Comparative investigations of ternary thermite Al/Fe2O3/CuO and Al/Fe2O3/Bi2O3 from pyrolytic,kinetics and combustion behaviors
2023-09-02ShiLiJilinChenToGuoWenDingLinJingMioYoJixingSongLifengXieYimingMo
Shi Li ,Ji-lin Chen ,To Guo ,* ,Wen Ding ,Lin Jing ,Mio Yo ,Ji-xing Song ,Li-feng Xie ,Yi-ming Mo
a College of Field Engineering,Army Engineering University of PLA,Nanjing,210007,China
b School of Mechanical Engineering,Nanjing University of Science and Technology,Nanjing,210094,China
c Xi'an Rare Metal Materials Research Institute Co.,Ltd.,Xi'an,710016,China
Keywords:Ternary thermite Thermal kinetics Reaction mechanism Combustion
ABSTRACT To develop new energy enhancement energetic materials with great combustion performance and thermal stability,two kinds of ternary thermite,Al/Fe2O3/CuO and Al/Fe2O3/Bi2O3,were prepared and analyzed via mechanical ball milling.The samples were characterized by SEM,XRD,TG-DSC,constant volume and constant pressure combustion experiments.The first exothermic peaks of Al/Fe2O3/CuO and Al/Fe2O3/Bi2O3 appear at 579 °C and 564.5 °C,respectively.The corresponding activation energies are similar.The corresponding mechanism functions are set as G(α)=[-ln(1-α)]3/4 and G(α) =[-ln(1-α)]2/3,respectively,which belong to the Avrami-Erofeev equation.Al/Fe2O3/CuO has better thermal safety.For small dose samples,its critical temperature of thermal explosion is 121.05 °C higher than that of Al/Fe2O3/Bi2O3.During combustion,the flame of Al/Fe2O3/CuO is spherical,and the main products are FeAl2O4 and Cu.The flame of Al/Fe2O3/Bi2O3 is jet-like,and the main products are Al2O3,Bi and Fe.Al/Fe2O3/Bi2O3 has better ignition and gas production performance.Its average ignition energy is 4.2 J lower than that of Al/Fe2O3/CuO.Its average step-up rate is 28.29 MPa/s,which is much higher than 6.84 MPa/s of Al/Fe2O3/CuO.This paper provides a reference for studying the thermal safety and combustion performance of ternary thermite.
1.Introduction
The mixture of aluminum powder(Al)and metal oxides or nonmetal oxides that can cause violent redox reactions and release a lot of heat after excitation is defined as thermite [1].Commonly used oxides include iron oxide (Fe2O3) [2],chromium oxide (Cr2O3) [3],molybdenum oxide (MoO3) [4],bismuth oxide (Bi2O3) [5],copper oxide(CuO)[6],etc.The choice of oxide will affect the combustion performance of thermite.
According to the existing research data [7],the adiabatic combustion temperature and gas production performance of thermite with different oxides can be understood.Fe2O3,Cr2O3,MoO3and WO3as oxidant thermite have advantages in adiabatic combustion temperature,but the gas production performance is poor.However,with Bi2O3and CuO as oxidants,thermite has an advantage in gas yield.
Many researchers have been trying to improve the thermite properties by adding various reactants to the thermite.At present,the research on dual-fuel thermite has made some progress.Wang et al.[8]replaced Al powder with Al-Mg alloy as the Al/Fe2O3fuel and its ignition performance became better.Shen et al.[9]found that the heat release of Al/B/Fe2O3was 3.6 times that of Al/Fe2O3and its impact sensitivity also decreased.Nie et al.[10]prepared a bimetallic thermite containing Al and Ni and found that the introduction of Ni powder improved the anti-aging properties of the thermite.At the same time,the phenomenon of Al powder sintering and agglomeration in the combustion process is improved.It can be seen that the use of multi-component fuels brings performance improvements to the thermite.
However,the research on double oxide thermite is still relatively limited.At present,the use of double oxides is mostly by adding low-energy oxides to the thermite to achieve energy dilution.Daniel et al.[11]achieved the purpose of regulating the combustion rate and the total amount of energy release by adding SiO2to Al/Fe2O3.Kobyakov et al.[12]found that adjusting the TiO2content in the range of 0-0.7 can achieve a smooth adjustment of the heat release of the Fe2O3/TiO2/Al composite thermite mixture.Yang et al.[13]assembled nano Al and MnO2/SnO2into MnO2/SnO2/Al ternary aluminothermic film.The change of the content ratio of MnO2and SnO2can achieve the effect of controlling the energy performance of the ternary aluminothermic film.Therefore,it is theoretically an effective method to introduce oxides with gas-generating advantages into traditional iron-based thermite to improve its gasgenerating properties.
In this work,CuO and Bi2O3were introduced into Al/Fe2O3thermite,respectively.The thermal analysis kinetic mechanism,thermal safety and combustion performance of ternary thermites were also studied.
Field emission scanning electron microscopy (FE-SEM) and Mapping images were used to observe the morphology and distribution of samples.The samples were tested by Thermogravimetric-differential scanning calorimetry (TG-DSC)with multiple heating rates,and the results were used to calculate the thermal analysis kinetic mechanism and to analyze its thermal safety.In addition,the ignition energy,combustion pressure,combustion time and combustion phenomenon of the thermite samples were studied through constant volume combustion experiments and constant pressure combustion experiments.Finally,composition,morphology and elemental distribution of combustion residues were characterized by FE-SEM,X-ray diffractometer(XRD) and Mapping.The combustion mechanism was further deduced.
2.Experiment
2.1.Materials and preparation
All reagents were of analytical grade and can be used directly in experiments.Nanometer Al powder (20-80 nm) was purchased from Siping Gaosda Nano material equipment Co,Ltd,after the TG(air atmosphere)test and calculation,the active aluminum content of nano Al powder is 68.84%.Absolute ethanol was purchased from Shanghai Jiuyi Chemical Reagent Co,Ltd.Fe2O3,CuO and Bi2O3were all micron-sized.
The materials were weighed according to the mass ratio.(Al:0.25,Fe2O3:0.6,CuO:0.15)(Al:0.25,Fe2O3:0.505,Bi2O3:0.245).The high content of Bi2O3ensures that the quality of Al powder consumed by Bi2O3is similar to that of CuO.The material was put into a ball mill jar,and alcohol was also added and stirred.The ball milling was set at a speed of 100 r/s for 30 min.The mixed solution was poured into petri dishes and dried in a drying cabinet at 50°C.The dried samples were scraped and ground.
2.2.Measurements and characterizations
The combustion residues were tested by XRD (Rigaku SMARTLAB9) with Cu-kα radiation.The morphologies of the thermite samples were observed by FE-SEM analysis (FEI quanta 400FEG/SU8220).The thermite samples were heated by TG-DSC (Netzsch STA 449F5)under Ar atmosphere at heating rates of 10 K/min,15 K/min,20 K/min,25 K/min,ranging from Room temperature to 1100°C.
2.3.Constant volume combustion experiment
Fig.1 shows a constant volume combustion experimental device,which consists of a DC power,an oscilloscope,a PCB sensor,a voltage detector and a current detector.The sample is placed in an Al2O3crucible.The DC power source,wire,Nickel chrome (Ni-Cr)wire and thermite sample form a closed loop.The voltage at both ends of the Ni-Cr wire is adjusted until the sample is excited and ignited.Through the data collected by the sensor,the current,voltage and pressure curves can be displayed in the oscilloscope.The output forms of voltage,current and pressure signals are all voltage signals,denoted asV1,V2,V3.When calculating,the voltage value isV1(V),the current value isV2(A),and the pressure value is 5.63V3(MPa).
2.4.Constant pressure combustion experiment
Fig.2 shows an experimental device for constant pressure combustion.DC Power forms a closed loop with wire and Ni-Cr wire.The voltage at the Ni-Cr wire is adjusted until the sample is excited and ignited.At the same time,a high-speed camera(FASTCAM SA-Z Japan)is turned on to capture the burning process at a frame rate of 10,000 fps.An attenuated film is added to the lens to make the shooting clearer.

Fig.2.Experimental device for constant pressure combustion.
3.Results and discussion
3.1.Morphology characterization
As shown in Fig.3,FE-SEM was used to observe the micromorphology of the samples prepared by mechanical ball milling.The Mapping image shows the elemental distribution of the sample.Fig.3(a) and Fig.3(b) are the microstructures of Al/Fe2O3/CuO and Al/Fe2O3/Bi2O3,respectively.Fig.3(c)-Fig.3(e)are the original SEM images of Fe2O3,CuO and Bi2O3,respectively.Fig.3(f) and Fig.3(g)are the elemental mapping images of Al/Fe2O3/CuO and Al/Fe2O3/Bi2O3,respectively.The smaller spherical particles in the picture are nano-Al powders,which are attached to the surface of oxide particles with larger particle size.The particles of nano-Al powder are complete,which shows that the crushing effect of ball milling on small particles is not obvious.It can be seen from the SEM image combined with the element distribution that there is partial agglomeration in the Al powder.The primary particle size of the oxide is in the micrometer scale.By comparing the original particle size of the oxide with the average particle size of the sample particles after ball milling,it can be found that the mechanical ball milling has a greater destructive effect on CuO and Bi2O3.The original samples of CuO and Bi2O3have larger particles,with a large number of particles exceeding 5 μm in size.The size of the large particles in the sample after ball milling is about 1um,indicating that the ball milling makes CuO and Bi2O3irregularly broken.The mapping image of the sample shows the distribution of Al,Cu,Fe,and Bi elements.The color map of the elements shows that the components are evenly distributed.
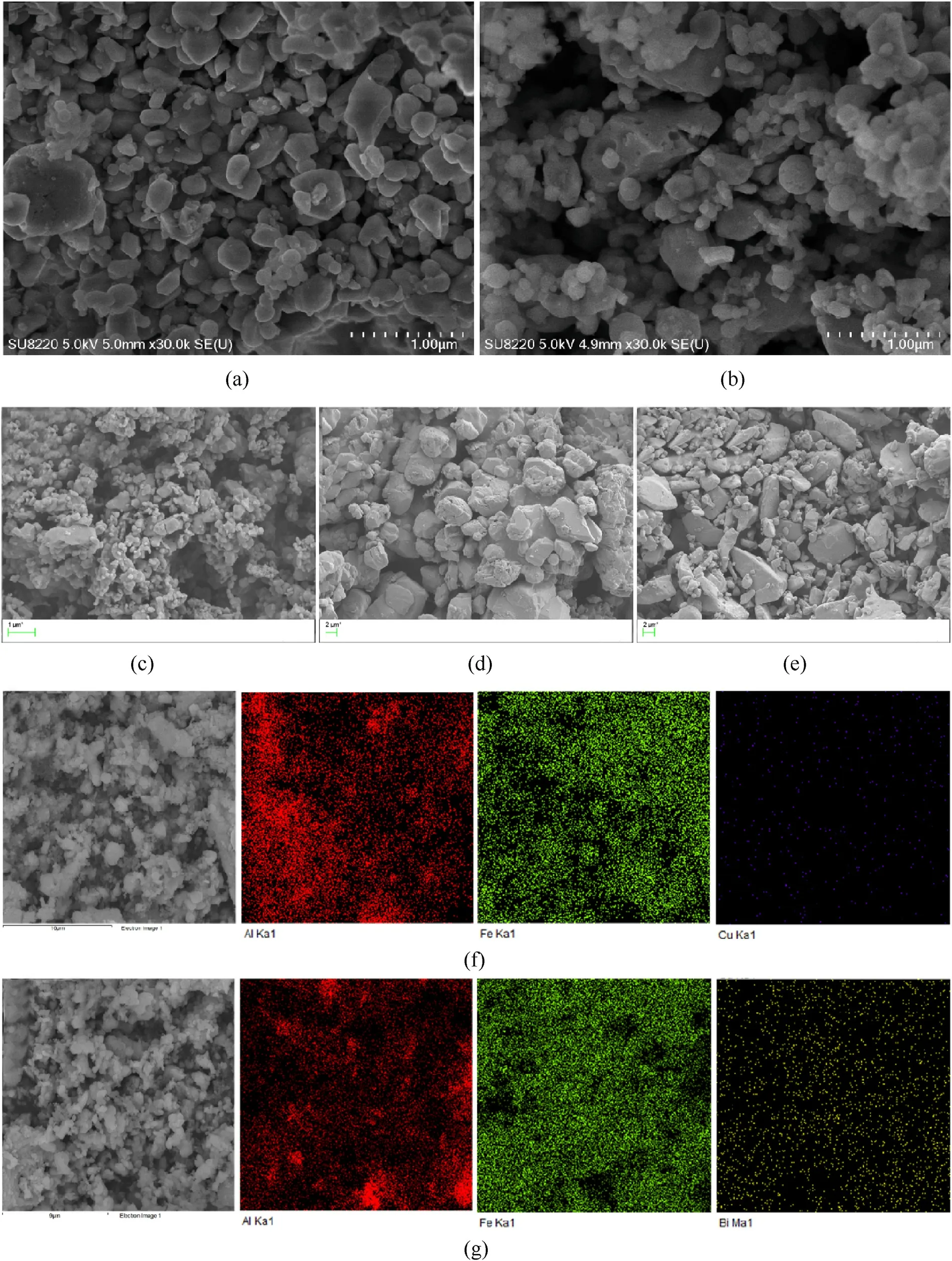
Fig.3.SEM and Mapping picture of the sample after ball milling: (a) and (f) Al/Fe2O3/CuO;(b) and (g) Al/Fe2O3/Bi2O3.(c) Fe2O3;(d) CuO;(e) Bi2O3.
3.2.DSC analysis
TG-DSC results reflect the thermal reactivity of thermite samples under linear heating conditions.Fig.4(a) shows the TG-DSC curve of the Al/Fe2O3/CuO thermite with a heating rate of 10 K/min.The mass of the sample in the experiment is 14.4571 mg.Two exothermic peaks and two endothermic peaks appear during the heating process.The peak temperature of endothermic peak A is 249.7°C and the TG curve drops at this time,indicating that mass loss occurs at this time.This is because solvents such as alcohol remaining in the sample are volatilized by heat.The Al powder melts and endotherms at 655.6°C,resulting in a sharp endothermic peak C.The first exothermic peak B appeared at 579.0°C,which was a solid-solid aluminothermic reaction.The second exothermic peak D appeared at 763.6°C,which was a solid-liquid phase aluminothermic reaction.

Fig.4.DSC curves of the samples: (a) Al/Fe2O3/CuO;(b)Al/Fe2O3/Bi2O3.
Fig.4(b)shows the TG-DSC curve of the Al/Fe2O3/Bi2O3thermite with a heating rate of 10 K/min.The mass of the sample in the experiment is 12.1312 mg.The endothermic peaks E and H appeared at 248.7°C and 660.5°C,respectively.The former was caused by the volatilization of the residual solvent in the sample and the latter was caused by the melting endotherm of the Al powder.Two exothermic peaks F and G appear at 564.5°C and 637.0°C,both of which are solid-state aluminothermic reactions.The third exothermic peak I appeared at 830°C,which was a liquidsolid phase aluminothermic reaction.
The aluminothermic reactions of Al/Fe2O3/Bi2O3and Al/Fe2O3/CuO can be divided into two parts.The first stage is dominated by solid-solid phase aluminothermic reaction,and the second stage is dominated by liquid-solid phase aluminothermic reaction.However,in the solid-solid phase thermite reaction stage,the two ternary thermites showed obvious differences.Al/Fe2O3/CuO showed only one exothermic peak,while Al/Fe2O3/Bi2O3showed two consecutive exothermic peaks.At this stage,the particles of the reactants are smaller,which can be considered as the local reaction of the nano-thermite.Referring to the previous research results[14,15],it can be found that the exothermic peak temperatures of nano-Al/Fe2O3and nano-Al/CuO are very close.The exothermic peak temperature difference between nano-Al/Fe2O3and nano-Al/Bi2O3is larger.Therefore,the following speculation can be made:in Al/Fe2O3/CuO,the exothermic peaks of Al/Fe2O3and Al/CuO will merge into one exothermic peak.In Al/Fe2O3/Bi2O3,there are two adjacent exothermic peaks,Al/Fe2O3and Al/Bi2O3.
3.3.Thermal safety analysis
It can be seen from Fig.3 that the particle size distribution of the internal components of the thermite sample is uneven and it belongs to the particle-type heterogeneous energetic material.Under thermal stimulation,hot spots tend to form at localized areas[16,17]where nano-Al powders contact small-sized oxides,also known as defects [18].The continuous input of external heat and the accumulation of heat generated by the hot spot enlarge the hot spot area [19].Meanwhile,the thermite reaction is a selfpropagating combustion [20,21].Therefore,the appearance of localized hot spots may further lead to overall thermal runaway or even explosion.In conclusion,the first exothermic peak in the DSC curve of the thermite sample is the key to determine its thermal safety.Its activation energy,mechanism function and critical temperature of thermal explosion need to be carefully studied.In addition,the quality of the sample being tested will affect the calculation of the critical temperature of thermal explosion [22].Therefore,the calculated critical temperature of thermal explosion is suitable for a small dose of the corresponding thermite.
3.3.1.Activation energy calculation
As shown in Fig.5,DSC with different heating rates was used to test the two samples to obtain the activation energy (E) of the thermite system,the most probable mechanism function (G(α))and to analyze its thermal safety.Erepresents the ease with which the thermite reaction occurs.G(α) represents the reaction mechanism of the thermite reaction.This work adopts the Kissinger method and Flynn-Wall-Ozawa(FWO)method to calculate the E of the thermite system.

Fig.5.Multiple heating rate DSC curves: (a) Al/Fe2O3/CuO;(b)Al/Fe2O3/Bi2O3.
The calculation formula of Kissinger method is [23-25].
Tais the peak reaction temperature (K),E1is the activation energy (J/mol),and β is the linear heating rate (K/min),Ais the preexponential factor (s-1),andRis the universal gas constant(8.314 J mol-1K-1).

Fig.6.Thermodynamic calculations fit straight lines: (a) and (b) Kissinger method;(c) and (d): FWO method.(e) and (f): the universal integration method.
The calculation formula of FWO method is [26,27].
Tais the peak reaction temperature (K),E2is the activation energy (J/mol),and β is the linear heating rate (K/min),Ais the preexponential factor (s-1),andRis the universal gas constant(8.314 J·mol-1·K-1).
lg β and 1/Taare linearly fitted to a straight line.The slope is used to calculate theE2.As shown in Fig.6(c) and Fig.6(d),the fitting equation of Al/Fe2O3/CuO isy=-11378.87x+14.35,the fitting equation of Al/Fe2O3/Bi2O3isy=-11296.14x+14.54.
3.3.2.Mechanism function
The thermal reaction of solid powder generally follows the laws of thermodynamics and the most probable mechanism function can describe the mechanism of thermal reaction.The method used to screen the mechanism function in thermite is the universal integration method [28].
The calculation formula of universal integration method is[29,30].
Tais the peak reaction temperature(K),T0is the initial reaction temperature (K),E0is the activation energy (J/mol),and β is the linear heating rate (K/min),A is the pre-exponential factor (s-1),andRis the universal gas constant (8.314 J·mol-1·K-1).
G(α) is the mechanism function which has 41 forms [30].The conversion ratio(αi)at different temperatures(Ti)was obtained by processing the DSC curve data.(i =1,2,3…12)The reaction starts temperature and end temperature take the closest value in the data set.
ln(G(α)/Ta-T0) and 1/Taare linearly fitted to obtain the correlation coefficient (R),the residual sum of squares (Q),activation energy (E0) and pre-exponential factor (A) under each mechanism function.There are two screening criteria.The first is that the smallerQis the better,the largerRis the better and the values ofE0andAare reasonable.The second rule is that the value ofE0is the closest to the value ofE1andE2(see Table 1).Note:To reduce computation,Q is computed for the first four data points.
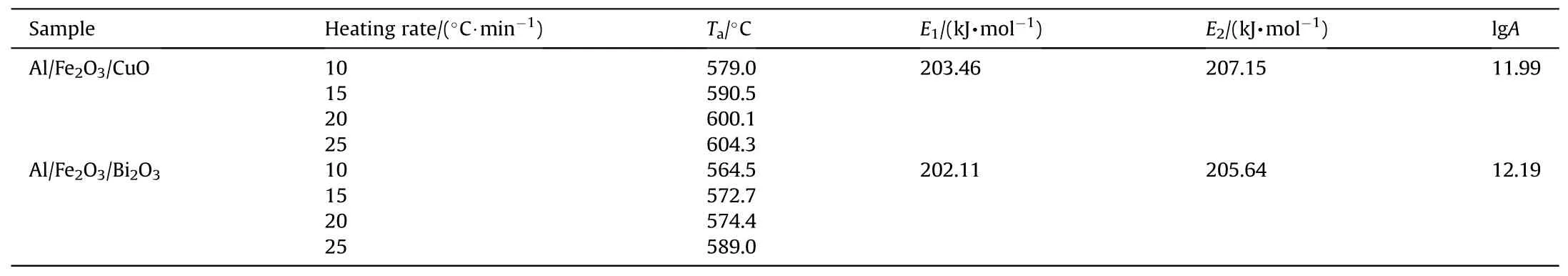
Table 1DSC curve data and activation energy results.
After the first criterion screening,the most probable mechanism functions of the thermal analysis kinetic equation of both ternary thermites are 14-16,28-30.The filtered data are shown in Table 2 and Table 3.

Table 2Screening results of mechanism function of Al/Fe2O3/CuO.

Table 3Screening results of mechanism function of Al/Fe2O3/Bi2O3.
Finally,the most probable mechanism function of Al/Fe2O3/CuO is No.15 function,G(α) =[-ln(1-α)]3/4,which belongs to the Avrami-Erofeev equation,n=3/4.The most probable mechanism function of Al/Fe2O3/Bi2O3is No.14 function,G(α) =[-ln(1-α)]2/3,which belongs to the Avrami-Erofeev equation,n=2/3.
The mechanism function obtained by screening is substituted into Eq.(3) and the fitting calculation is carried out.Fig.6(e) and Fig.6(f) show that the fitting equation of Al/Fe2O3/CuO isy= -24615.33x+25.18,and the fitting equation of Al/Fe2O3/Bi2O3isy=-252574.91x+26.70.
3.3.3.Critical temperature of thermal explosion
Energetic materials could burn and explode due to external thermal stimulation during storage and transportation [31].As a kind of energetic material,it is necessary to evaluate the thermal safety of thermite.The critical temperature of thermal explosion(Tb)is one of the key parameters.The calculation method ofTbcan be expressed as followed [32,33]:
whereEis the activation energy (the average ofE1andE2),which can reflect the difficulty of exciting the chemical reaction.Ta0is the peak temperature when the heating rate(β) is close to 0 and it is also the self-accelerating decomposition temperature of energetic materials.a,b and c are fitting coefficients.After calculation,the Tbof Al/Fe2O3/CuO is 594.80°C.The Tbof Al/Fe2O3/CuO is 473.75°C.
In conclusion,the self-accelerating decomposition temperature of a small dose of Al/Fe2O3/CuO thermite is 564.3°C,and the critical temperature of thermal explosion is 594.80°C.The selfaccelerating decomposition temperature of a small dose of Al/Fe2O3/Bi2O3thermite is 451°C,and the critical temperature of thermal explosion is 473.75°C.In contrast,for small doses of ternary thermite,Al/Fe2O3/CuO is more difficult to change from thermal decomposition or combustion to explosion and thus has higher safety.
3.4.Combustion performance analysis
3.4.1.Constant volume combustion experiment
The constant volume combustion experiment was carried out through the experimental device shown in Fig.1.Electric heating ignition,laser ignition [34]and impact ignition [35]are the most common ignition methods for energetic materials at present.The methods used in the combustion experiments in this work are all electric heating ignition.After the switch is closed,waveforms such as voltage (E),current (I) flowing through the Ni-Cr wire and burning pressure(P)can be obtained in the oscilloscope.From the time the switch is closed until the pressure starts to rise,the electric energy consumed during this period is considered to be all used to stimulate ignition,which is defined as ignition energy (W),which can be expressed asW=∫EIdt.Ignition energy can reflect the ignition performance of thermite,that is the difficulty of being ignited under the action of external energy.Combustion pressure is used to measure the gas production performance of thermite.The amount of samples in each group was 30 mg and each sample was tested twice.The average value of the obtained results is taken as the final result,and the specific calculation data are shown in Table 4.

Table 4Ignition energy and boost performance of thermite.
Fig.7 shows the pressure changes of Al/Fe2O3/CuO and Al/Fe2O3/Bi2O3during combustion,respectively.It can be seen that there is an ignition delay time after the switch is closed,the thermite sample is excited to ignite and the pressure in the container rises rapidly.The ignition energy of Al/Fe2O3/CuO is 16.48 J,the peak pressure is 0.40 MPa and the average pressure increase rate is 6.84 MPa/s.The ignition energy of Al/Fe2O3/Bi2O3is 12.28 J,the peak pressure is 0.91 MPa and the average pressure increase rate is 28.29 MPa/s.
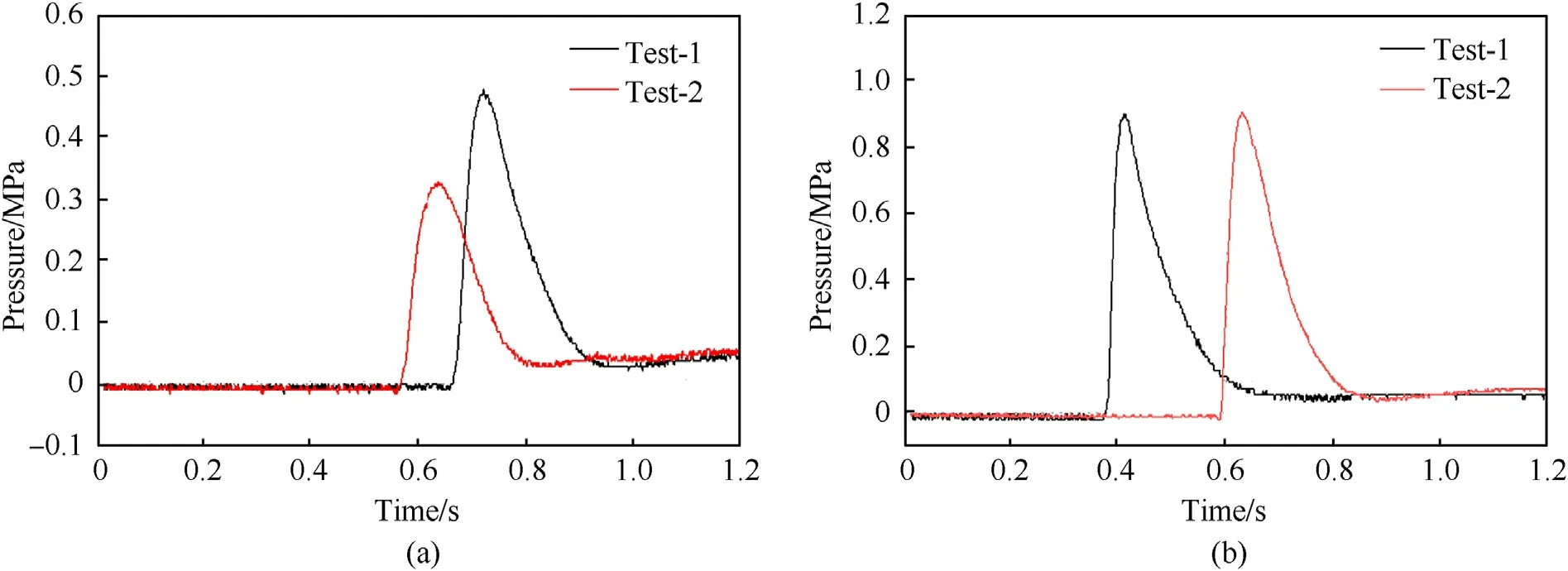
Fig.7.Combustion pressure-time curve: (a) Al/Fe2O3/CuO;(b) Al/Fe2O3/Bi2O3.
In comparison,Al/Fe2O3/Bi2O3is easier to stimulate ignition.At the same time,its gas production performance is better than that of Al/Fe2O3/CuO.The gas production performance of the thermite mainly depends on the combustion rate and the melting and boiling point of the product.The boiling points of Bi,Bi2O3and Cu are 1560°C,1890°C and 2567°C,respectively.During the combustion process,Bi has a lower boiling point and forms gas at high temperature,increasing the gas yield.This increases the pressure in the container,making the contact between the reactants more intimate,and promoting the combustion.It can also be seen from the experimental results of combustion that the combustion rate of Al/Fe2O3/Bi2O3is higher than that of Al/Fe2O3/CuO under constant volume conditions.
3.4.2.Constant pressure combustion experiments
Fig.8 shows the combustion images of two groups of thermites captured by a high-speed camera.The recording time of the initial burning picture was taken as the starting point of burning and was recorded as 0 ms.The amount of samples in each group was 30 mg.

Fig.8.Constant pressure combustion experiments phenomenon: (a) Al/Fe2O3/CuO;(b)Al/Fe2O3/Bi2O3.
The constant pressure combustion experiments has no container constraints and can be regarded as burning under constant pressure conditions.The combustion duration of the two thermites is about 70 ms,indicating that the combustion of the thermite is a very rapid process.Fig.8(a) shows the combustion phenomenon of Al/Fe2O3/CuO.The combustion flame is spherical and sparks are scattered during the combustion process.This indicates that a small amount of gas is generated during the combustion process.Fig.8(b)shows the combustion phenomenon of Al/Fe2O3/Bi2O3.Its combustion flame is jet-like and accompanied by an explosion sound.This means that a large amount of gas generated in the combustion process.A fireball appears on top of the flame in the picture.At the end of the combustion,yellow smoke appeared over the experimental unit.It is inferred that the fireball is Bi metal gasified at high temperature.
The combustion phenomenon in the open environment is in good agreement with the pressure data measured in the constant volume combustion experiment.Al/Fe2O3/Bi2O3has higher combustion gas yield than Al/Fe2O3/CuO.At the same time,it also shows that the pressure has an effect on the combustion rate.By comparing the burning time of two kinds of thermite in constant pressure combustion experiment and constant volume combustion experiment,it can be found that the higher the pressure,the faster the corresponding combustion rate.
3.4.3.Combustion product analysis
After the constant pressure combustion experiment and constant volume combustion experiment,the residues after combustion of the thermite samples were collected.Fig.9 is an electron microscope image and XRD of the combustion residue.Fig.9(a)-Fig.9(c) show the combustion residues of Al/Fe2O3/CuO.Fig.9(b)shows that the combustion residues of Al/Fe2O3/CuO are mainly FeAl2O4and Cu.The marked area in Fig.9(c)illustrates the overflow of active Al following the rupture of the Al2O3shell in the thermite reaction.The reactants gradually sintered together during the combustion process.Corresponding to the phenomenon of largescale agglomeration of combustion products in Fig.9(a).From the Mapping images,it can be seen that Al,Fe and O elements are packed together,which corresponds to the FeAl2O4phase shown in XRD.At the same time,the distribution of Cu elements is displayed on the particle surface,which corresponds to the Cu phase shown in XRD.It is presumed that a small amount of Cu gasified and solidified on the surface of the product during the combustion.Fig.9(d)-Fig.9(f)show the combustion residues of Al/Fe2O3/Bi2O3.Fig.9(e)shows that the combustion products of Al/Fe2O3/Bi2O3are mainly Al2O3,Bi and Fe.Similar to Al/Fe2O3/CuO,the combustion products of Al/Fe2O3/Bi2O3also have sintering and agglomeration phenomenon.However,the surface of the product particles is rougher with more fine particles attached.It is presumed that the generated Bi vapor cooled and adhered to the surface of the residue.
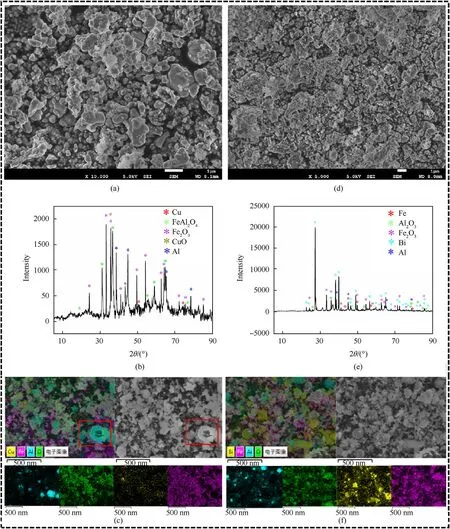
Fig.9.Combustion residue analysis chart: (a)-(c) Al/Fe2O3/CuO;(d)-(f) Al/Fe2O3/Bi2O3.
3.4.4.Combustion mechanism analysis
At present,there are two mainstream views on the occurrence of aluminothermic reaction.One is that the oxide is decomposed at high temperature to release oxygen(O2).The Al powder particles are oxidized and burned with O2as the medium [36].The other is that the Al powder particles are in direct contact with the oxide.The Al powder particles are oxidized and burned in the medium of lattice oxygen [36].
Fig.10 shows the combustion mechanism of thermite.Electric heating wire excitation ignition is a fast-heating method to realize ignition.Electric energy is converted into heat energy by using electric wire as the medium.The particle size distribution of the internal components of thermite is not uniform.When the temperature of the electric wire exceeds the critical temperature of the thermal explosion of the thermite,the hot spot will be formed at the small particles inside and the position distribution is random.This also corresponds to the initial reaction mechanism function obtained in the previous screening,that is random nucleation and subsequent growth.
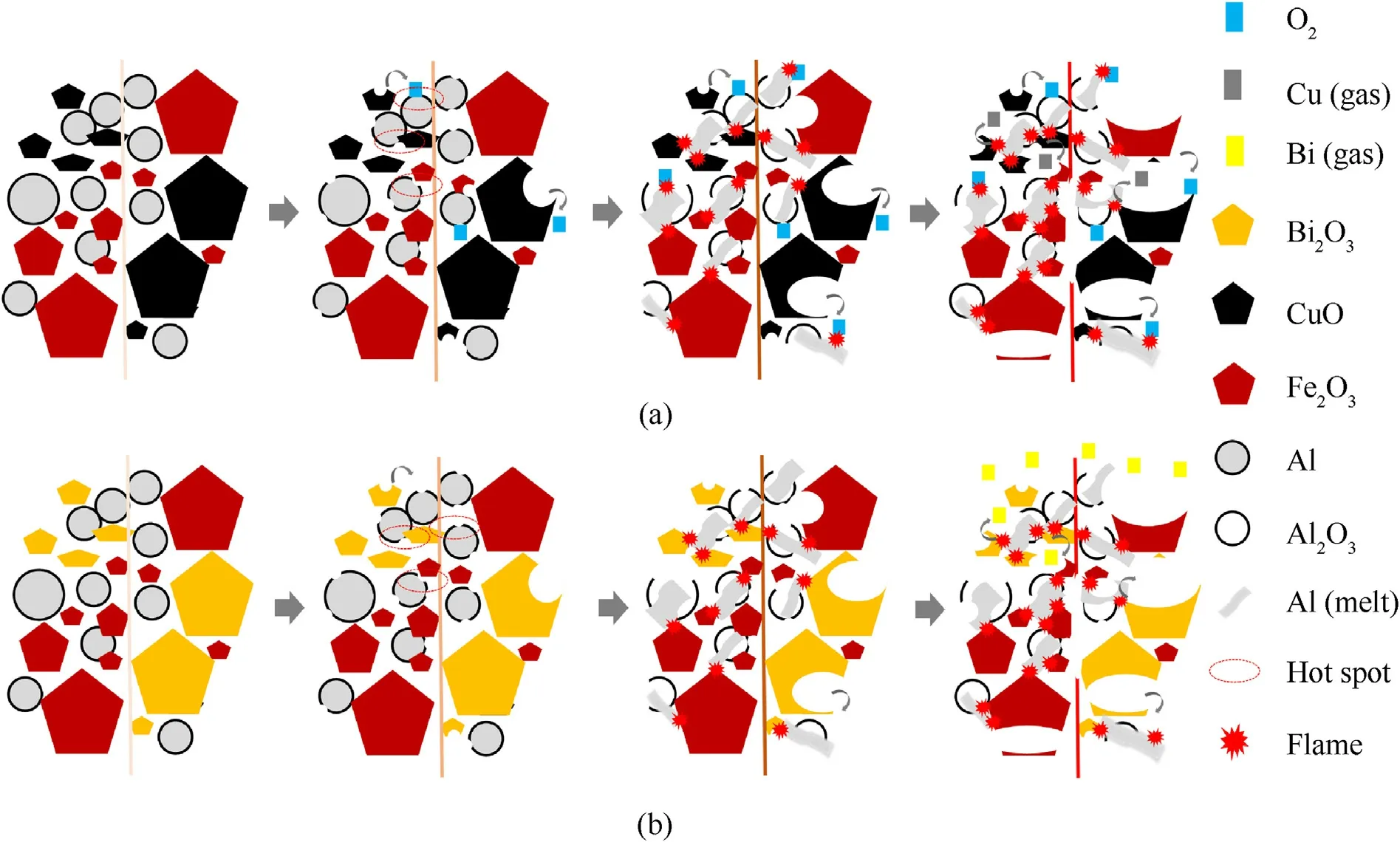
Fig.10.Combustion mechanism analysis diagram: (a) Al/Fe2O3/CuO;(b) Al/Fe2O3/Bi2O3.
According to the previous calculation results,the thermal critical explosion temperatures of Al/Fe2O3/CuO and Al/Fe2O3/Bi2O3are 594.8°C and 473.75°C,respectively.At this time,the Al powder has not yet melted.But the Al2O3shell on the surface of the Al powder will undergo phase transformation and transform into a higher density crystal form.This leads to the rupture of the alumina shell,resulting in the appearance of an exposed active Al surface[37].In Al/Fe2O3/CuO,CuO begins partial thermal decomposition [38],releasing a small amount of O2.So there are two ways to form hot spots[36].One is the condensed phase diffusion mediated by lattice oxygen[37],that is,the exposed active Al is in direct contact with the oxide with smaller particle size to produce aluminothermic reaction.The other is the gas phase diffusion mediated by free O2[39],that is,the exposed active Al is oxidized by the O2released internally.However,in Al/Fe2O3/Bi2O3,the oxides are not thermally decomposed [36].Therefore,the formation of internal hot spots depends on the direct contact between exposed active Al and oxides with smaller particle size[40]to realize the condensed phase diffusion of lattice oxygen[39].Under the continuous heating of the electric heating wire,the number of hot spots increases gradually.Eventually,this will cause the thermite to ignite and burn continuously.
In the process of combustion,the overall temperature rises rapidly.When the temperature is higher than the melting point of Al,the volume of active Al expands due to melting.The outer Al2O3shell is ruptured in a large area due to radial pressure[41],resulting in the eruption of partially molten Al inside,as marked in Fig.9.Molten Al reacts with oxides,sintered and agglomerated[42,43]to form large particles.
As can be seen from Fig.8,a large amount of gas is produced during combustion.Corresponding to the pressure curve in Fig.7,the injection phenomenon of Al/Fe2O3/Bi2O3is more severe than that of Al/Fe2O3/CuO.Sparks spattered around during the combustion of Al/Fe2O3/CuO,and fireballs ejected upward during the combustion of Al/Fe2O3/Bi2O3.Combined with the XRD and Mapping images in Fig.9,it is inferred that sparks is a part of Cu vapor and the fireball is Bi vapor.The distribution range of Bi is obviously higher than that of Cu,which also confirms the increase of gas production.
Combined with the XRD test results of combustion products,it is inferred that the reactions that occur after ignition include:
A large amount of gas produced during combustion can cause the flame sputtering phenomenon shown in Fig.8.This will lead to the incomplete reaction of many samples due to outward spray,resulting in a lot of reaction phases in the residues.
4.Conclusions
In this work,Al/Fe2O3/CuO and Al/Fe2O3/Bi2O3ternary thermite were prepared by mechanical ball milling.The thermal safety and combustion performance of thermite were analyzed by SEM,XRD,TG-DSC,constant volume combustion test and constant pressure combustion test.It is feasible for CuO and Bi2O3to introduce Al/Fe2O3to form ternary thermite to obtain excellent gas production performance.And it shows different effects.
(1) The activation energies of the first exothermic peak of Al/Fe2O3/CuO and Al/Fe2O3/Bi2O3are similar,and the reaction mechanism functions belong to Avrami-Erofeev equation,which are set asG(α)=[-ln(1-α)]3/4andG(α) =[-ln(1-α)]2/3,respectively.
(2) Al/Fe2O3/CuO has higher thermal safety.Thermal critical explosion temperature was calculated based on DSC data of small dose samples.The critical temperature of thermal explosion of Al/Fe2O3/CuO is about 121.05°C higher than that of Al/Fe2O3/Bi2O3.In the application of large-scale gas demand engineering,good gas production and high safety are equally important.
(3) Al/Fe2O3/Bi2O3has better ignition and gas production performance.The ignition energy of Al/Fe2O3/Bi2O3is lower,and its average boost rate is about 4.14 times that of Al/Fe2O3/CuO.It has more application value in the field of microthruster fuel.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors declare that they have no known possible financial disputes or personal relationships that may have influenced the work reported in this paper.This work is supported by the National Natural Science Foundation of China,project number: 51704302 and the Natural Science Foundation of Shaanxi Province,China,project number: Grant No.2020JC-50.
杂志排行
Defence Technology的其它文章
- Numerical simulation of flow field characteristics and theimprovement of pressure oscillation of rotating detonation engine
- Tuning energy output of PTFE/Al composite materials through gradient structure
- The dynamic response of a high-density polyethylene slow-release structure under launching overload
- Adaptive saturated tracking control for solid launch vehicles in ascending based on differential inclusion stabilization
- Impact point prediction guidance of ballistic missile in high maneuver penetration condition
- Deep learning-based method for detecting anomalies in electromagnetic environment situation
