大麻素Ⅱ型受体对LPS诱导小鼠黑质区COX-2和iNOS基因表达的影响
2023-08-26王炳超孙琳朱天立马泽刚
王炳超 孙琳 朱天立 马泽刚

[摘要]目的探索大麻素Ⅱ型(CB2)受體对脂多糖(LPS)诱导小鼠黑质(SN)区炎症反应的作用。方法将18只8周龄雄性野生型(WT)C57BL/6小鼠随机分为WT对照组、WT LPS组和WT LPS+JWH133(CB2受体激动剂)组,12只8周龄雄性CB2受体敲除(CB2-KO)C57BL/6小鼠随机分为CB2-KO对照组和CB2-KO LPS组。对照组小鼠单次双侧SN立体定位注射生理盐水,其余各组小鼠注射等体积的LPS,然后连续腹腔注射JWH133或生理盐水14 d。应用实时荧光定量PCR技术检测各组小鼠SN中环氧化酶2(COX-2)和诱导型一氧化氮合酶(iNOS)基因的表达。结果与WT对照组相比,WT LPS组小鼠SN区COX-2和iNOS基因表达水平升高,差异有统计学意义(F=20.9、21.4,q=5.536、5.518,P<0.01);JWH133能明显抑制LPS诱导的COX-2和iNOS基因表达上调(q=5.170、4.553,P<0.05);与WT LPS组相比,CB2-KO LPS组小鼠COX-2和iNOS基因表达明显上调,差异有统计学意义(q=4.150、5.496,P<0.05)。结论激活CB2受体能够抑制LPS诱导小鼠SN区COX-2和iNOS基因的表达,缺失CB2受体能够促进LPS诱导小鼠SN区COX-2和iNOS基因的表达。
[关键词]受体,大麻酚,CB2;脂多糖类;炎症;环氧化酶2;一氧化氮合酶
[中图分类号]R338.2[文献标志码]A[文章编号]2096-5532(2023)03-0371-04
doi:10.11712/jms.2096-5532.2023.59.086[开放科学(资源服务)标识码(OSID)]
[网络出版]https://kns.cnki.net/kcms2/detail/37.1517.r.20230731.1044.001.html;2023-07-3117:11:34
EFFECT OF CANNABINOID TYPE Ⅱ RECEPTORS ON LIPOPOLYSACCHARIDE-INDUCED GENE EXPRESSION OF CYCLOO-XYGENASE-2 AND INDUCIBLE NITRIC OXIDE SYNTHASE IN THE SUBSTANTIA NIGRA OF MICEWANG Bingchao, SUN Lin, ZHU Tianli, MA Zegang (Department of Physiology, School of Basic Medicine, Qingdao University, Qingdao 266071, China)
[ABSTRACT]ObjectiveTo investigate the effect of cannabinoid receptor-2 (CB2) on lipopolysaccharide (LPS)-induced inflammatory response in the substantia nigra (SN) of mice. MethodsA total of 18 male C57BL/6 wild-type (WT) mice, aged 8 weeks, were randomly divided into WT control group, WT LPS group, and WT LPS+JWH133 (a CB2 receptor agonist) group, and 12 male CB2 receptor-knockout (CB2-KO) C57BL/6 mice were randomly divided into CB2-KO control group and CB2-KO LPS group. The mice in the control group received a single stereotactic injection of normal saline into the bilateral SN, and those in the other groups were injected with an equal volume of LPS, followed by the intraperitoneal injection of JWH133 or normal saline for 14 consecutive days. Quantitative real-time PCR was used to measure the mRNA expression levels of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) in the SN. ResultsCompared with the WT control group, the WT LPS group had significant increases in the mRNA expression levels of COX-2 and iNOS in the SN (F=20.9,21.4;q=5.536,5.518;P<0.01). JWH133 significantly inhibited the upregulated mRNA expression of COX-2 and iNOS induced by LPS (q=5.170,4.553;P<0.05). Compared with the WT LPS group, the CB2-KO LPS group had significant increases in the mRNA expression levels of COX-2 and iNOS (q=4.150,5.496;P<0.05). ConclusionActivation of CB2 receptor can inhibit LPS-induced mRNA expression of COX-2 and iNOS in the SN of mice, while deletion of CB2 receptor can promote LPS-induced mRNA expression of COX-2 and iNOS.
[KEY WORDS]receptor, cannabinoid, CB2; lipopolysaccharides; inflammation; cyclooxygenase 2; nitric oxide synthase
炎症是免疫系统保护生物体免受有害因素伤害的一种生物反应,然而过度的炎症反应可能导致机体损伤和疾病的产生[1-2]。近年来研究发现,神经炎症是帕金森病(PD)发病的关键因素[3]。有临床研究证实,在PD病人死后的中脑黑质(SN)中,除了观察到多巴胺(DA)能神经元丢失之外,还检测到活化的胶质细胞和大量的炎症因子[4-5],这表明神经炎症参与了PD的发病。因此,有效抑制胶质细胞介导的炎症反应,可能有助于PD的治疗。大麻素Ⅱ型(CB2)受体属于G蛋白偶联受体,在中枢神经系统中主要表达于胶质细胞[6]。最近CB2受体成为治疗PD的关键靶点[7]。本实验室研究发现,激活CB2受体能够抑制1-甲基-4-苯基-吡啶阳离子引起的星形胶质细胞环氧化酶2(COX-2)和诱导性一氧化氮合酶(iNOS)的表达,抑制星形胶质细胞的炎症反应[8]。iNOS和COX-2是调节炎症反应的关键酶,参与炎症因子的过度产生[9]。然而CB2受体在PD体内模型中研究较少,激活该受体能否抑制脂多糖(LPS)诱导的SN区COX-2和iNOS基因表达,目前尚不清楚。因此,本研究应用LPS制备PD小鼠炎症模型,探讨CB2受体对LPS诱导小鼠SN区COX-2和iNOS基因表达的影响。现将结果报告如下。
1材料与方法
1.1实验动物及试剂
SPF級雄性健康C57BL/6野生型(WT)以及CB2受体敲除(CB2-KO)小鼠,8周龄,体质量18~22 g,其中WT小鼠购于北京维通利华公司,CB2-KO小鼠由美国巴罗神经研究所赠予。小鼠每3~4只一笼,饲养环境:室温23~26 ℃,湿度为40%~60%,12-12 h昼夜循环光照,可自由饮水进食。实验开始前小鼠需要适应饲养环境1周。JWH133(CB2受体激动剂)购于美国APE x BIO公司;LPS购于美国Sigma公司;TRIzol购于美国Life Technologies公司;RNA逆转录试剂盒、SYBR Green购于南京诺唯赞生物科技股份有限公司;PCR引物购于日本Takara公司;其他试剂均为国产分析纯。
1.2动物分组及处理
将18只8周龄雄性WT C57BL/6小鼠随机分为WT对照组(A组)、WT LPS组(B组)和WT LPS+JWH133组(C组),12只8周龄雄性CB2-KO C57BL/6小鼠随机分为CB2-KO对照组(D组)和CB2-KO LPS组(E组),每组6只。小鼠预先腹腔注射JWH133(100 μg·kg-1·d-1)或生理盐水2 d。24 h后,参照以往文献的方法,以脑立体定位注射的方式,单次双侧SN区注射0.2 g/L的LPS 0.6 μL建立PD动物模型[10-11],对照组小鼠注射等体积的生理盐水。SN区注射LPS 1 h后,再连续腹腔注射JWH133(100 μg·kg-1·d-1)或生理盐水14 d[12]。
1.3实时荧光定量PCR(qRT-PCR)检测COX-2和iNOS mRNA水平
药物处理后,用异氟烷完全麻醉小鼠,迅速断头取脑,用刀片将小脑部位切除,然后沿大脑腹侧视神经根部垂直切开脑组织,SN的轮廓在大脑的冠状切面即可见到,用眼科镊夹取出SN置于离心管中,加入500 μL的TRIzol,提取小鼠SN总RNA。取1 μg总RNA使用反转录试剂盒进行反转录,加入4×gDNA wiper Mix 4 μL,加入RNase free water至16 μL,混匀。反应(42 ℃ 2 min,4 ℃ 3 min)完成后加入5×HiScript Ⅲ qRT SuperMix 4 μL,总体积20 μL,混匀。反应(37 ℃ 15 min,85 ℃ 5 s)后得到cDNA。采用SYBR Green染料法定量检测COX-2、iNOS以及GAPDH基因的表达[13]。采用2-△△CT法计算目的基因相对表达量。qRT-PCR检测所用引物及序列见表1。
1.4统计学处理
应用GraphPad Prism 7.0软件对数据进行统计处理。实验所得数据以±s表示,多组间比较采用单因素方差分析(One-way ANOVA),并应用Turkey法进行两两比较,P<0.05表示差异有统计学意义。
2结果
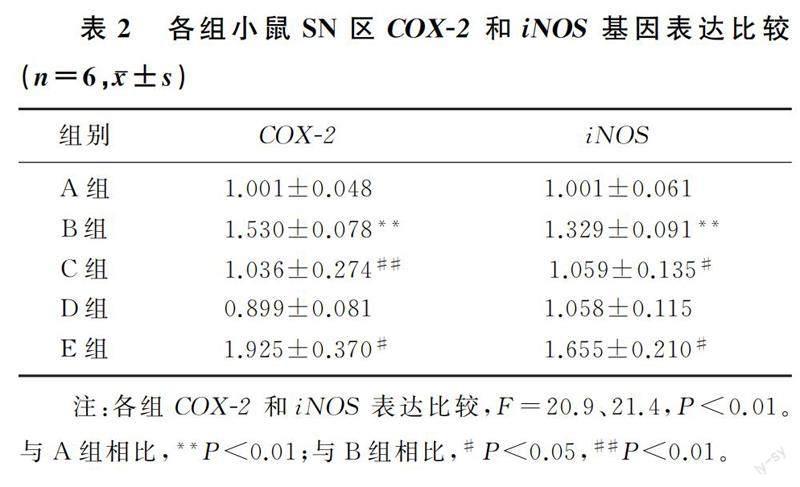
与WT对照组相比较,WT LPS组小鼠SN区COX-2和iNOS基因表达水平升高,差异有统计学意义(F=20.9、21.4,q=5.536、5.518,P<0.01);JWH133处理能够明显抑制LPS诱导的COX-2和iNOS基因表达上调(q=5.170、4.553,P<0.05);与WT LPS组小鼠比较,CB2-KO LPS组小鼠SN区COX-2和iNOS基因表达明显上调,差异有统计学意义(q=4.150、5.496,P<0.05);CB2-KO对照组小鼠COX-2和iNOS基因表达水平与WT对照组相比较差异无统计学意义(q=1.071、0.940,P>0.05)。见表2。
3讨论
PD是继阿尔茨海默病(AD)之后最常见的慢性神经退行性疾病,其病理学特征是中脑SN中DA能神经元的进行性丢失[14]。PD炎症反应的特征是SN中胶质细胞激活,引起COX-2和iNOS等炎症递质增加,诱导产生大量的炎症因子,造成对DA能神经元的损伤。有研究表明,SN区微量注射LPS可以引起局部的免疫反应,并产生PD特征性的黑质纹状体通路局部病变[10-11]。因此,本实验应用脑立体定位技术在SN区注射LPS建立PD小鼠模型,并且采用qRT-PCR方法检测SN中COX-2和iNOS基因表达水平。COX-2和iNOS是炎症反应的重要参与者。当有炎症刺激时,COX-2表达增加,催化合成更多的前列腺素,产生的前列腺素一方面加重了炎症反应,另一方面诱导iNOS表达增加,从而催化产生更多的一氧化氮,进一步加重炎症反应,造成神经元的死亡[9]。所以,有效地抑制SN区COX-2和iNOS的表达,可对DA能神经元发挥保护作用。CB2受体是内源性大麻素系统(ECS)的组成部分之一,ECS由两种内源性大麻素和大麻素Ⅰ、Ⅱ型(CB1和CB2)受体以及合成和降解它们的酶组成[15-16]。不同于CB1受体的激活,CB2受体的激活没有精神副作用,而且广泛地分布在神经胶质细胞中[17]。因此,CB2受体及其特定配体近年来获得更多的关注。最近的研究证实,CB2受体可以调节中枢神经系统的免疫功能和神经炎症反应[18-19]。LI等[20]在脑出血模型中研究发现,激活胶质细胞的CB2受体可抑制iNOS等表达的增加,发挥神经保护作用。ASO等[21]研究发现,应用CB2受体激动剂JWH133的AD小鼠在主动回避测试和V迷宫记忆检测中表现出认知缺陷的部分缓解,这种认知能力的改善伴随炎症因子的减少。既然CB2受体在神经炎症中发挥保护作用,那么是否可以通过激活胶质细胞的CB2受体抑制PD模型中SN区的炎症反应?
本實验选用对CB2受体具有高选择性的合成激动剂JWH133,探讨了激活CB2受体对LPS诱导的COX-2和iNOS基因表达的影响。实验结果显示,LPS处理后小鼠SN区COX-2和iNOS基因表达水平显著增加。JWH133腹腔注射2周可以抑制LPS处理引起的COX-2和iNOS基因表达升高,说明激活CB2受体可以抑制炎症递质的产生,缓解炎症反应。与LPS处理的WT小鼠相比较,CB2-KO小鼠经LPS处理后脑SN区COX-2和iNOS基因的表达明显增加。在LPS诱导下SN区COX-2和iNOS基因表达明显上调,而敲除CB2受体又能进一步促进COX-2和iNOS基因表达,说明CB2受体的缺失加剧了LPS诱导的炎症反应,证实CB2受体激活在PD的发病中发挥了抗炎作用,但其具体机制还有待进一步探究。在临床研究方面,虽然有不同的试验分析了基于大麻的药物在PD病人中的应用[22-24],但目前尚无专门研究CB2受体作用或CB2受体激动剂对PD病人影响的临床试验。然而,人类全基因组关联研究分析显示,CB2受体基因CNR2与PD相关[25]。此外,在PD病人的死后脑组织中发现了CB2受体表达的改变[26]。CB2受体在PD中的保护作用提示,CB2受体可能是PD潜在的新治疗靶点。
[参考文献]
[1]METSIOS G S, MOE R H, KITAS G D. Exercise and inflammation[J]. Best Practice & Research Clinical Rheumatology, 2020,34(2):101504.
[2]YONG H Y F, RAWJI K S, GHORBANI S, et al. The benefits of neuroinflammation for the repair of the injured central nervous system[J]. Cellular & Molecular Immunology, 2019,16(6):540-546.
[3]KWON H S, KOH S H. Neuroinflammation in neurodegene-rative disorders: the roles of microglia and astrocytes[J]. Translational Neurodegeneration, 2020,9(1):42.
[4]XU S B, LU J N, SHAO A W, et al. Glial cells: role of the immune response in ischemic stroke[J]. Frontiers in Immuno-logy, 2020,11:294.
[5]LEE S L, HSU J Y, CHEN T C, et al. Erinacine A prevents lipopolysaccharide-mediated glial cell activation to protect dopaminergic neurons against inflammatory factor-induced cell death in vitro and in vivo[J]. International Journal of Molecular Sciences, 2022,23(2):810.
[6]STASIULEWICZ A, ZNAJDEK K, GRUDZIE? M, et al. Aguide to targeting the endocannabinoid system in drug design[J]. International Journal of Molecular Sciences, 2020,21(8):2778.
[7]VAN NIEKERK G, MABIN T, ENGELBRECHT A M. Anti-inflammatory mechanisms of cannabinoids: an immunometabolic perspective[J]. Inflammopharmacology, 2019,27(1):39-46.
[8]JIA Y, DENG H, QIN Q Y, et al. JWH133 inhibits MPP+-induced inflammatory response and iron influx in astrocytes[J]. Neuroscience Letters, 2020,720:134779.
[9]ZHANG W Q, LU J H, WANG Y Y, et al. Canagliflozin attenuates lipotoxicity in cardiomyocytes by inhibiting inflammation and ferroptosis through activating AMPK pathway[J]. International Journal of Molecular Sciences, 2023,24(1):858.
[10]HUMBERT-CLAUDE M, DUC D, DWIR D, et al. Tollip, an early regulator of the acute inflammatory response in the substantia nigra[J]. Journal of Neuroinflammation, 2016,13(1):303.
[11]DU Z R, GU Y, XIE X M, et al. GPER and IGF-1R mediate the anti-inflammatory effect of genistein against lipopolysaccharide (LPS)-induced nigrostriatal injury in rats[J]. The Journal of Steroid Biochemistry and Molecular Biology, 2021,214:105989.
[12]CHUNG Y C, SHIN W H, BAEK J Y, et al. CB2 receptor activation prevents glial-derived neurotoxic mediator production, BBB leakage and peripheral immune cell infiltration and rescues dopamine neurons in the MPTP model of Parkinsons disease[J]. Experimental & Molecular Medicine, 2016,48(1):e205.
[13]JIANG M C, CHEN X H, ZHAO X, et al. Involvement of IGF-1 receptor signaling pathway in the neuroprotective effects of Icaritin against MPP(+)-induced toxicity in MES23.5 cells[J]. European Journal of Pharmacology, 2016,786:53-59.
[14]TOLOSA E, GARRIDO A, SCHOLZ S W, et al. Challenges in the diagnosis of Parkinson's disease[J]. Lancet Neurology, 2021,20(5):385-397.
[15]DI MARZO V, PISCITELLI F. The endocannabinoid system and its modulation by phytocannabinoids[J]. Neurotherapeutics, 2015,12(4):692-698.
[16]BISOGNO T, MACCARRONE M. Endocannabinoid signaling and its regulation by nutrients[J]. BioFactors, 2014,40(4):373-380.
[17]NAGOOR MEERAN M F, SHARMA C, GOYAL S N, et al. CB2 receptor-selective agonists as candidates for targeting infection, inflammation, and immunity in SARS-CoV-2 infections[J]. Drug Development Research, 2021,82(1):7-11.
[18]JEAN-GILLES L, BRAITCH M, LATIF M L, et al. Effects of pro-inflammatory cytokines on cannabinoid CB1 and CB2 receptors in immune cells[J]. Acta Physiologica, 2015,214(1):63-74.
[19]CAPOZZI A, CAISSUTTI D, MATTEI V, et al. Anti-inflammatory activity of a CB2 selective cannabinoid receptor agonist: signaling and cytokines release in blood mononuclear cells[J]. Molecules, 2021,27(1):64.
[20]LI L, TAO Y H, FENG Z, et al. Inflammatory regulation by driving microglial M2 polarization: neuroprotective effects of cannabinoid receptor-2 activation in intracerebral hemorrhage[J]. Frontiers in Immunology, 2017,8:112.
[21]ASO E, JUVS S, MALDONADO R, et al. CB2 cannabinoid receptor agonist ameliorates Alzheimer-like phenotype in AβPP/PS1 mice[J]. Journal of Alzheimers Disease: JAD, 2013,35(4):847-858.
[22]PAES-COLLI Y, AGUIAR A F L, ISAAC A R, et al. Phytocannabinoids and Cannabis-based products as alternative pharmacotherapy in neurodegenerative diseases: from hypothesis to clinical practice[J]. Frontiers in Cellular Neuroscience, 2022,16:917164.
[23]OIKONOMOU P, JOST W H. Randomized controlled trials on the use of cannabis-based medicines in movement disorders: a systematic review[J]. Journal of Neural Transmission, 2022,129(10):1247-1256.
[24]PEBALL M, WERKMANN M, ELLMERER P, et al. Nabilone for non-motor symptoms of Parkinsons disease: a randomized placebo-controlled, double-blind, parallel-group, enriched enrolment randomized withdrawal study (The NMS-Nab Study)[J]. Journal of Neural Transmission, 2019,126(8):1061-1072.
[25]LIU Q R, CANSECO-ALBA A, ZHANG H Y, et al. Cannabinoid type 2 receptors in dopamine neurons inhibits psychomotor behaviors, alters anxiety, depression and alcohol prefe-rence[J]. Scientific Reports, 2017,7(1):17410.
[26]G?MEZ-G?LVEZ Y, PALOMO-GARO C, FERNNDEZ-RUIZ J, et al. Potential of the cannabinoid CB(2) receptor as a pharmacological target against inflammation in Parkinsons disease[J]. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 2016,64:200-208.
(本文編辑马伟平)