H3N2亚型犬流感病毒实验感染模型的建立
2023-08-15黄程杨龙峰孙鹏程慧敏杨志远林健祝洪伟刘立新孙厚民李加凤赵际成段会娟潘洁刘月焕
黄程,杨龙峰,孙鹏,程慧敏,杨志远,林健,祝洪伟,刘立新,孙厚民,李加凤,赵际成,段会娟,潘洁,刘月焕
H3N2亚型犬流感病毒实验感染模型的建立

1北京市农林科学院畜牧兽医研究所,北京 100097;2北京市延庆区动物疫病预防控制中心,北京 102100;3青岛易邦生物工程有限公司,山东青岛 266113
【目的】建立H3N2亚型犬流感病毒(canine influenza virus, CIV)实验感染模型,了解犬流感的发病特征,为疫苗效力评价奠定基础。【方法】6—13月龄CIV血凝抑制(haemagglutination inhibition, HI)抗体阴性(HI<1﹕10)比格犬26只,其中3只鼻腔喷雾PBS作为阴性对照,另23只分5组(10、103、105、106、107EID50/只),分别为3、5、5、5、5只/组,每只犬各鼻腔喷雾H3N2亚型CIV(A/canine/China/Huabei-170607/2017(H3N2),简称HB株)病毒液1mL。观察临床症状、肺脏病变和肺脏组织病理学变化,计算肺实变率,检测病毒和HI抗体效价。【结果】10 EID50剂量感染组,3只犬均未出现明显临床症状,肺脏均无明显眼观病变,肺脏实变率0%,无组织病理学变化,病毒检测均为阴性,HI抗体效价几何平均值(geometric mean titer, GMT)为1﹕15.9;103EID50剂量感染组,5只犬中有4只喘息、流鼻汁和咳嗽,1只犬未表现出临床症状,2只犬肺脏出现轻微实变,实变率平均为1.4%,4只犬病毒分离阳性,HI抗体效价GMT为1﹕320;105EID50剂量感染组,5只犬在攻毒后5 d开始出现流鼻汁和咳嗽等临床症状,肺脏均有实变,实变率平均为4.2%,肺泡间隔增宽,病毒分离均为阳性,HI抗体效价GMT为1﹕2 940.7;106EID50剂量感染组,5只犬在攻毒后4 d体温升高、流鼻汁、咳嗽,临床症状出现较105EID50剂量感染组早1 d,肺脏实变程度增加,实变率平均为17.9%,肺泡间隔增宽,病毒分离均为阳性,HI抗体效价GMT为1﹕2 228.7;107EID50剂量感染组,5只犬在攻毒后3 d表现出体温升高、流鼻汁、咳嗽等严重的临床症状,症状出现较106EID50剂量感染组早1 d,肺脏严重实变,实变率平均为29.0%,肺泡间隔增宽,病毒分离均为阳性,HI抗体效价GMT为1﹕2 940.7;对照犬均未出现明显临床症状,肺脏均无明显眼观病变,无组织病理学变化,病毒检测均为阴性,HI抗体效价均<1﹕10。【结论】106EID50剂量病毒是能引起犬明显发病的最小病毒接种剂量,建立起H3N2亚型CIV实验感染模型。
比格犬;犬流感病毒;H3N2亚型;肺脏;感染模型
0 引言
【研究意义】犬流感(canine influenza,CI)是由正黏病毒科甲型流感病毒属的犬流感病毒(canine influenza virus,CIV)引起的犬接触性呼吸道传染病[1-2]。感染犬表现发热、精神沉郁、咳嗽和流鼻汁等临床症状,CIV的致病特征是高水平的病毒复制和广泛的组织损伤[3]。尽管该病致死率不高,但犬只多死于肺部继发感染[4],因此本病仍不可被忽视。甲型流感病毒具有跨物种传播的特性[5-6]。犬作为伴侣动物,给人类和周围其他动物提供了病毒跨物种传播的机会,对人类健康构成潜在威胁,具有重要的公共卫生意义[7-8]。此外,CIV造成的呼吸道损伤对警犬嗅觉有一定影响,使得嗅觉减退[9]。【前人研究进展】2004年,在美国佛罗里达州的赛犬中首次暴发H3N8亚型犬流感,研究发现这一亚型起源于马流感病毒(equine influenza virus, EIV)[5,10],H3N8亚型犬流感主要在北美洲和欧洲流行[11-12]。2007年韩国首次暴发H3N2亚型犬流感疫情,该亚型最早于2005年从禽类传播至中国犬类,在犬之间水平传播[1]。2009—2010年,LI和LIN等[13-14]在浙江、江苏、北京、辽宁等地也相继分离到H3N2亚型CIV,且与韩国H3N2亚型CIV高度同源。流行趋势表明H3N2亚型犬流感已经成为包括中国在内的亚洲地方性动物传染病[15-16]。不同物种的流感病毒,包括禽流感病毒、人流感病毒等,可与CIV重配[17],从而促进了犬流感病毒的重组,使流感病毒的致病性、流行性发生改变[18-20]。NA等[21]使用106.75EID50剂量CIV接种7周龄比格犬,观察到犬出现高热、流鼻汁的临床症状,犬的肺脏和气管出现病理变化。Liu等采用不同感染剂量接种10周龄比格犬,可观察到106EID50接种剂量的犬临床症状明显,病理变化典型[22]。Wu等对6周龄豚鼠接种106TCID50剂量的CIV,发现CIV可在豚鼠呼吸道复制[23]。【本研究切入点】虽然已有评价H3N2 CIV致病性的相关报道[21-23],但方法相对复杂,观察到的临床症状轻,目前尚无系统、可行和易重复的评价方法。鉴于感染用毒株和评价方法不同,有必要建立CIV人工感染模型。【拟解决的关键问题】利用分离得到的H3N2亚型CIV(A/canine/China/Huabei-170607/2017(H3N2),简称HB株),以1mL/只犬,经鼻腔接种不同病毒含量的H3N2亚型CIV,统计分析临床症状、肺脏实变率、病毒复制和血凝抑制(haemagglutination inhibition, HI)抗体效价,来探究H3N2亚型CIV的致病性和建立犬的发病模型。
1 材料与方法
1.1 毒株
H3N2亚型CIV(HB株,F3代)为北京市农林科学院畜牧兽医研究所动物疫病研究室分离、鉴定和保存,EID50为107.3/0.1 mL,经纯净性检验,无细菌、支原体和外源病毒污染。
1.2 SPF鸡胚及实验动物
(1)SPF鸡胚 10日龄SPF鸡胚,购自北京勃林格殷格翰维通生物技术有限公司。
(2)犬 26只6—13月龄CIV HI抗体阴性(HI<1﹕10)比格犬,雌雄各13只,购自青岛博隆实验动物有限公司。
(3)试验地点 青岛易邦生物工程有限公司负压动物舍,实验动物使用许可证号SYXK(鲁)2016-0016。北京市农林科学院畜牧兽医研究所。
(4)试验时间 2019年7月至2021年2月
1.3 试剂
PBS(0.01 mol·L-1,pH7.0—7.2)、胰蛋白胨磷酸盐肉汤(TPB)、0.5%鸡红细胞和H3亚型CIV HI试验抗原,均由北京市农林科学院畜牧兽医研究所动物疫病研究室制备。
1.4 方法
1.4.1 试验设计 26只犬随机分组进行鼻腔喷雾攻毒,在负压动物舍饲养,自由采食和饮水。试验分组见表1。
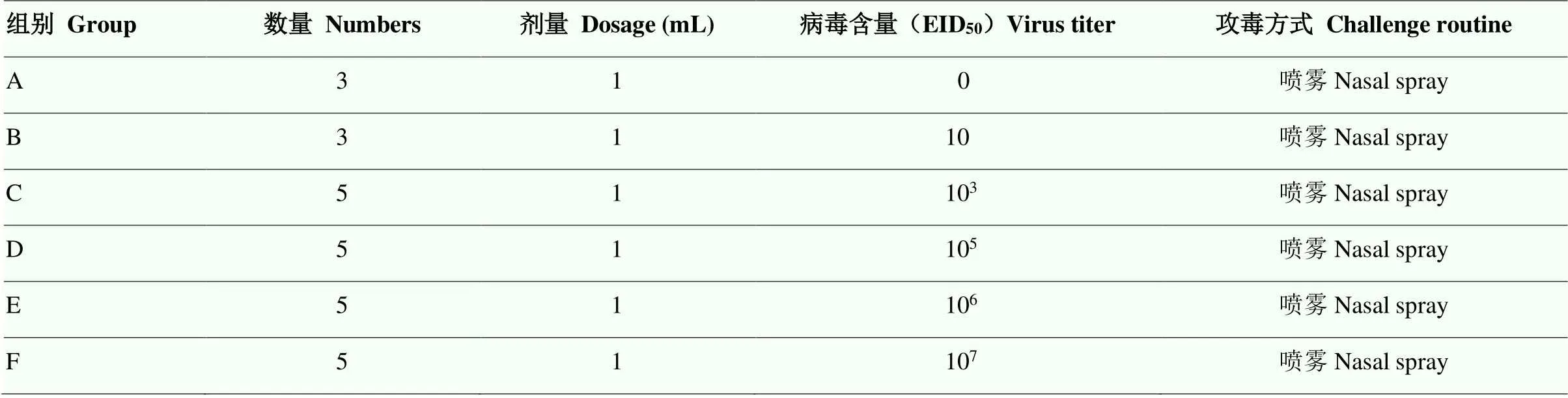
表1 试验分组
A组为阴性对照 Group A was the non-challenged group
1.4.2 临床症状观察 每天定时观察1次,连续监测14 d。观察精神状态、采食和粪便等,测定直肠温度,并填写临床症状积分表,积分标准见表2。
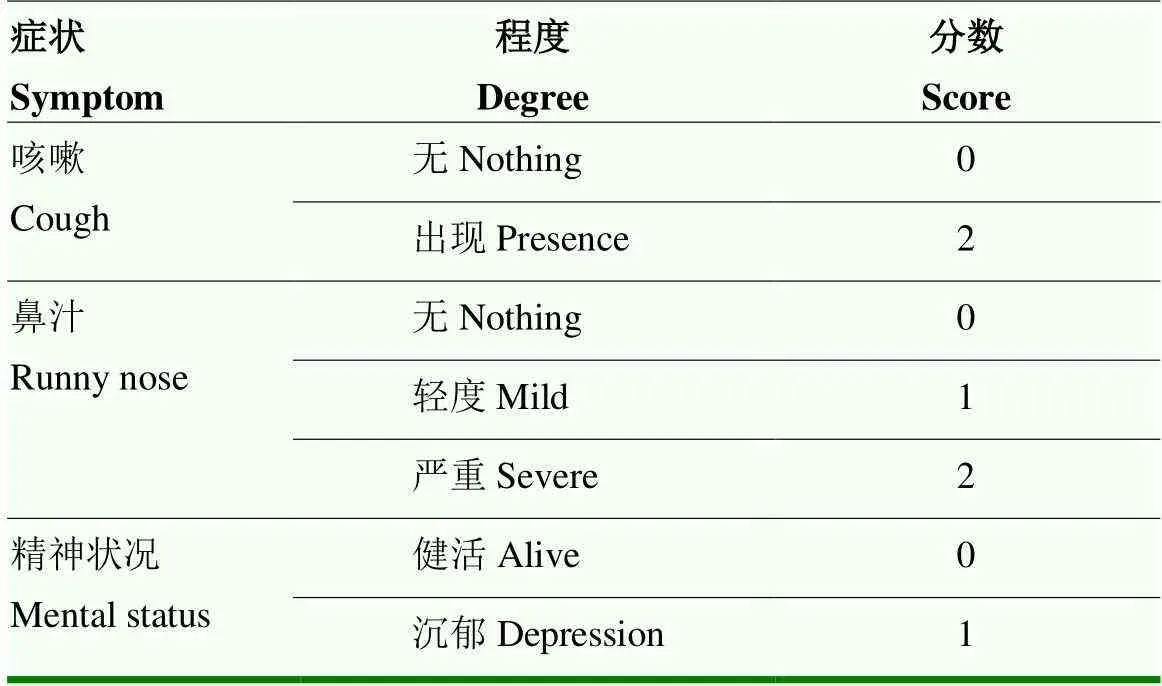
表2 临床症状评分标准
1.4.3 肺脏病变观察与实变率计算 攻毒后14 d,试验犬麻醉后静脉注射10%氯化钾致死,剖检观察肺脏病变。取实变肺组织,称重,按照肺脏实变重量/肺脏总重量乘以100%计算肺脏实变率。同时取病变肺脏,10%中性福尔马林溶液固定,制作石蜡切片,H.E.染色[24],观察组织病理学变化。
1.4.4 病毒分离 攻毒后5 d,采集鼻拭子,每份拭子离心后经0.22 μm滤膜过滤,滤液经尿囊腔接种5枚10日龄SPF鸡胚,每胚0.2 mL。置36—37℃孵化至96 h,收获鸡胚尿囊液,测定HA效价。5枚接种鸡胚中如有1枚鸡胚尿囊液HA效价不低于1﹕16,可判定为病毒分离阳性。
1.4.5 HI抗体测定 攻毒后14 d,采血,离心,分离血清。血清经白陶土和红血球处理后,测定HI抗体效价[25],计算HI抗体效价几何平均值(geometric mean titer, GMT)。
2 结果
2.1 临床症状
体温结果如图1。10 EID50攻毒组的犬体温均未超过39.5℃,在正常体温范围内。103EID50攻毒组2只犬在攻毒后6 d体温高于40℃,超出正常体温。105EID50攻毒组1只犬在攻毒后11 d体温高于40℃,超出正常体温。另外,106EID50和107EID50攻毒组在攻毒后3—6 d体温与其他剂量攻毒组显著差异。106EID50攻毒组的5只犬在攻毒后2—6 d体温陆续高于40℃,超出正常体温。107EID50攻毒组的5只犬在攻毒后3—6 d体温陆续高于40℃,超出正常体温。对照组犬体温均未高于39.5℃,在正常体温范围内。
临床症状评分结果如图2。10 EID50攻毒组犬均未出现咳嗽等呼吸道症状。103EID50攻毒组4只犬在攻毒后2 d开始出现轻微的咳嗽、喘息和流鼻汁,而剩余1只犬未表现临床症状,临床症状积分在第6天最高,达1.6分,后续逐渐恢复,第12天后未见明显临床症状。105EID50攻毒组的所有犬在攻毒后5 d开始出现流鼻汁、精神沉郁、咳嗽,临床症状积分在第7天最高,达2.6分,后续逐渐恢复,第13天后未见明显临床症状。106EID50攻毒组所有犬在攻毒后4 d开始出现呼吸系统症状,包括流鼻汁、精神沉郁、咳嗽,临床症状积分在第9天出现最高峰,达2.4分,第12天后未见明显临床症状。107EID50攻毒组5只犬在攻毒后3d开始出现呼吸困难症状,临床症状积分在第6天达到最高,即2.4分,第12天后未见明显临床症状。对照组犬均未出现咳嗽、喘息和流鼻汁等症状。
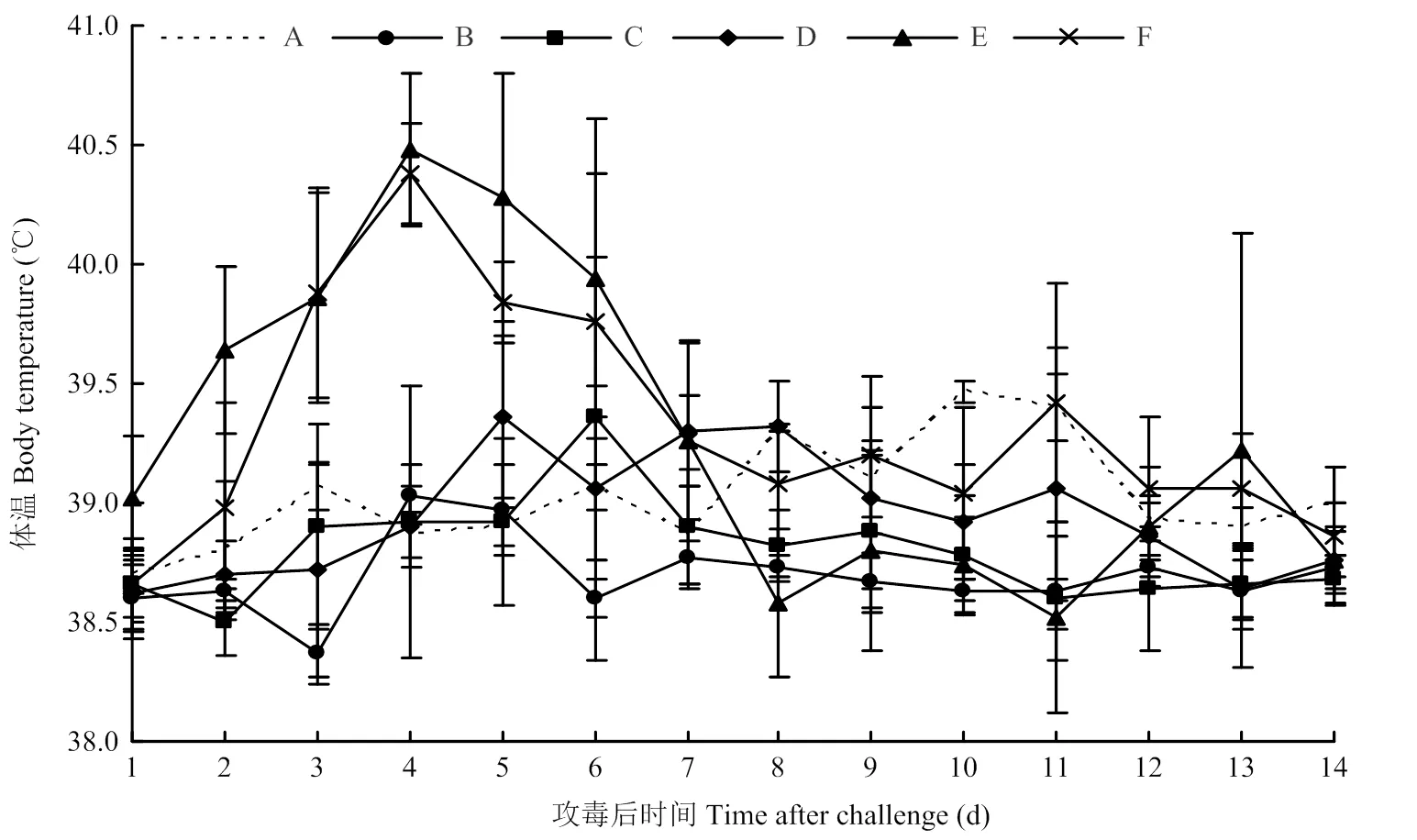
A:阴性对照Non-challenged group;B:10 EID50;C:103 EID50;D:105 EID50;E:106 EID50;F:107 EID50。下同 The same as below
2.2 病理变化
攻毒后14d剖检,10 EID50攻毒组3只犬肺脏均无明显眼观病变,实变率为0%;103EID50攻毒组,2只犬肺脏出现红褐色硬币状大小实变,分别位于尖叶和膈叶,实变率平均为1.4%;105EID50攻毒组,5只犬肺脏均有小面积实变,实变率平均为4.2%;106EID50攻毒组5只犬的肺脏均有红褐色树叶状大小实变,主要位于尖叶和心叶,实变率平均为17.9%,显著高于105EID50攻毒组;107EID50攻毒组5只犬的肺脏均有严重暗灰色大面积片状实变,主要位于尖叶和心叶,实变率平均为29.0%,实变面积进一步增大;而对照组3只犬肺脏均无明显眼观病变(表3和图3)。
组织病理学变化显示对照组和10 EID50攻毒组犬肺脏均无病理变化,103—107EID50感染组犬肺脏充血和水肿,肺泡间隔增宽,有明显的淋巴细胞浸润(图4)。
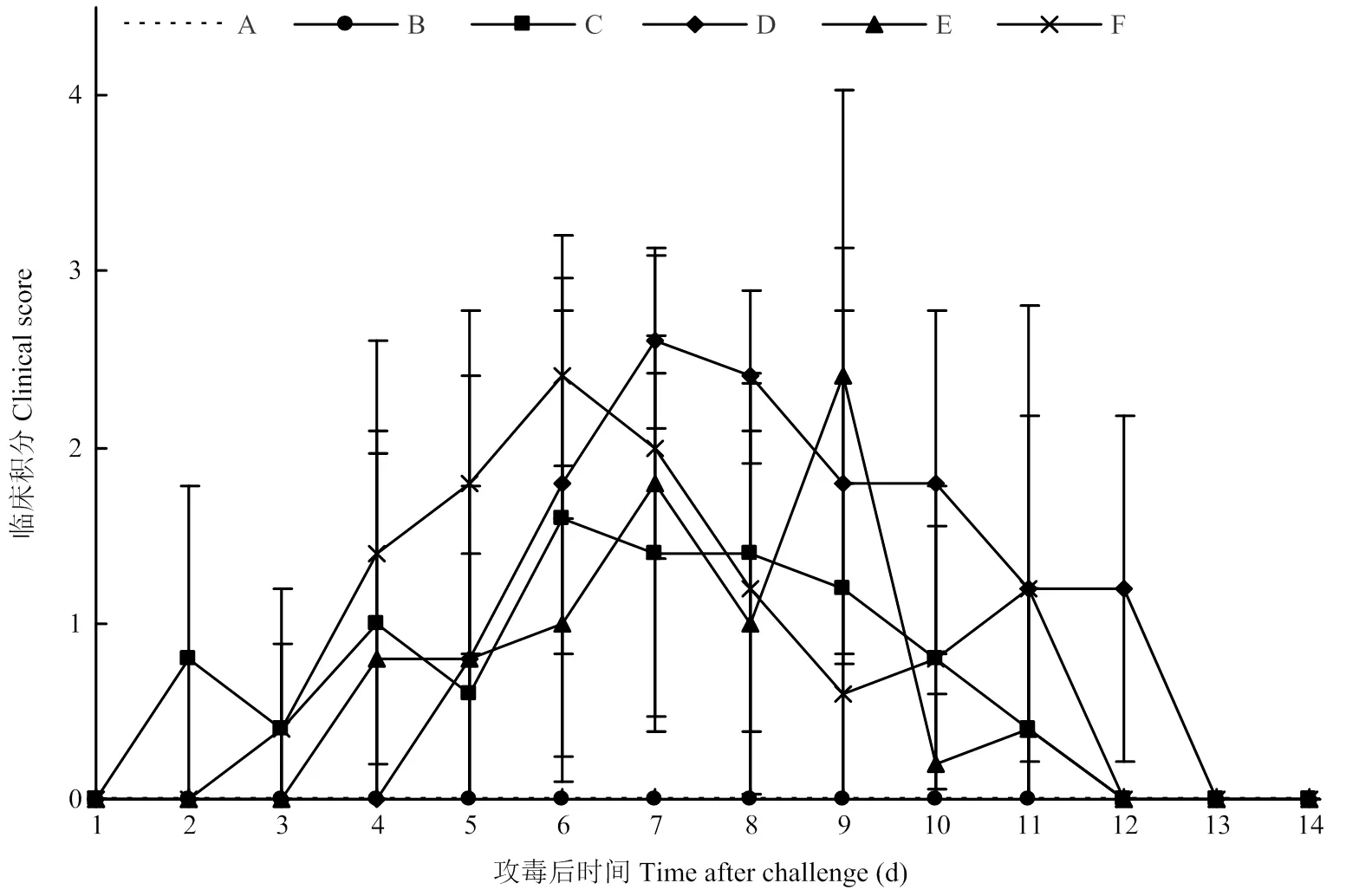
图2 临床症状评分

表3 肺脏实变率
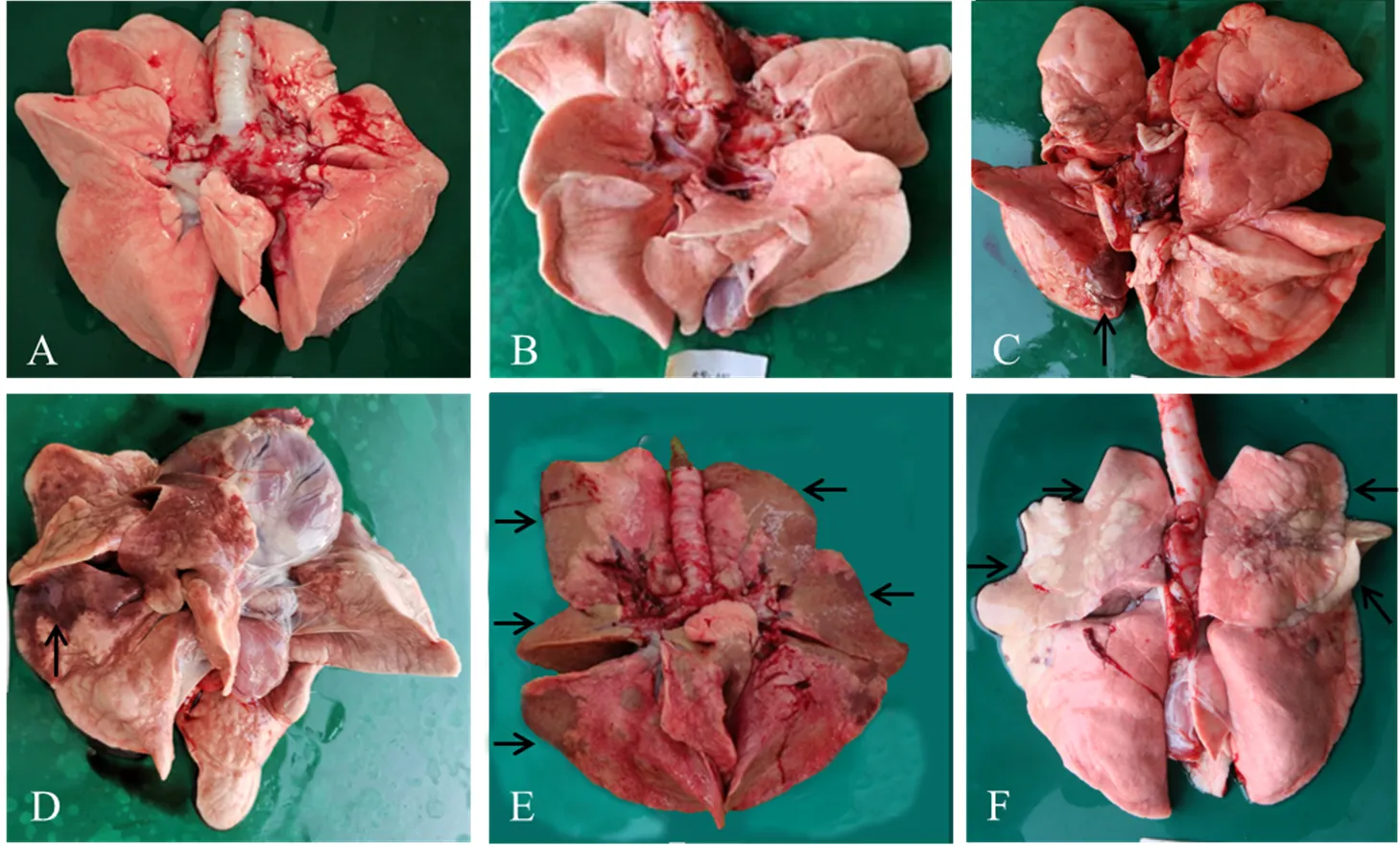
图3 试验犬肺脏大体病变
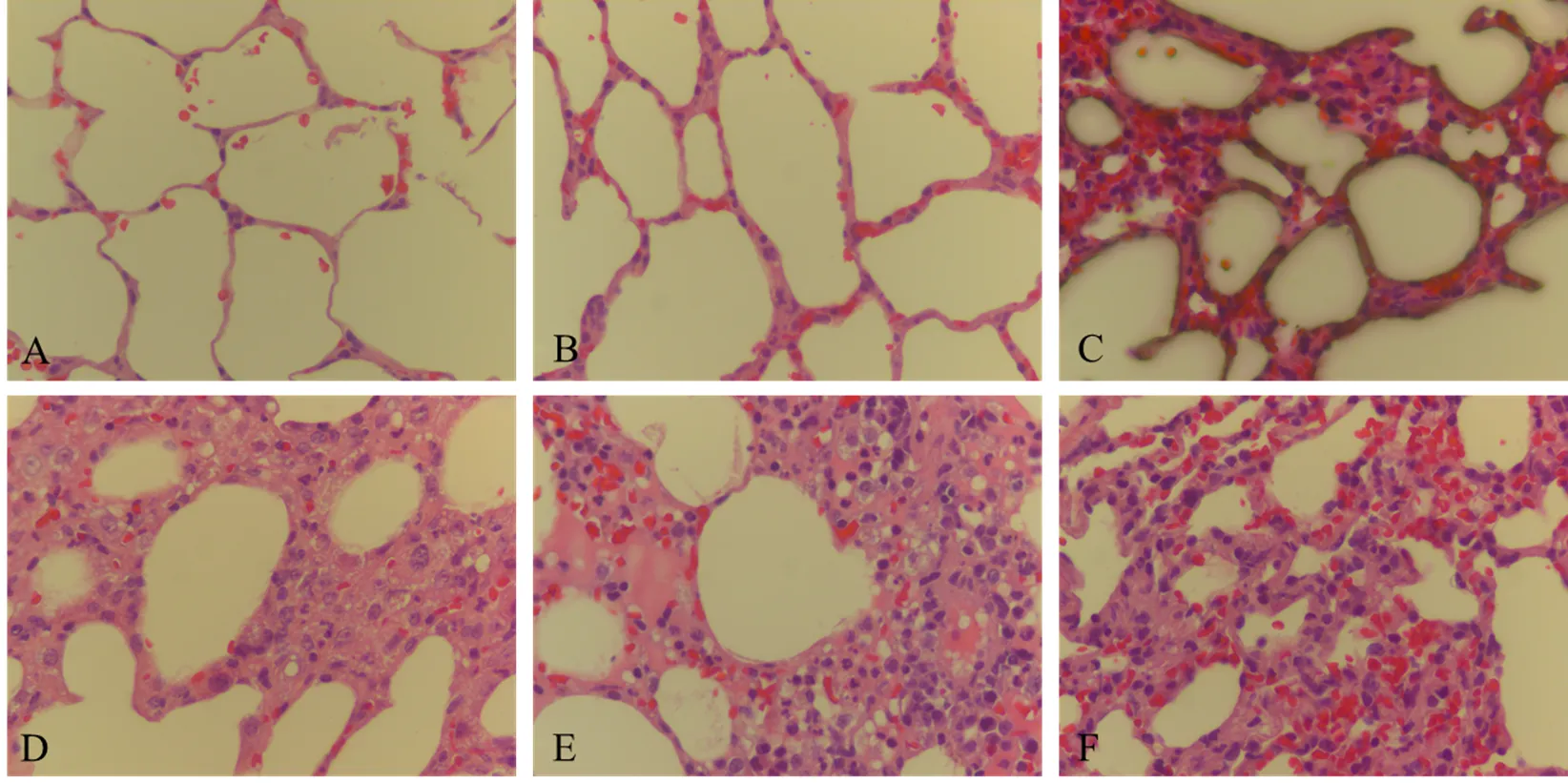
图4 试验犬肺脏组织病理学变化
2.3 病毒分离和HI抗体效价
病毒分离结果见表4。10 EID50攻毒组攻毒后第5天病毒分离均为阴性,103EID50攻毒组4只犬在攻毒后第5天病毒分离阳性,105EID50、106EID50和107EID50攻毒组,所有犬在攻毒后第5天病毒分离均为阳性。
HI抗体效价结果见表4。攻毒后第14天,10 EID50攻毒组犬HI抗体效价GMT为1﹕15.9,但该攻毒组犬未表现出临床症状。103EID50攻毒组犬HI抗体效价GMT为1﹕320。105EID50攻毒组犬HI抗体效价GMT为1﹕2 940.7,显著高于103EID50攻毒组。106EID50攻毒组犬HI抗体效价GMT为1﹕2 228.7。107EID50攻毒组犬HI抗体效价GMT为1﹕2 940.7。对照组HI抗体效价均小于1﹕10。
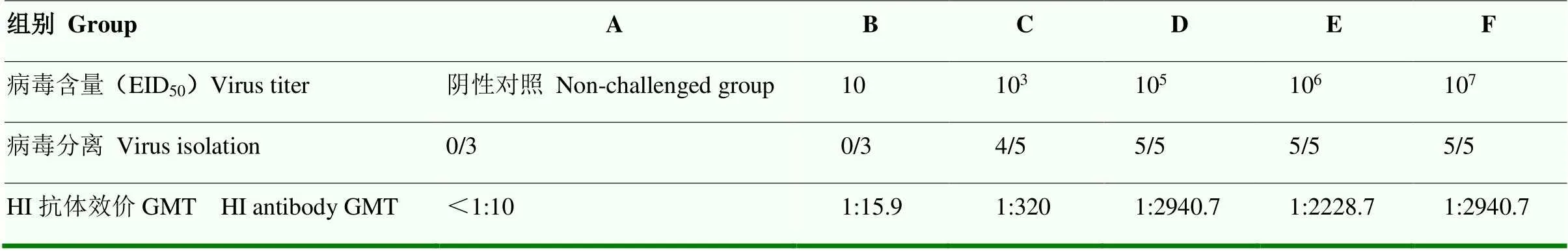
表4 病毒分离和HI抗体效价结果
3 讨论
犬流感作为一种新发传染病,近年来在北京等地都有发生,对公共卫生安全具有潜在威胁。目前主要的CIV亚型为H3N8和H3N2,H3N2亚型犬流感在中国广泛流行[9]。开展H3N2亚型CIV的致病性研究,可为了解该病发病机制、疫苗研究和评价奠定基础。
3.1 犬人工感染H3N2亚型CIV后的症状
流感病毒主要感染上呼吸道及支气管上皮细胞,但严重时可蔓延至细支气管和肺泡,引起间质性肺炎[26]。本试验证实H3N2亚型CIV能引起犬的高烧、呼吸急促、咳嗽和严重的肺实变,这与SONG、LIU等[1,27]对H3N2 CIV致病性研究结果基本一致。
目前,LUO等[28]参考H3N8 CIV的临床评分系统研究建立H3N2 CIV临床症状评分。他结合该临床症状评分系统和肺脏病变对H3N2亚型CIV的最小感染剂量进行研究,将临床症状评分和肺脏病变面积作为发病判定标准。但其方法繁琐。因此本试验采用肺脏实变率进行肺脏病变评价,探讨肺脏实变率是否可作为发病判定标准。本试验前期研究表明,107EID50剂量攻毒后第7、11、14天,犬肺脏均出现明显实变,且病变主要分布在尖叶和心叶。攻毒后第7天,肺脏出现红褐色点状或片状实变。攻毒后第11和14天,肺脏出现暗灰色片状实变,且实变面积大,易于观察。随着时间推移,肺脏实变由红褐色转变为暗灰色,且病变加重。此外,CIV感染犬的病毒复制情况初步研究表明,105、106、107EID50剂量各感染5只犬,在攻毒后第3—6天所有犬病毒分离均为阳性,第7天分别1只、2只、2只犬病毒分离阳性,第10天病毒分离均为阴性。本试验中结合前人的研究结果,统计分析临床症状、肺脏大体病变病毒分离、HI抗体检测等研究结果,采取临床症状观察、攻毒后第5天病毒分离、攻毒后第14天肺脏实变率3个方面来研究CIV致病性。
3.2 不同病毒含量的H3N2亚型CIV对犬肺脏的影响
本试验结果表明,10 EID50攻毒组犬未发病,随着攻毒剂量的增大,犬表现的临床症状越明显,表明H3N2亚型CIV致病力与病毒含量呈正相关。临床症状一般从感染后第2—5天开始,在第6—7天达到峰值,随后逐渐减轻。106EID50和107EID50攻毒组临床症状积分分别在第9天和第6天最高。结合前期攻毒后7天HI抗体效价数据,攻毒剂量越大,产生HI抗体越早,HI抗体效价也越高。CIV感染犬后第5天,10 EID50攻毒组病毒分离阴性,103EID50攻毒组4/5犬病毒分离阳性,105EID50、106EID50和107EID50攻毒组所有犬都排毒。犬表现的临床症状与排毒情况也基本一致,10EID50攻毒组犬无明显异常,103EID50攻毒组4/5犬表现出精神沉郁、咳嗽等临床症状,105EID50、106EID50和107EID50攻毒组所有犬都表现出明显的犬流感临床症状。肺脏病变结果表明,10EID50攻毒组犬的肺脏部均未出现实变,攻毒剂量越大的组,犬肺脏实变越严重。犬在感染107EID50剂量CIV后,虽然临床症状从第7天开始逐渐恢复,直至第14天左右无明显临床症状,但肺脏实变却加重。
另外,试验结果表明,随着攻毒剂量的增加,犬表现的临床症状越明显,肺脏病变越严重,攻毒剂量与发病严重程度呈正相关。105EID50剂量感染犬均能表现出流鼻汁、咳嗽等临床症状,且所有犬肺脏都出现实变,但3/5犬肺脏仅出现轻微实变,4/5犬体温在正常范围;106EID50剂量感染犬都能表现出发烧、咳嗽等临床症状,所有犬肺脏出现大面积实变,因此106EID50攻毒剂量可观察到较明显临床症状和肺脏实变。另一方面,与LUO等[28]对2014年H3N2 CIV研究中感染犬的肺脏实变面积相比,本试验感染犬的肺脏实变更明显,或许与2017年分离的H3N2亚型CIV(HB株)毒力增强有关。
肺脏实变率计算法是基于肺脏病变28分计数法[29]进行简单化处理,在清楚地看到肺脏病变前提下,称重方法相对简单,广泛应用于评判肺脏发生病变的程度。本研究通过肺脏实变率,发现虽然攻毒剂量越大,实变率越高;但肺脏实变情况与攻毒剂量未见显著的线性相关关系,推断CIV感染动物后,剂量达到发病阈值,即可引起发病,肺脏实变面积可能与感染持续时间相关。动物感染流感病毒表现出的肺脏损伤是机体免疫诱导细胞因子所造成的[30],这种肺脏实变颜色和区域面积随时间的变化与CIV发病机理及免疫应答是否有关,仍需进一步研究。
通过上述研究,临床症状观察和体温检测具有人为因素的误差,二者可作为H3N2亚型CIV感染动物试验的参照。106EID50剂量感染犬出现严重的肺脏实变。因此,肺脏的实变程度作为犬发病与否的重要标准。肺脏实变率因人为测定和动物个体差异造成一定的误差,因此本研究初步将肺脏实变率不小于8%作为攻毒犬发病判定标准。
4 结论
应用H3N2亚型CIV(HB株)人工感染6—13月龄比格犬后,体温测定、临床症状和肺脏病变观察、病毒分离和抗体检测结果表明,106EID50剂量病毒是可感染犬,且能引起犬明显发病的最小病毒接种剂量。该实验模型为犬流感发病机制研究和疫苗效力评价奠定了基础。
[1] SONG D, KANG B, LEE C, JUNG K, HA G, KANG D, PARK S, PARK B, OH J. Transmission of avian influenza virus (H3N2) to dogs. Emerging Infectious Diseases, 2008, 14(5): 741-746.
[2] GIBBS E P J, ANDERSON T C. Equine and canine influenza: a review of current events. Animal Health Research Reviews, 2010, 11(1): 43-51.
[3] GONZALEZ G, MARSHALL J F, MORRELL J, ROBB D, MCCAULEY J W, PEREZ D R, PARRISH C R, MURCIA P R. Infection and pathogenesis of canine, equine, and human influenza viruses in canine tracheas. Journal of Virology, 2014, 88(16): 9208-9219.
[4] TENG Q Y, ZHANG X, XU D W, ZHOU J W, DAI X G, CHEN Z G, LI Z J. Characterization of an H3N2 canine influenza virus isolated from Tibetan mastiffs in China. Veterinary Microbiology, 2013, 162(2/3/4): 345-352.
[5] CRAWFORD P C, DUBOVI E J, CASTLEMAN W L, STEPHENSON I, GIBBS E P J, CHEN L M, SMITH C, HILL R C, FERRO P, POMPEY J, BRIGHT R A, MEDINA M J, JOHNSON C M, OLSEN C W, COX N J, KLIMOV A I, KATZ J M, DONIS R O, GROUP I G. Transmission of equine influenza virus to dogs. Science, 2005, 310(5747): 482-485.
[6] ABDELWHAB E M, ABDEL-MONEIM A S. Orthomyxoviruses. Recent Advances in Animal Virology. Singapore: Springer Singapore, 2019: 351-378.
[7] LYOO K S, KIM J K, KANG B, MOON H, KIM J, SONG M, PARK B, KIM S H, WEBSTER R G, SONG D. Comparative analysis of virulence of a novel, avian-origin H3N2 canine influenza virus in various host species. Virus Research, 2015, 195: 135-140.
[8] PARRISH C R, MURCIA P R, HOLMES E C. Influenza virus reservoirs and intermediate hosts: dogs, horses, and new possibilities for influenza virus exposure of humans. Journal of Virology, 2015, 89(6): 2990-2994.
[9] 张伟, 张罗. 嗅觉功能障碍的诊断与治疗. 首都医科大学学报, 2013, 34(6): 814-819.
ZHANG W, ZHANG L. Diagnosis and treatment of olfactory disorder. Journal of Capital Medical University, 2013, 34(6): 814-819. (in Chinese)
[10] TU L Q, ZHOU P, LI L T, LI X Z, HU R J, JIA K, SUN L S, YUAN Z G, LI S J. Evaluation of protective efficacy of three novel H3N2 canine influenza vaccines. Oncotarget, 2017, 8(58): 98084-98093.
[11] PAYUNGPORN S, CRAWFORD P C, KOUO T S, CHEN L M, POMPEY J, CASTLEMAN W L, DUBOVI E J, KATZ J M, DONIS R O. Influenza A virus (H3N8) in dogs with respiratory disease, Florida. Emerging Infectious Diseases, 2008, 14(6): 902-908.
[12] KRUTH S A, CARMAN S, WEESE J S. Seroprevalence of antibodies to canine influenza virus in dogs in Ontario. The Canadian Veterinary Journal, 2008, 49(8): 800-802.
[13] LI S, SHI Z, JIAO P, ZHANG G, ZHONG Z, TIAN W, LONG L P, CAI Z, ZHU X, LIAO M, WAN X F. Avian-origin H3N2 canine influenza A viruses in Southern China. Infect Genetic Evology, 2010, 10(8): 1286-1288.
[14] LIN Y, ZHAO Y B, ZENG X J, LU C P, LIU Y J. Genetic and pathobiologic characterization of H3N2 canine influenza viruses isolated in the Jiangsu Province of China in 2009-2010. Veterinary Microbiology, 2012, 158(3/4): 247-258.
[15] ALI A, DANIELS J B, ZHANG Y, RODRIGUEZ-PALACIOS A, HAYES-OZELLO K, MATHES L, LEE C W. Pandemic and seasonal human influenza virus infections in domestic cats: prevalence, association with respiratory disease, and seasonality patterns. Journal of Clinical Microbiology, 2011, 49(12): 4101-4105.
[16] RODRIGUEZ L, NOGALES A, REILLY E C, TOPHAM D J, MURCIA P R, PARRISH C R, MARTINEZ SOBRIDO L. A live-attenuated influenza vaccine for H3N2 canine influenza virus. Virology, 2017, 504: 96-106.
[17] VOORHEES I E H, GLASER A L, TOOHEY-KURTH K, NEWBURY S, DALZIEL B D, DUBOVI E J, POULSEN K, LEUTENEGGER C, WILLGERT K J E, BRISBANE-COHEN L, RICHARDSON-LOPEZ J, HOLMES E C, PARRISH C R. Spread of canine influenza A(H3N2) virus, United States. Emerging Infectious Diseases, 2017, 23(12): 1950-1957.
[18] LYU Y L, SONG S K, ZHOU L W, BING G X, WANG Q, SUN H R, CHEN M Y, HU J Y, WANG M Y, SUN H L, PU J, XIA Z F, LIU J H, SUN Y P. Canine influenza virus A(H3N2) clade with antigenic variation, China, 2016-2017. Emerging Infectious Diseases, 2019, 25(1): 161-165.
[19] LI Y G, ZHANG X H, LIU Y X, FENG Y, WANG T C, GE Y, KONG Y Y, SUN H Y, XIANG H Y, ZHOU B, FANG S S, XIA Q, HU X Y, SUN W Y, WANG X F, MENG K Y, LV C X, LI E T, XIA X Z, HE H B, GAO Y W, JIN N Y. Characterization of canine influenza virus A (H3N2) circulating in dogs in China from 2016 to 2018. Viruses, 2021, 13(11): 2279.
[20] WASIK B R, VOORHEES I E H, PARRISH C R. Canine and feline influenza. Cold Spring Harbor Perspectives in Medicine, 2021, 11(1): a038562.
[21] NA W, XIE X, YEOM M, KANG A, KIM H O, LIM J W, PARK G, YOON S W, JEONG D G, KIM H K, HAAM S, LIU Y J, SONG D. Morphological features and pathogenicity of mutated canine influenza viruses from China and South Korea. Transboundary and Emerging Diseases, 2020, 67(4): 1607-1613.
[22] LIU Y B, FU C, LU G, LUO J, YE S T, OU J J, WANG X B, XU H B, HUANG J, WU L Y, ZHANG X, WU P X, LI S J. Comparison of pathogenicity of different infectious doses of H3N2 canine influenza virus in dogs. Frontiers in Veterinary Science, 2020, 7: 580301.
[23] WU M H, SU R S, GU Y X, YU Y N, LI S, SUN H P, PAN L Q, CUI X X, ZHU X H, YANG Q Z, LIU Y W, XU F X, LI M L, LIU Y, QU X Y, WU J, LIAO M, SUN H L. Molecular characteristics, antigenicity, pathogenicity, and zoonotic potential of a H3N2 canine influenza virus currently circulating in South China. Frontiers in Microbiology, 2021, 12: 628979.
[24] 中华医学会. 临床技术操作规范病理学分册. 北京:人民军医出版社, 2012, 33.
Chinese Medical Association. Pathological volume of clinical technical operation specification. Beijing: People's Military Medical Publishing House, 2012, 33. (in Chinese)
[25] KLOPFLEISCH R, WERNER O, MUNDT E, HARDER T, TEIFKE J P. Neurotropism of highly pathogenic avian influenza virus A/ chicken/Indonesia/2003 (H5N1) in experimentally infected pigeons (livia f. domestica). Veterinary Pathology, 2006, 43(4): 463-470.
[26] HSIEH Y C, WU T Z, LIU D P, SHAO P L, CHANG L Y, LU C Y, LEE C Y, HUANG F Y, HUANG L M. Influenza pandemics: past, present and future. Journal of the Formosan Medical Association, 2006, 105(1): 1-6.
[27] LIU Y B, FU C, YE S T, LIANG Y X, QI Z H, YAO C W, WANG Z, WANG J, CAI S Q, TANG S Y, CHEN Y, LI S J. The inactivated vaccine of reassortant H3N2 canine influenza virus based on internal gene cassette from PR8 is safe and effective. Veterinary Microbiology, 2021, 254: 108997.
[28] LUO J, LU G, YE S T, OU J J, FU C, ZHANG X, WANG X B, HUANG J, WU P X, XU H B, WU L Y, LI S J. Comparative pathogenesis of H3N2 canine influenza virus in beagle dogs challenged by intranasal and intratracheal inoculation. Virus Research, 2018, 255: 147-153.
[29] FENG Z X, SHAO G Q, LIU M J, WU X S, ZHOU Y Q, GAN Y. Immune responses to the attenuated168 strain vaccine by intrapulmonic immunization in piglets. Agricultural Sciences in China, 2010, 9(3): 423-431.
[30] BETAKOVA T, KOSTRABOVA A, LACHOVA V, TURIANOVA L. Cytokines induced during influenza virus infection. Current Pharmaceutical Design, 2017, 23(18): 2616-2622.
Establishment of a Canine Experimental Infection Model with a H3N2 Subtype Canine Influenza Virus
HUANG Cheng1, YANG LongFeng2, SUN Peng3, CHENG HuiMin1, YANG ZhiYuan1, LIN Jian1, ZHU HongWei3, LIU LiXin1, SUN HouMin3, LI JiaFeng3, ZHAO JiCheng1, DUAN HuiJuan1, PAN Jie1, LIU YueHuan1
1Institute of Animal Husbandry and Veterinary Medicine, Beijing Municipal Academy of Agricultural and Forestry Sciences, Beijing 100097;2Yanqing District Animal Disease Prevention and Control Center of Beijing Municipality, Beijing 102100;3YEBIO Bioengineering Co., Ltd of Qingdao, Qingdao 266113, Shandong
【Objective】In this research, an experimental animal infection model of canine influenza virus (CIV, H3N2 subtype) was established to better understand the pathogenesis of canine influenza, so as to lay the foundation for vaccine efficacy evaluation. 【Method】26 beagles aged 6-13 months with negative CIV Haemagglutination Inhibition (HI) antibody (HI<1﹕10) were selected, three beagles of whichwere challenged by 1mL nasal spray of PBS, and 23 beagles of which were challenged by1mL nasal spray of H3N2 CIV (A/canine/China/Huabei-170607/2017(H3N2),HB strain for short) with 5 groups (10, 103, 105, 106, and 10750% EID50) as 3, 5, 5, 5, and 5 beagles each group, respectively.Clinical symptoms, lung lesions, histopathological changes of lung, calculating the proportion of consolidation mass, HI antibody titer and virus shedding were examined at 14 days after virus challenge together with three control beagles. 【Result】 3 beagles inoculated with a dose of 10 EID50H3N2 CIV did not show any clinical symptoms, gross lesions in lungs and histopathological changes, the consolidation rate was 0%, and the virus shedding was not detected. The geometric mean titer (GMT) of HI antibodies was 1﹕15.9. However, 4/5 of beagles inoculated with a dose of 103EID50H3N2 CIV showed the clinical symptoms and virus shedding, such as puffing, runny nose and cough. 2/5 of beagles showed light lung consolidation, whose rate was 1.4%. The GMT of HI antibodies was 1:320. All beagles (5/5) infected with a dose of 105EID50H3N2 CIV showed clinical symptoms at day 5 after challenge, such as runny nose and cough, virus shedding, lung consolidation, and widened alveolar septum, and the consolidation rate was 4.2%. The GMT of HI antibodies was 1﹕2 940.7. 5/5 of beagles infected with a dose of 106EID50all showed severe clinical symptoms at day 4 after challenge, such as cough and elevated body temperature, virus shedding, obvious pathological features in the lungs, and the GMT of HI antibodies was 1﹕2 228.7. The clinical symptoms appeared earlier 1 day than that in 105EID50dose infection group, and the degree of lung consolidation increased. The lung consolidation ratio was 17.9%. 5/5 of beagles infected with doses of 107EID50all showed severe clinical symptoms at day 3 after challenge which appeared earlier 1 day than that in 106EID50dose infection group, virus shedding and widened alveolar septum in the lungs, the GMT of HI antibodies was 1﹕2 940.7, and the consolidation rate of lung was 29.0%. The control beagles did not show any clinical symptoms, gross lesions and histopathological changes in lungs, the virus shedding was not detected, and GMT of HI antibodies was <1﹕10. 【Conclusion】The dose of 106EID50was the minimum virus inoculation dose that could cause obvious pathogenesis in beagles. An experimental animal infection model of CIV subtype H3N2 in beagles was established.
beagles; canine influenza virus; H3N2 subtype; lung; infection model

10.3864/j.issn.0578-1752.2023.13.015
2022-03-28;
2022-08-02
北京市农林科学院科技创新能力建设专项(KJCX20200211)、北京市农林科学院畜牧兽医研究所事业基金(XMSSYJJ202110)、北京市农林科学院青年基金(QNJJ202025)
黄程,E-mail:huangc_2019@163.com。通信作者刘月焕,E-mail:liuyuehuan@sina.com
(责任编辑 林鉴非)
