Oxidative stress values of frozen-thawed rabbit semen in the control and different taxifolin groups.
3.4.Oxidative stress values
The values belonging to oxidative stress parameters of frozenthawed rabbit spermatozoa supplemented with different doses of taxifolin are given in Table 2.The MDA,which is an important marker of LPO,level of the 200 µM taxifolin group was significantly higher than the 50 and 100 µM taxifolin groups (P<0.001).The differences observed between the groups in GSH level,GSH-Px and catalase activities did not reach the statistical significance.
4.Discussion
One of the main purposes of artificial insemination is to enhance the economic gain to higher levels by increasing the genetic productivity in animals,thus increasing the number of animals with high yield characteristics.For this purpose,the worldwide spread of artificial insemination is only possible with the use of frozen semen[21].However,in almost all species,irreversible damages occur in spermatozoa during freeze of semen or storage of it in a cold environment without freezing.These damages are observed in the DNA,membrane integrity,motility,viability and fertilization ability of the spermatozoa[22].Sudden temperature changes,osmotic stress,cryoprotective toxicity and high reactive oxygen species (ROS)-induced oxidative stress during cryopreservation of spermatozoa are associated with the formation of these damages[23].Oxidative stress is defined as the deterioration of the balance between oxidants and antioxidants in favor of oxidants.Oxidants and antioxidants are in balance in spermatozoa as in other cells.However,increasing ROS during cold storage and freezing processes disrupts this balance[24].This situation has recently led researchers to conduct studies on adding antioxidants to extenders during cryopreservation of semen in many species,including rabbits.However,the question “which antioxidant is more effective?” is still up-to-date,so researches continue.In this context,in the present study,the role of taxifolin in reducing the damages caused by the freeze-thawing process of rabbit semen was investigated.Taxifolin,also known as dihydroquercetin,is a flavonoid.Taxifolin with high antioxidant feature has scavenging effect on free radicals in the body[25].It is claimed that it shows this effect by binding free radicals to itself[26].Taxifolin has promising pharmacological activities in the treatment of inflammation,tumors,microbial infections,oxidative stress,cardiovascular and liver disorders[27].Since the studies on the in vitro effects of taxifolin on the storage of semen of different species are very limited (two scientific studies to our knowledge),the results obtained from this study are also discussed with the findings on the in vitro effects of quercetin,a taxifolin-like flavonoid,in the semen of different species.

Figure 1.Flow cytometric dot blot image for dead and live spermatozoa of frozen-thawed rabbit semen in the control and different taxifolin (TAX) groups.A: the control group;B: the taxifolin-50 µM group;C: the taxifolin-100 µM group;D: the taxifolin-200 µM group.

Figure 2.The boxplot image for dead (A) and live (B) spermatozoa of frozen-thawed rabbit semen in the control and different taxifolin (TAX) groups.a;Different from the taxifolin-50 µM group (P<0.01).b;Different from both the control and taxifolin-50 µM groups (P<0.05).
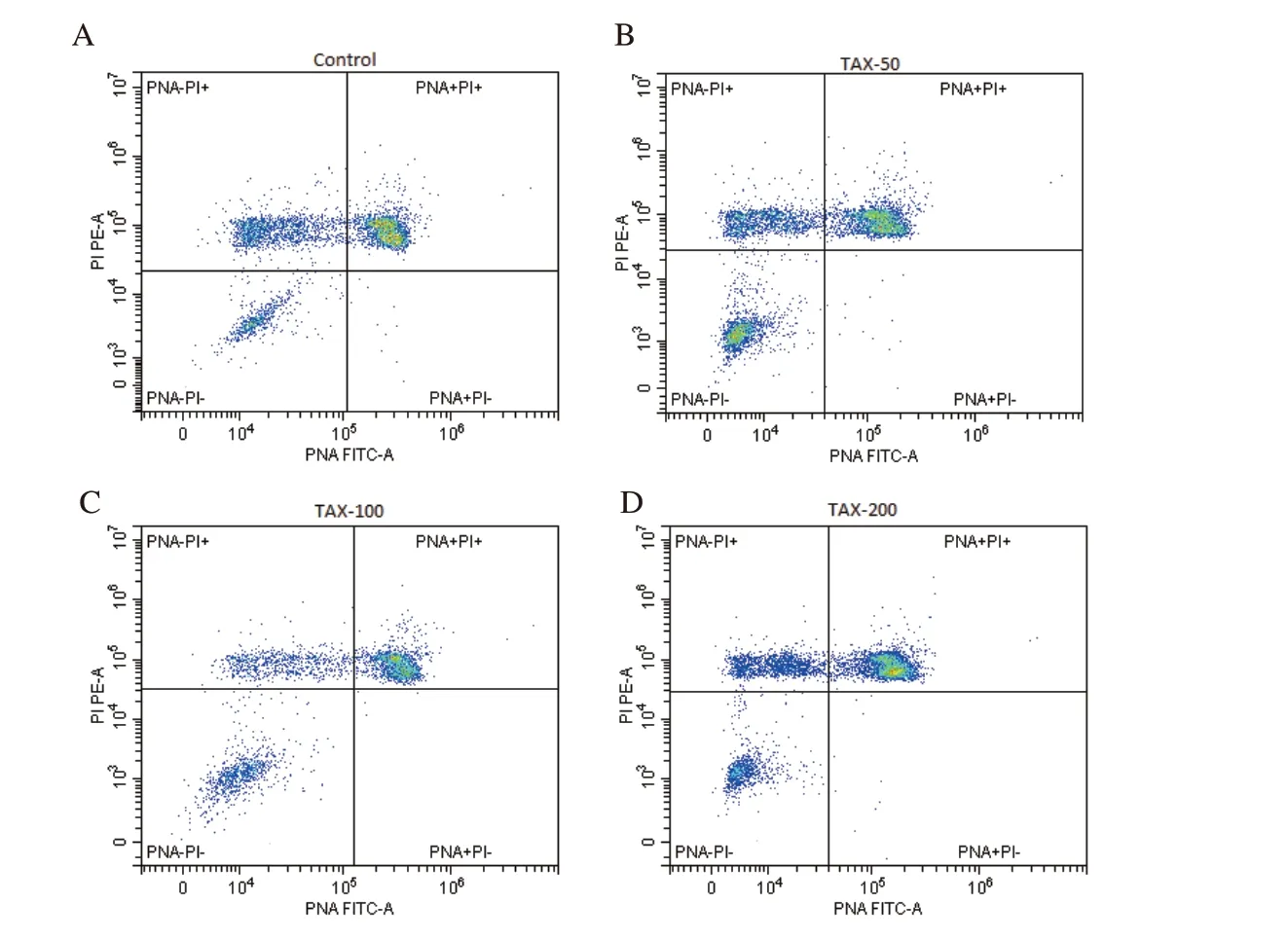
Figure 3.Flow cytometric dot blot image for acrosomal damage of frozen-thawed rabbit semen in the control and different taxifolin (TAX) groups.A: the control group;B: the taxifolin-50 µM group;C: the taxifolin-100 µM group;D: the taxifolin-200 µM group.

Figure 4.The boxplot image for acrosomal damage of frozen-thawed rabbit semen in the control and different taxifolin (TAX) groups.a;Different from the control group (P<0.01).b;Different from the taxifolin-50 µM group(P<0.01).
Conventional analyses such as motility,plasma membrane integrity,acrosomal integrity,viability,morphological and mitochondrial activity are now routinely used to evaluate the structure and functionality of fresh,chilled or frozen-thawed spermatozoa.In this study,the addition of 50 µM taxifolin showed a significant increase in total,progressive and rapid motility rates and a significant decrease in static spermatozoon rate compared to both control and other doses of taxifolin.In a study performed by Sevim and Sarıözkan[12],rabbit semen diluted with a Tris-buffer solution containing 10,100 and 500 µM taxifolin was stored at 4 ℃ for 24 h,and 100 µM dose was observed to have the highest motility rates during liquid-storage when compared to the control group.In another study conducted in rams[13],semen was frozen by using different extenders including different doses of taxifolin,and it was claimed that the highest post-thaw total motility rate was obtained in the group containing 3% glycerol and 10 µM taxifolin;however,the 500 µM dose was found to be toxic.On the other hand,it was reported that the motility rates obtained from frozen-thawed human[28],dog[29],stallion[30] and ram[31] semen supplemented with quercetin at different doses were higher than the quercetinfree group.Findings from this study related to the motility are consistent with the studies on taxifolin and quercetin.In this study,the positive effect of low-dose taxifolin supplementation on motility is directly related to lowering of the high level of ROS and LPO caused by freeze-thawing process through antioxidant enzymes,which was evidenced in the present study.Increasing ROS damages mitochondria,which play a key role in adenosine triphosphate(ATP) metabolism,leading to ATP deficiency,which in turn leads to motility losses[32].Therefore,reduction of ROS by taxifolin may contribute to the protection of post-thaw spermatozoa motility by preventing mitochondrial damage (increase in HMMP,decrease in LMMP was showed in this study) and consequently ATP deficiency.In this study,it was observed that low-dose taxifolin (50 µM)significantly increased HMMP,high-dose taxifolin (200 µM)significantly decreased viability of spermatozoa.In a study conducted in rams,the highest and lowest rates of viability and mitochondrial activity were found in frozen-thawed semen containing low (10 µM) and high (500 µM) doses of taxifolin,respectively[13].On the other hand,it has been reported in the studies conducted in rabbit[33],buffalo[34],human[35],mouse[36] and rooster[37] semen that liquid-or frozen-storage procedure-induced decreases in viability and/or mitochondrial activity of spermatozoa were able to prevent by quercetin addition.However,Silva et al[31]reported that different doses of quercetin decreased the HMMP value of frozen-thawed ram semen in a dose-dependent manner.The findings of this study are compatible with the results of the study performed in frozen-thawed ram spermatozoa performed by Bucak et al[13].Mitochondria,which are responsible for spermatozoon metabolism and energy production,are damaged due to increased ROS caused by cryopreservation.Due to cytoplasmic antioxidant enzyme deficiency,spermatozoa do not have the ability to adequately repair the damages in mitochondria,viability and vitality induced by increased ROS[38].However,it is well-known that exogenous antioxidants have positive effects in preventing or repairing this circumstance.Therefore,in this study,it may be stated that low-dose taxifolin supplementation protects mitochondria from ROS attacks through its antioxidant effect and maintains mitochondrial activity,vitality and viability in spermatozoa at an optimum level.On the other hand,in the present study,the decrease determined in the viability rates of spermatozoa supplemented with 200 µM taxifolin shows that high-dose creates a toxic effect supporting the study of Bucak et al[13].
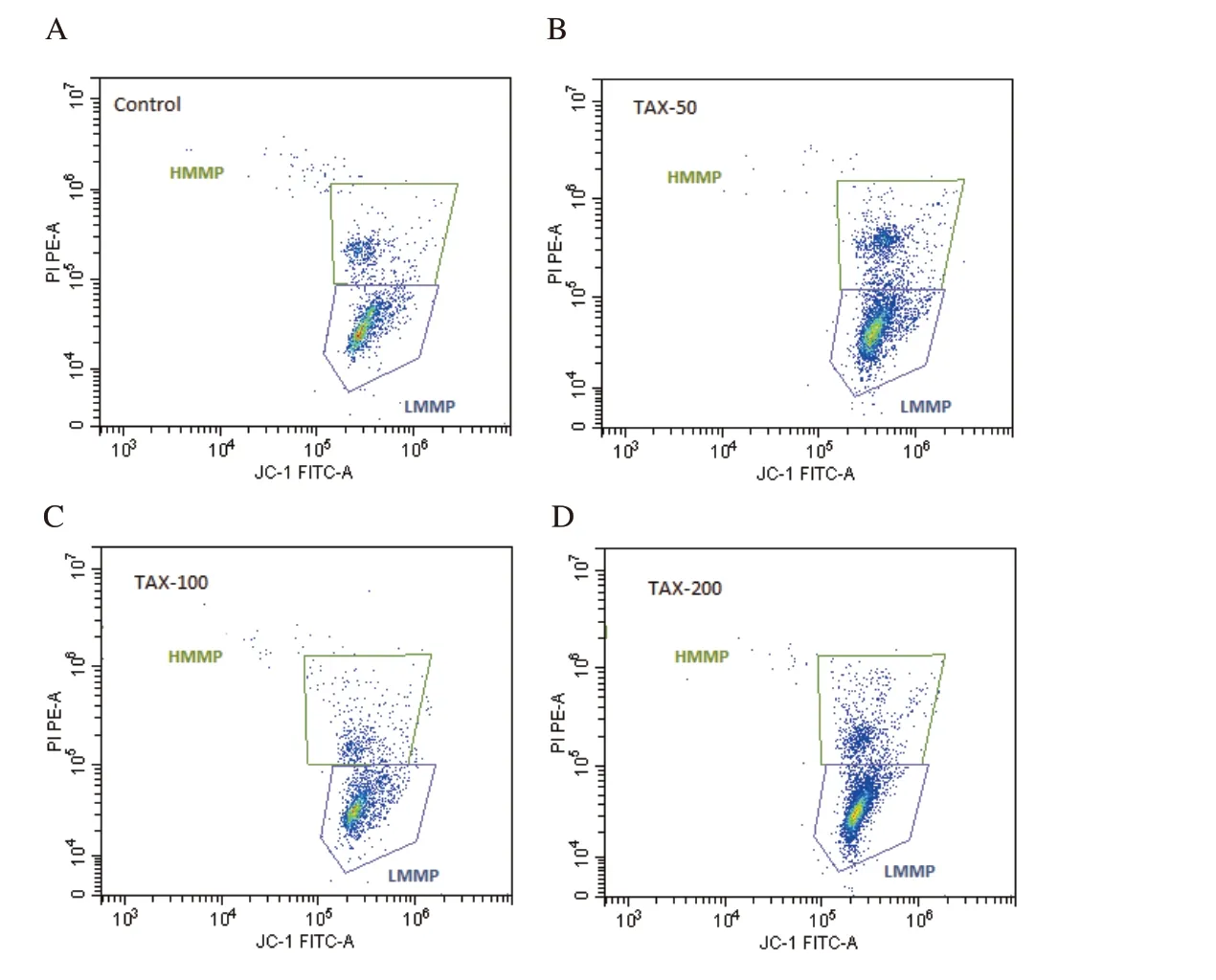
Figure 5.Flow cytometric dot blot image for high and low mitochondrial membrane potential of frozen-thawed rabbit semen in the control and different taxifolin (TAX) groups.A: the control group;B: the taxifolin-50 µM group;C: the taxifolin-100 µM group;D: the taxifolin-200 µM group.HMMP: high mitochondrial membrane potential;LMMP: low mitochondrial membrane potential.
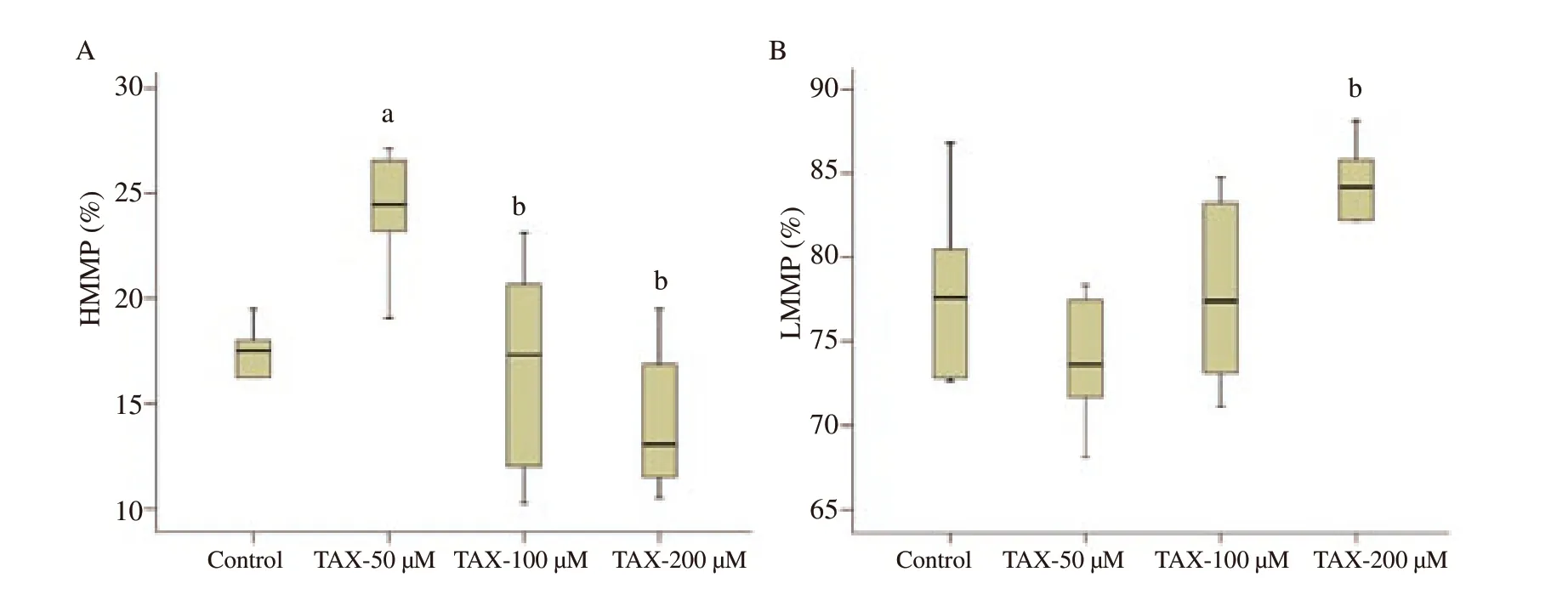
Figure 6.The boxplot image for high (A) and low (B) mitochondrial membrane potential of frozen-thawed rabbit semen in the control and different taxifolin (TAX) groups.a;Different from the control group (P<0.01).b;Different from taxifolin-50 µM group (P<0.01).HMMP: high mitochondrial membrane potential;LMMP: low mitochondrial membrane potential.
In the present study,low-dose taxifolin supplementation provided a significant decrease in acrosomal damage.Consistent with our study,it was reported that acrosomal damage in liquid-stored rabbit spermatozoa at 4 ℃ was prevented by taxifolin addition at 10 (low)and 100 (medium) µM doses[12].In addition,it was suggested that the addition of low-dose taxifolin during freezing of ram semen resulted in the lowest acrosomal damage after thawing,although it was not statistically significant compared to the control[13].It was observed that less acrosomal damage occurred in liquid-and/or frozen-stored of rabbit[33] and buffalo[34] semen supplemented with quercetin.In this study,the findings related to the decrease in the rates of acrosomal damaged spermatozoa after the addition of taxifolin are in line with the findings of the studies conducted in rabbits[12] and rams[13] for the in vitro effect of taxifolin.This positive effect may be associated with the reduction of cryopreservationinduced increments in peroxidative damages in acrosomal membranes by taxifolin addition to the semen extender.
In this study,it was determined that 50 and 100 µM doses of taxifolin significantly decreased the level of MDA,while the 200 µM dose significantly increased it.Also,the MDA level of the 200 µM taxifolin group was significantly higher than the 50 and 100 µM taxifolin groups.The numerical differences observed between the groups in GSH level,GSH-Px and catalase activities did not reach the statistical significance.In a study conducted in Merino rams,which was consistent with our study findings,it was observed that the addition of taxifolin to semen resulted in significant decreases in LPO levels and significant increases in antioxidant gene expressions after freezing-thawing;however,it did not cause any change in total GSH and antioxidant power levels[13].In semen freeze studies in humans[35],buffalo[34],goats[39] and roosters[37],it has been suggested that the addition of quercetin to the extender significantly reduces the free radicals and by-products of LPO in thawed semen.In this study,the decrease observed in MDA level after addition of 50 and 100 µM taxifolin may be explained by the prevention of peroxidation of lipids in the spermatozoon membrane as a result of preventing or reducing the formation of unstable compounds in the cells through the effective scavenging of ROS and other free radicals by the strong antioxidant effect of taxifolin.As it is known,MDA emerges as a by-product of LPO.However,the fact that high-dose taxifolin (200 µM) supplementation increases LPO and thus MDA level may be considered as an indication of overdose.This result obtained regarding the oxidative stress parameters is similar to the findings of the study conducted on ram semen,in which the effect of taxifolin was examined.
Although the measurement of cold-shock damages by spermatological,oxidative stress and flow-cytometric analyses are the strengths of this study,the absence of molecular analyses (such as protein and mRNA expressions of CatSper ion channel) and fertility experiments can be considered as the factors limiting the comprehensiveness of the study.
In conclusion,the findings of this study clearly show that the addition of 50 µM taxifolin to rabbit semen freezing extender before dilution makes an important contribution to improving spermatozoa quality parameters such as motility,plasma membrane integrity and acrosomal integrity,metabolic parameter such as mitochondrial activity and oxidative stress parameters such as MDA.The basic mechanism underlying these improvements seems to be related to the reduction of cold-shock induced LPO emerging during the freeze-thawing process of semen by strong antioxidant effect of taxifolin.It is thought that the taxifolin used to improve the freezing quality of rabbit semen may also be used for short and long-term storage of human semen.
Conflict of interest statement
The authors declare no conflict of interest.
Funding
This study was financially supported by Fırat University Scientific Research Projects Coordination Unit (Grant No: VF.21.02).
Authors’ contributions
İrfan Yılmaz (this study was summarized from his master’s thesis)was involved in conceptualization,investigation,formal analysis,resources,and writing original draft preparation.Gaffari Türk was involved in supervision,funding acquisition,statistical analyses,writing-reviewing and editing.Aslıhan Çakır Cihangiroğlu,Tutku Can Acısu,İbrahim Halil Güngör,Gözde Arkalı and Edanur Güler Ekmen were involved in investigation.
杂志排行
Asian Pacific Journal of Reproduction的其它文章
- The role of small non-coding RNAs (sncRNAs) in male infertility: A scoping review
- Reproductive outcomes of water pipe smoking: A scoping review
- Predictors of antenatal health service utilization among left-behind wives of male outmigrants: Evidence from Patna District,India
- Methanolic pomegranate dried peel extract improves cryopreserved semen quality and antioxidant capacity of rams
