中性粒细胞与淋巴细胞比值对动脉性肺动脉高压的预后价值
2023-08-14杨莹莹郑璐杨海波赵荫涛
杨莹莹,郑璐,杨海波,赵荫涛
中性粒细胞与淋巴细胞比值对动脉性肺动脉高压的预后价值
杨莹莹,郑璐,杨海波,赵荫涛
郑州大学第一附属医院心血管内科,河南郑州 450000
研究中性粒细胞与淋巴细胞比值(neutrophil-to-lymphocyte ratio,NLR)与动脉性肺动脉高压(pulmonary arterial hypertension,PAH)患者预后的关系。回顾性分析2020年11月至2022年10月郑州大学第一附属医院收治的58例PAH患者的临床资料,中位随访时间10个月(1~22个月),定义心力衰竭加重再入院、全因死亡、心肺移植为终点事件。根据随访结果绘制受试者操作特征曲线(receiver operating characteristic curve,ROC曲线)评价NLR预测患者预后不良的敏感度和特异性,生存分析使用Kaplan-Meier法,采用多因素Cox回归探讨影响PAH患者预后的危险因素。终点事件患者的男性占比、中性粒细胞、NLR、N末端脑钠肽前体、尿酸、C反应蛋白(C-reaction protein,CRP)均显著高于无终点事件患者(<0.05)。相关性结果显示NLR与CRP呈正相关(=0.490,<0.05)。ROC曲线结果显示,NLR截断值为2.72时预测PAH患者预后不良的敏感度和特异性分别为75.0%和73.8%,曲线下面积为0.765。NLR<2.72患者的生存率显著高于NLR≥2.72患者(<0.05)。多因素Cox回归分析结果显示,性别(=11.476,95%:3.26~40.4)、NLR(=3.657,95%:1.117~11.973)均是PAH患者预后不良的独立危险因素。高水平NLR提示PAH患者预后不良,是发生不良事件的独立危险因素。
动脉性肺动脉高压;中性粒细胞;淋巴细胞;预后
动脉性肺动脉高压(pulmonary arterial hypertension,PAH)是一种进展性疾病,其平均肺动脉压(mean pulmonary arterial pressure,mPAP)≥25mmHg(1mmHg=0.133kPa),可导致肺血管阻力(pulmonary vascular resistance,PVR)慢性升高、右心室衰竭和早期死亡[1];其病理表现为血管内皮功能障碍、血管收缩、肺小动脉闭塞性重塑及原位血栓形成。研究表明炎症在PAH的发病机制中起重要作用,而中性粒细胞与淋巴细胞比值(neutrophil-to-lymphocyte ratio,NLR)是炎症状态的一个简单且易于评估的指标[2-3]。基于人群的研究表明,NLR增加是心力衰竭和心血管死亡的独立预测因素[4-5]。本文拟研究NLR与PAH严重程度及预后的关系,现将结果报道如下。
1 资料与方法
1.1 一般资料
回顾性选取2020年11月至2022年10月郑州大学第一附属医院收治的PAH患者。纳入标准:①年龄>18岁;②符合《中国肺动脉高压诊断与治疗指南(2021版)》[6]中PAH的诊断标准;③临床资料完整。排除标准:①其他类型的肺动脉高压,如左心疾病所致肺动脉高压、肺部疾病或低氧所致肺动脉高压、慢性血栓栓塞性肺动脉高压、其他肺动脉阻塞性病变所致肺动脉高压等;②住院期间诊断感染性疾病并应用抗生素;③既往痛风病史;④临床资料不完整或失访。PAH是指海平面、静息状态下,经右心导管检查测定的肺动脉平均压≥25mmHg,肺小动脉楔压≤15mmHg及PVR>3WU(1WU=80dyn·s/cm5)[6]。研究共纳入经右心导管诊断的PAH患者58例,其中女52例,男6例;中位年龄35(31,51)岁;特发性肺动脉高压(idiopathic pulmonary arterial hypertension,IPAH)9例,先天性心脏病相关肺动脉高压(congenital heart disease associated with pulmonary arterial hypertension,CHD-PAH)12例,结缔组织病相关肺动脉高压(connective tissue disease associated with pulmonary arterial hypertension,CTD-PAH)37例。所有患者均签署右心导管检查知情同意书,均经肺血管专科医生行规范化肺动脉高压靶向治疗,对CHD-PAH患者经风湿免疫科专科医生规范化诊疗并门诊随访。PAH靶向治疗中,单药治疗:内皮素受体拮抗剂9例;磷酸二酯酶Ⅴ型抑制剂(phosphodiesterase type 5 inhibitor,PDE5抑制剂)3例。联合治疗:内皮素受体拮抗剂+PDE5抑制剂30例;内皮素受体拮抗剂+前列腺素受体激动剂4例;内皮素受体拮抗剂+可溶性鸟苷酸环化酶激动剂4例;内皮素受体拮抗剂+PDE5抑制剂+前列腺素受体激动剂4例;内皮素受体拮抗剂+可溶性鸟苷酸环化酶激动剂+前列腺素受体激动剂4例。
1.2 方法
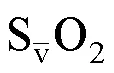
1.3 统计学方法
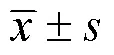
2 结果
2.1 PAH患者的临床基线数据
共16例患者到达随访终点,终点事件患者的男性占比、中性粒细胞、NLR、NT-proBNP、UA、CRP均显著高于无终点事件患者(<0.05),见表1。
2.2 NLR与临床指标的相关性分析
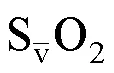
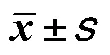
表1 PAH患者的基线资料比较[M(Q1,Q3),,n(%)]
注:1mmHg=0.133kPa;1WU=80dyn·s/cm5

表2 NLR与临床指标的相关性
2.3 NLR对PAH患者预后不良的预测价值
ROC曲线结果显示,NLR截断值为2.72时预测PAH患者预后不良的敏感度和特异性分别为75.0%和73.8%,曲线下面积为0.765。
2.4 不同NLR水平PAH患者的生存曲线
NLR<2.72患者的生存率显著高于NLR≥2.72患者(<0.05),见图1。
2.5 多因素Cox回归分析
多因素Cox回归分析结果显示,性别(=11.476,95%:3.26~40.4)、NLR(=3.657,95%:1.117~11.973)均是PAH患者预后不良的独立危险因素(<0.05),见表3。
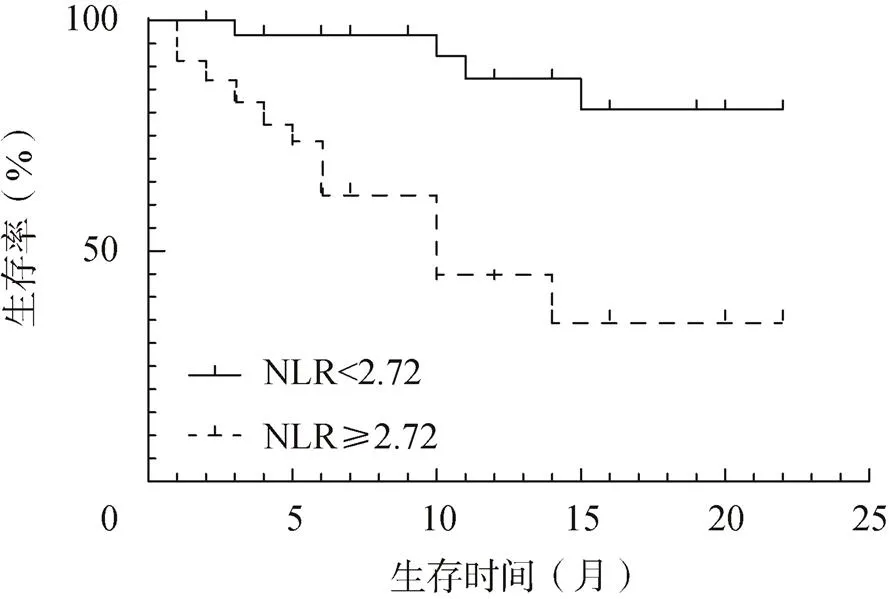
图1 不同NLR水平PAH患者的生存曲线
3 讨论
PAH患者全身炎症反应标记物水平普遍升高。研究表明,PAH患者血管周围炎症细胞聚集,包括巨噬细胞、树突状细胞、T和B淋巴细胞及肥大细胞;其血液循环中炎症细胞因子和趋化因子升高,如白细胞介素-1β、白细胞介素-6和肿瘤坏死因子-α[7]。NLR是一种基于炎症的生物标记物,它与心力衰竭、急性心肌梗死、冠心病、感染性心内膜炎和急性肺栓塞等多种疾病的预后相关[8-12]。
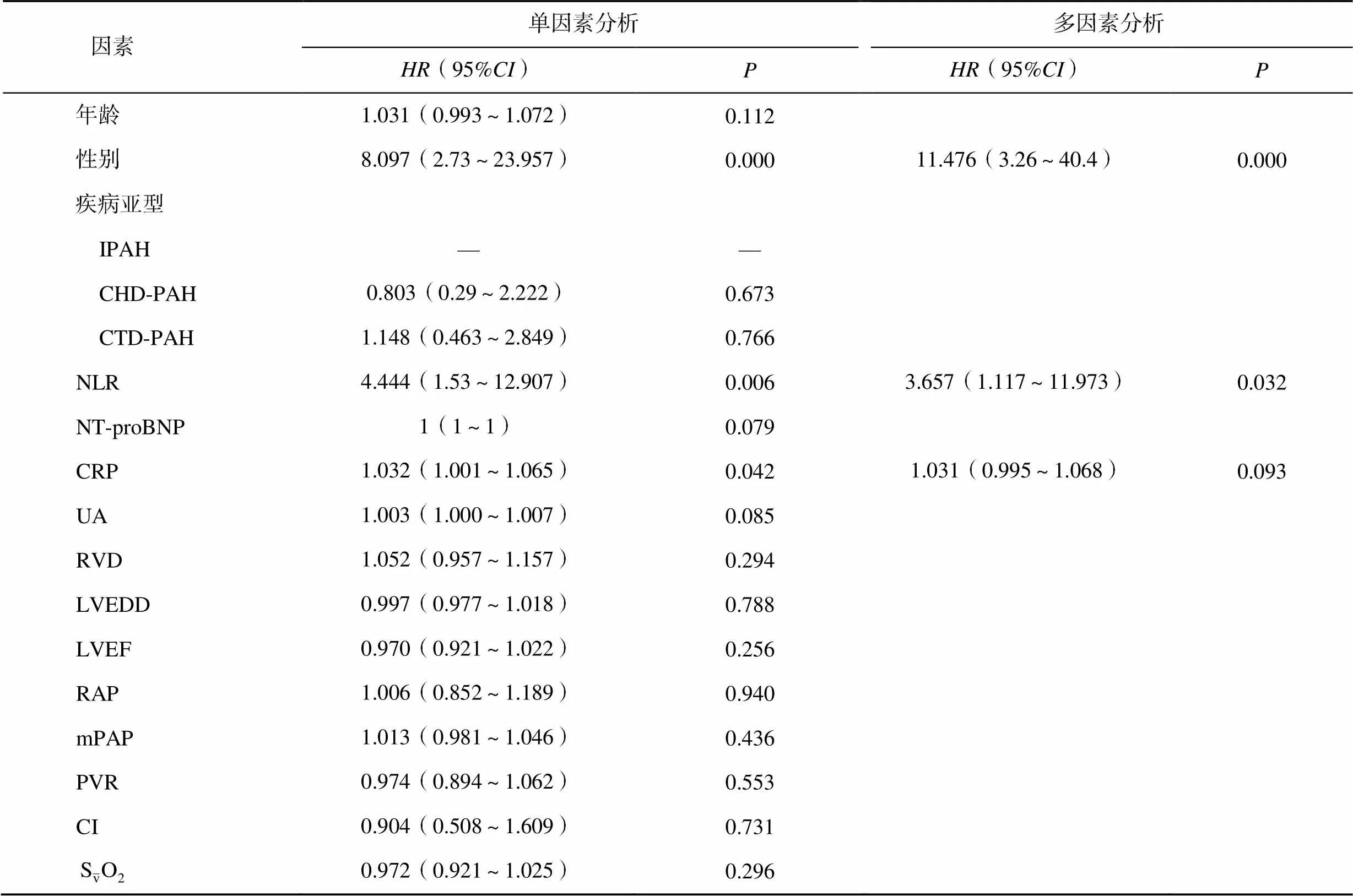
表3 影响PAH患者预后的Cox回归分析
本研究结果显示,NLR与NT-proBNP、UA、RVD、LVEDD、LVEF及血流动力学参数无显著相关。Harbaum等[13]研究结果显示,NLR与疾病严重程度可能相关,NLR与6min步行距离、RAP呈正相关,与NT-proBNP呈负相关。多因素Cox回归分析结果显示性别和NLR均是PAH患者不良预后的独立预测因素,与既往研究相似[13-14]。NLR预测不良预后的最佳截断值为2.72,不同NLR水平患者的生存率差异显著。Harbaum等[13]和Jutras-Beaudoin等[14]研究结果显示NLR是此类患者预后不良的独立预测因子,但截断值均>4,可能与其研究人群种族不同且年龄相对较大有关。研究表明NLR在不同年龄、性别、种族中有所差异[15-16],因此NLR值的差异可能源于潜在的患者特征。
本研究结果显示虽然女性的PAH患病率明显高于男性,但男性发生终点事件的风险是女性的11.476倍,提示男性预后更差。随访期间不良预后患者的基线NLR显著高于无终点事件患者。研究表明,与健康受试者相比,从IPAH患者中分离出的中性粒细胞释放更多介质,如弹性蛋白酶或白三烯B4[17]。中性粒细胞弹性蛋白酶由肺血管平滑肌细胞产生,抑制该酶可通过诱导平滑肌细胞凋亡预防和逆转实验性PAH[18-19]。PAH患者的白三烯B4水平升高,降低其水平可逆转实验性PAH[20]。缺氧严重抑制中性粒细胞凋亡[21]。PAH患者因氧弥散功能障碍而处于缺氧状态。无胸腺大鼠T淋巴细胞的缺乏可导致PAH的发生,提示恢复缺失的淋巴细胞群可通过限制炎症来预防PAH[22]。
综上,NLR是PAH患者预后不良的独立危险因素,高水平NLR提示PAH患者预后不良,NLR有望成为PAH临床管理的炎症指标。
[1] RUBIN L J. Pulmonary arterial hypertension[J]. Proc Am Thorac Soc, 2006, 3(1): 111–115.
[2] PRICE L C, WORT S J, PERROS F, et al. Inflammation in pulmonary arterial hypertension[J]. Chest, 2012, 141(1): 210–221.
[3] SHAH N, PARIKH V, PATEL N, et al. Neutrophil lymphocyte ratio significantly improves the Framingham risk score in prediction of coronary heart disease mortality: Insights from the national health and nutrition examination survey-Ⅲ[J]. Int J Cardiol, 2014, 171(3): 390–397.
[4] FEST J, RUITER T R, KOERKAMP B G, et al. The neutrophil-to-lymphocyte ratio is associated with mortality in the general population: The rotterdam study[J]. Eur J Epidemiol, 2019, 34(5): 463–470.
[5] KIM S, ELIOT M, KOESTLER D C, et al. Association of neutrophil-to-lymphocyte ratio with mortality and cardiovascular disease in the Jackson heart study and modification by the Duffy antigen variant[J]. JAMA Cardiol, 2018, 3(6): 455–462.
[6] 中华医学会呼吸病学分会肺栓塞与肺血管病学组, 中国医师协会呼吸医师分会肺栓塞与肺血管病工作委员会, 全国肺栓塞与肺血管病防治协作组, 等. 中国肺动脉高压诊断与治疗指南(2021版)[J]. 中华医学杂志, 2021, 101(1): 11–51.
[7] RABINOVITCH M, GUIGNABERT C, HUMBERT M, et al. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension[J]. Circ Res, 2014, 115(1): 165–175.
[8] UTHAMALINGAM S, PATVARDHAN E A, SUBRAMANIAN S, et al. Utility of the neutrophil to lymphocyte ratio in predicting long-term outcomes in acute decompensated heart failure[J]. Am J Cardiol, 2011, 107(3): 433–438.
[9] NÚÑEZ J, NÚÑEZ E, BODÍ V, et al. Usefulness of the neutrophil to lymphocyte ratio in predicting long-term mortality in ST segment elevation myocardial infarction[J]. Am J Cardiol, 2008, 101(6): 747–752.
[10] PAPA A, EMDIN M, PASSINO C, et al. Predictive value of elevated neutrophil-lymphocyte ratio on cardiac mortality in patients with stable coronary artery disease[J]. Clin Chim Acta, 2008, 395(1-2): 27–31.
[11] TURAK O, ÖZCAN F, IŞLEYEN A, et al. Usefulness of neutrophil-to-lymphocyte ratio to predict in-hospital outcomes in infective endocarditis[J]. Can J Cardiol, 2013, 29(12): 1672–1678.
[12] KAYRAK M, ERDOĞAN H I, SOLAK Y, et al. Prognostic value of neutrophil to lymphocyte ratio in patients with acute pulmonary embolism: A restrospective study[J]. Heart Lung Circ, 2014, 23(1): 56–62.
[13] HARBAUM L, BAASKE K M, SIMON M, et al. Exploratory analysis of the neutrophil to lymphocyte ratio in patients with pulmonary arterial hypertension[J]. BMC Pulm Med, 2017, 17(1): 72.
[14] JUTRAS-BEAUDOIN N, TORO V, LAJOIE A C, et al. Neutrophil-lymphocyte ratio as an independent predictor of survival in pulmonary arterial hypertension: An exploratory study[J]. CJC Open, 2021, 4(4): 357–363.
[15] HOWARD R, KANETSKY P A, EGAN K M. Exploring the prognostic value of the neutrophil-to-lymphocyte ratio in cancer[J]. Sci Rep, 2019, 9(1): 19673.
[16] AZAB B, CAMACHO-RIVERA M, TAIOLI E. Average values and racial differences of neutrophil lymphocyte ratio among a nationally representative sample of United States subjects[J]. PLoS One, 2014, 9(11): e112361.
[17] ROSE F, HATTAR K, GAKISCH S, et al. Increased neutrophil mediator release in patients with pulmonary hypertension--Suppression by inhaled iloprost[J]. Thromb Haemost, 2003, 90(6): 1141–1149.
[18] KIM Y M, HAGHIGHAT L, SPIEKERKOETTER E, et al. Neutrophil elastase is produced by pulmonary artery smooth muscle cells and is linked to neointimal lesions[J]. Am J Pathol, 2011, 179(3): 1560–1572.
[19] NICKEL N P, SPIEKERKOETTER E, GU M, et al. Elafin reverses pulmonary hypertension via caveolin-1- dependent bone morphogenetic protein signaling[J]. Am J Respir Crit Care Med, 2015, 191(11): 1273–1286.
[20] TIAN W, JIANG X, TAMOSIUNIENE R, et al. Blocking macrophage leukotriene B4 prevents endothelial injury and reverses pulmonary hypertension[J]. Sci Transl Med, 2013, 5(200): 200ra117.
[21] WALMSLEY S R, COWBURN A S, CLATWORTHY M R, et al. Neutrophils from patients with heterozygous germline mutations in the von Hippel Lindau protein (pVHL) display delayed apoptosis and enhanced bacterial phagocytosis[J]. Blood, 2006, 108(9): 3176–3178.
[22] TAMOSIUNIENE R, TIAN W, DHILLON G, et al. Regulatory T cells limit vascular endothelial injury and prevent pulmonary hypertension[J]. Circ Res, 2011, 109(8): 867–879.
Prognostic value of neutrophil-to-lymphocyte ratio in pulmonary arterial hypertension
Department of Cardiovascular Medicine, the First Affiliated Hospital of Zhengzhou University, Zhengzhou 450000, Henan, China
To study the relationship between neutrophil-to-lymphocyte ratio (NLR) and prognosis of patients with pulmonary arterial hypertension (PAH).The clinical data of 58 patients with PAH admitted to the First Affiliated Hospital of Zhengzhou University from November 2020 to October 2022 were retrospectively analyzed. The median follow-up time was 10 months (1-22 months), and the end events were defined as aggravated readmission of heart failure, all-cause death, and cardiopulmonary transplantation. According to the follow-up results, receiver operating characteristic (ROC) curve was drawn to evaluate the sensitivity and specificity of NLR in predicting poor prognosis of patients. Kaplan-Meier method was used for survival analysis. Multivariate Cox regression was used to investigate the risk factors affecting the prognosis of patients with PAH.The male proportion, neutrophils, NLR, N-terminal pro-brain natriuretic peptide, uric acid, and C-reaction protein (CRP) in patients with endpoint events were significantly higher than those without endpoint events (<0.05). The correlation results showed that NLR was positively correlated with CRP (=0.490,<0.05). ROC curve results showed that when the NLR truncation value was 2.72, the sensitivity and specificity of predicting poor prognosis in PAH patients were 75.0% and 73.8%, respectively, and the area under the curve was 0.765. The survival rate of patients with NLR<2.72 was significantly higher than that of patients with NLR≥2.72 (<0.05). Multivariate Cox regression analysis showed that gender (=11.476, 95%: 3.26-40.4) and NLR (=3.657, 95%: 1.117-11.973) were independent risk factors for poor prognosis in PAH patients.High NLR levels suggest poor prognosis and are an independent risk factor for adverse events.
Pulmonary arterial hypertension; Neutrophil; Lymphocyte; Prognosis
R563
A
10.3969/j.issn.1673-9701.2023.22.016
赵荫涛,电子信箱:ytzhao@126.com
(2022–11–02)
(2023–07–17)
