碳纳米管在作物研究中的应用进展与分析
2023-07-28刘学文高军涛杨自尚
刘学文,高军涛,李 赫,杨自尚,陈 静
•专论与综述•
碳纳米管在作物研究中的应用进展与分析
刘学文1,高军涛2,李 赫1,杨自尚1,陈 静1※
(1. 河南农业大学机电工程学院,郑州 450002;2. 中电科新防务技术有限公司,郑州 450047)
碳纳米管在农作物研究中的应用对象已经涵盖了大米、玉米、小麦、番茄、葡萄等常见的农作物。由于研究目的不同,使用的碳纳米管种类、分散方式、浓度也不同,导致文献中出现了试验结果多样化的问题。为了建立一个较为统一的碳纳米管试验框架,该研究首先以作物培育、抗逆、生理指标监测和农业基因工程为纲,对碳纳米管在作物研究中的应用进展进行了综述,并归纳了文献中使用的碳纳米管参数及其应用效果。通过综合分析,论证了作物研究中存在碳纳米管有效浓度未知、特异性作用不明显、间接生物毒性研究不足的问题,并针对性地给出了作物研究中的碳纳米管试验方案建议,包括碳纳米管的选型、培养基的制备以及表征方法。最后,与以往利用原始和共价修饰的碳纳米管进行作物培育不同,在未来,利用非共价修饰的碳纳米管或碳纳米管的非共价堆积作用进行作物研究是重要的发展方向。
纳米;作物;碳纳米管;表面修饰;作物培育;生物毒性
0 引 言
碳纳米管(carbon nanotube,CNT)因管壁层数不同而分为单壁碳纳米管(single-walled carbon nanotube,SWCNT)和多壁碳纳米管(multi-walled carbon nanotube,MWCNT),其优良的光学特性、生物相容性和尺寸特性已经被广泛认可并被应用于许多领域[1-2]。在结构上,SWCNT可以被视为由石墨烯沿某一矢量方向卷曲而成的单层管状结构,直径0.4~2 nm(更大直径的SWCNTs在技术上是可行的,但容易导致缺陷甚至表面塌陷),长度一般在微米到亚毫米量级;MWCNT可以被视为多个不同直径的SWCNTs的同轴嵌套形式,层数为几层到几十层不等。各管层通过范德瓦尔斯力分开,管层间距为0.34~0.41 nm[3]。MWCNT的外直径2~100 nm,内直径1~3 nm,长度一般在微米到亚毫米量级。也有报道称可以制备长度为厘米尺度的SWCNTs[4-5]和MWCNTs[6-8]。
近年来,CNTs与农作物相互作用已经成为研究热点,主要集中在CNTs对作物生长发育的促进和生物毒性等方面[9-10],目标物种已经涵盖了大米、玉米、小麦、番茄、葡萄等常见的农作物。植物的生长发育是个非常复杂的过程,受众多内在外在因素的影响,因此文献中关于CNTs影响作物生长发育的形式具有多样性的特点,其中积极作用包括提高种子发芽率,增大芽长和根长,增加叶片的数量,增加花和果实数量以及提高应对胁迫环境的能力等;但当CNTs浓度高时,也会对作物生长产生氧化损伤、发芽率降低等负面影响[11]。因此,有必要对文献中的农作物的种类、生长阶段、培育方式以及碳纳米管的种类、分散方式、施用方法、浓度进行总结归纳,以期发现规律、为后续研究者建立一个较为统一的研究框架。最后,本文也探讨了CNTs在作物研究中可能的应用范围以及发展趋势。
1 作物对碳纳米管的吸收
植物细胞壁是限制外源性生物分子递送到植物体内的屏障,由于细胞壁孔隙因植物种类、组织类型不同而不同,所以外源性分子穿过细胞壁的难易程度和通量也不同。按照现有的数学模型[12-13],直径超过20 nm的纳米材料进入植物是非常困难的[14]。
但许多文献报道了大尺寸CNTs可以穿过植物细胞壁,SMIRNOVA等发现超声分散的外径(outside diameter,OD)20~70 nm,内径(inner diameter,ID)5~10 nm,长度≥2 μm的MWCNTs分散液可以进入Onobrychis arenaria的根部和叶部[15]。WILD等使用超声分散的外径110~170 nm(长度≤9 μm)的MWCNTs分散液水培小麦时发现MWCNTs可以刺穿表皮和根毛细胞壁,贯穿整个根部的伸长区、根毛和分枝以及根冠,MWCNTs进入细胞质的深度约为4 μm。延长培育时间(最长28天)会导致穿透细胞壁的MWCNTs数量增加,但进入的深度并未超过4 μm[16]。KHODAKOVSKAYA等发现平均直径20 nm,长度0.5~1 μm的MWCNTs可以进入烟叶细胞[17]。
因此,CNTs在细胞壁处的积累可能改变了细胞壁的性质,主要有以下几种途径:
1)CNTs在细胞壁和角质层上造成了新的、更大的孔隙,并在纤维微丝中产生破裂和破坏,最终促进了更大尺寸CNTs的吸收[18]。
2)CNTs会诱导活性氧(reactive oxygen species,ROS)大量爆发[19],其中,OH自由基能够在植物多糖中裂解糖键,引起细胞壁木糖葡聚糖聚合物的分裂,导致植物细胞壁松动,气孔增大[20]。类似地,环境胁迫诱导的ROS爆发也会增加对大尺寸纳米颗粒(nanoparticles,NPs)的吸收[21-22]。
3)表面修饰也可以辅助大尺寸CNTs穿透细胞壁。例如,Serag等验证了用纤维素酶修饰过尖端和侧壁的杯状堆叠碳纳米管(直径范围60~100 nm)可以穿过拟南芥细胞壁,并认为是通过修饰于CNTs表面的纤维素酶和细胞壁之间的局部接触位点进入的,其中,纤维素的局部水解起到了辅助作用[23]。
虽然作物对CNTs的吸收已被试验验证,但CNTs穿透作物细胞壁的原理及其在细胞间转运的动力学原理尚缺乏透彻研究。
2 碳纳米管在作物研究中的应用
不同修饰物修饰的CNTs在作物研究中的应用(如图 1所示)也不同:1)未修饰的或表面羧基化的CNTs主要用来研究对作物培育的影响,羧化的CNTs比未修饰的CNTs具有更优的可移动性,使得芥菜种子发芽更快、发芽率更高[24];2)生物大分子(如蛋白质、核酸[25]等)修饰的或聚合物(如壳聚糖、聚乙二醇PEG、聚乙烯亚胺PEI、聚氨基胺PAMAM[26]等)与生物大分子共同修饰的CNTs常被用来研制生物传感器[27]或进行DNA/RNA递送等;3)表面活性剂(如月桂醇硫酸钠SDS、胆酸钠SC[28]、脱氧胆酸钠SDC等)或单纯聚合物[29]修饰的CNTs对农作物影响鲜有报道。下面是碳纳米管在作物研究中具体应用实例的详细介绍。
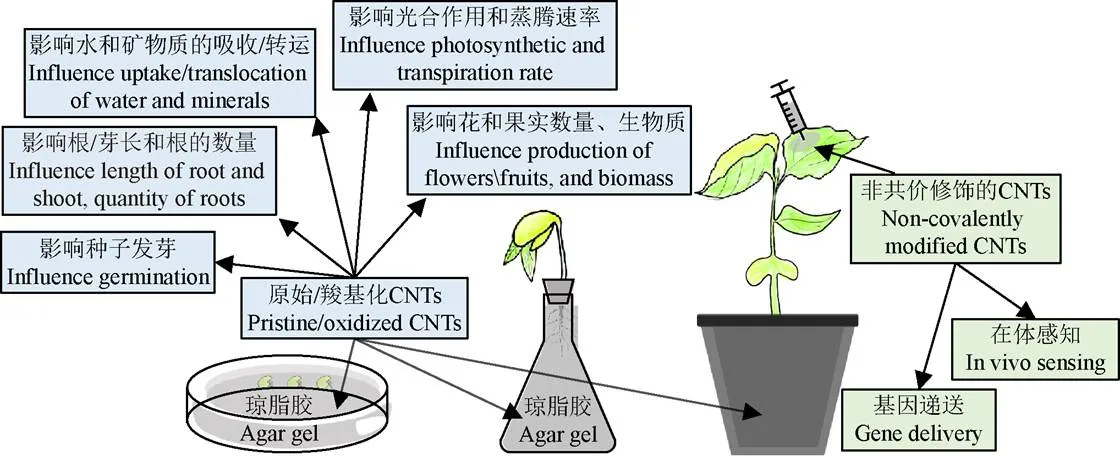
图1 碳纳米管在作物研究中的应用
2.1 碳纳米管在作物培育中的应用
CNTs的作用包括[30-32]:提高种子发芽率,增加芽长和根长,增加根和叶片的数量,增加花和果实数量,增强光合作用率,提高蒸腾速率,增加作物的生物质,增强水和矿物质的摄取以及提高转运速率等。
2.1.1 碳纳米管对作物种子萌发的影响
2.3~23 mg/L CNTs处理过的芥菜种子比蒸馏水处理过的发芽率更高,50%的种子萌芽所需时间缩短了44%,而46 mg/L的CNTs明显出现生物毒性[24];SMIRNOVA等使用0.1~10 mg/L未修饰的MWCNTs分散液浸泡萌发Onobrychis arenaria种子,种子的发芽率有明显提升,根和茎的长度均明显增加[15]。Haghighi等用10~40 mg/L未修饰的MWCNTs(直径8~15 nm,长度>10 μm)分散液直接萌发种子,发现番茄、洋葱和小萝卜的平均发芽时间延长;根长和茎长增加;40 mg/L的CNTs对洋葱和小萝卜产生生物毒性[33]。Cañas等发现未修饰的SWCNTs会抑制番茄的根伸长,增强洋葱和黄瓜的根伸长,PABS-CNTs会抑制生菜的根系伸长,而卷心菜和胡萝卜不受上述两种SWCNTs的影响[29]。
CNTs对种子萌发的影响呈现明显的浓度依赖和种类依赖,低浓度的CNTs对种子的萌发产生积极影响。
2.1.2 碳纳米管对作物幼苗生长的影响
Tiwari等利用磁力搅拌的MWCNTs-琼脂混合物(CNTs直径6~9 nm,长度5 μm)培养玉米种子,发现MWCNTs使黑色层种皮和根部细胞穿孔,因而水的输送增强。在正常培养基中,低剂量MWCNTs被认为是有益的,可改善根部吸水性、植物生物量和必需的Ca、Fe营养素的浓度,但在高浓度下抑制了玉米幼苗的生长[34]。赛闹汪青等使用1 000 mg/L的MWCNTs培育水稻幼苗发现直径30 nm以下的MWCNTs抑制了水稻幼苗的生长,但直径大的影响更小,而直径大于50 nm的MWCNTs却显著优化了水稻幼苗的生长[35]。
CNTs对幼苗生长的影响存在浓度依赖,同时也存在直径尺寸依赖,参照CNTs影响种子萌发的试验也可以发现:使用大直径CNTs的文献中,较少提及CNTs对作物培育的毒性。
2.1.3 碳纳米管对花和果实数量的影响
在补充MWCNTs的土壤中种植长春花,MWCNTs通过诱导早期花卉发育和更高的花卉产量来刺激植物繁殖系统。在补充MWCNTs(浓度50 mg/L,200 mg/L,OD 13~18 nm,长度1~12 µm)和石墨烯的土壤中种植的长春花,总花产量分别提高了37%和58%[36]。在补充表面弱羧化MWCNTs的土壤中生长的番茄植物产生的花朵和果实是对照土壤的两倍[37]。González-G等发现MWCNTs(OD 30~50 nm,长度10~20 μm)增加了番茄作物的果实产量和干枝生物量。由于抗坏血酸、类黄酮的含量和谷胱甘肽过氧化物酶的活性增加,抗氧化系统得到改善。碳纳米管的应用也提高了净光合作用和水分利用效率[38]。包兴成等发现4~12 mg/L的羧化MWCNTs(OD 20~30 nm,长度10~30μm)对夏黑葡萄的生长和果实品质均有促进作用,其中,8 mg/L的CNTs作用效果最佳[39]。
与前述对种子萌发的影响不同,上述文献中并未发现高浓度的CNTs对种子萌芽率、根长、芽长、花和果实数量出现抑制作用。
2.1.4 碳纳米管对作物培育的负面作用机理
CNTs会引起植株内ROS过量爆发,导致植株氧化损伤。例如:Tan等发现用CNTs培养的水稻细胞ROS增加,细胞活力降低[40]。MWCNTs与草甘膦联合的水培拟南芥的根系和地上部的生长受到显著影响,丙二醛含量高,代谢抗氧化分子上调表明MWCNTs与草甘膦联合施用引起强烈的氧化损伤,抗氧化酶活性的降低表明ROS与抗氧化防御系统之间由于ROS的不断产生而失衡[41]。
CNTs促进细胞壁非流动性Ca2+流失,将引起后续植物损伤:Tiwari等研究发现CNTs与Fe2+同时存在时,细胞内Fe2+的含量增加,而Ca2+的含量降低[34]。另外,CNTs可以将Fe3+还原成Fe2+,Fe2+会继续与Ca2+发生交换[42]。由于根部Ca2+主要固化于细胞壁,因此,CNTs的存在会加重细胞壁Ca2+的流失,可能造成根部细胞壁被穿孔,体内营养物质外渗,更易被病菌侵染等后果。
文献中CNTs的作用结果因作物种类、CNTs类型和浓度的不同而不同,甚至出现相互矛盾的现象。通过分析CNTs产生负面作用的原理发现,当试验中的培养基、培养方式、作物生长特性不同时,产生差异性结果是必然的。表1给出了典型文献中的CNTs参数。总体来看,大直径的、短型的、合适浓度的CNTs对作物的培育能够起到积极作用。
2.2 碳纳米管在作物对抗胁迫环境中的应用
Martínez等发现MWCNTs在盐胁迫下可以进入成株的细胞,积累量较高,认为MWCNTs对NaCl处理植物生长的积极影响是水分吸收增加的结果[30]。Pandey等发现MWCNTs减少了NaCl引起的毒性作用,盐胁迫条件下栽培的作物长期施用MWCNTs改善了期望的显性性状:长春花发育了更高的花数和叶数,棉花增加了纤维生物量。干旱胁迫试验表明,缺水条件下,在成熟期长春花植株中引入MWCNTs可提高植物存活率,且无叶片枯萎症状[36]。贺君杰等发现5 mg/L的MWCNTs可以通过调节重金属转运ATP酶5(ATPase5)基因的表达减少铜离子吸收、提高根部非蛋白硫醇的水平以螯合过量的铜离子等方式缓解苜蓿铜胁迫[43]。
CNTs在作物抗逆中的作用既有植物生理作用也有基因表达调控作用,机理较复杂。
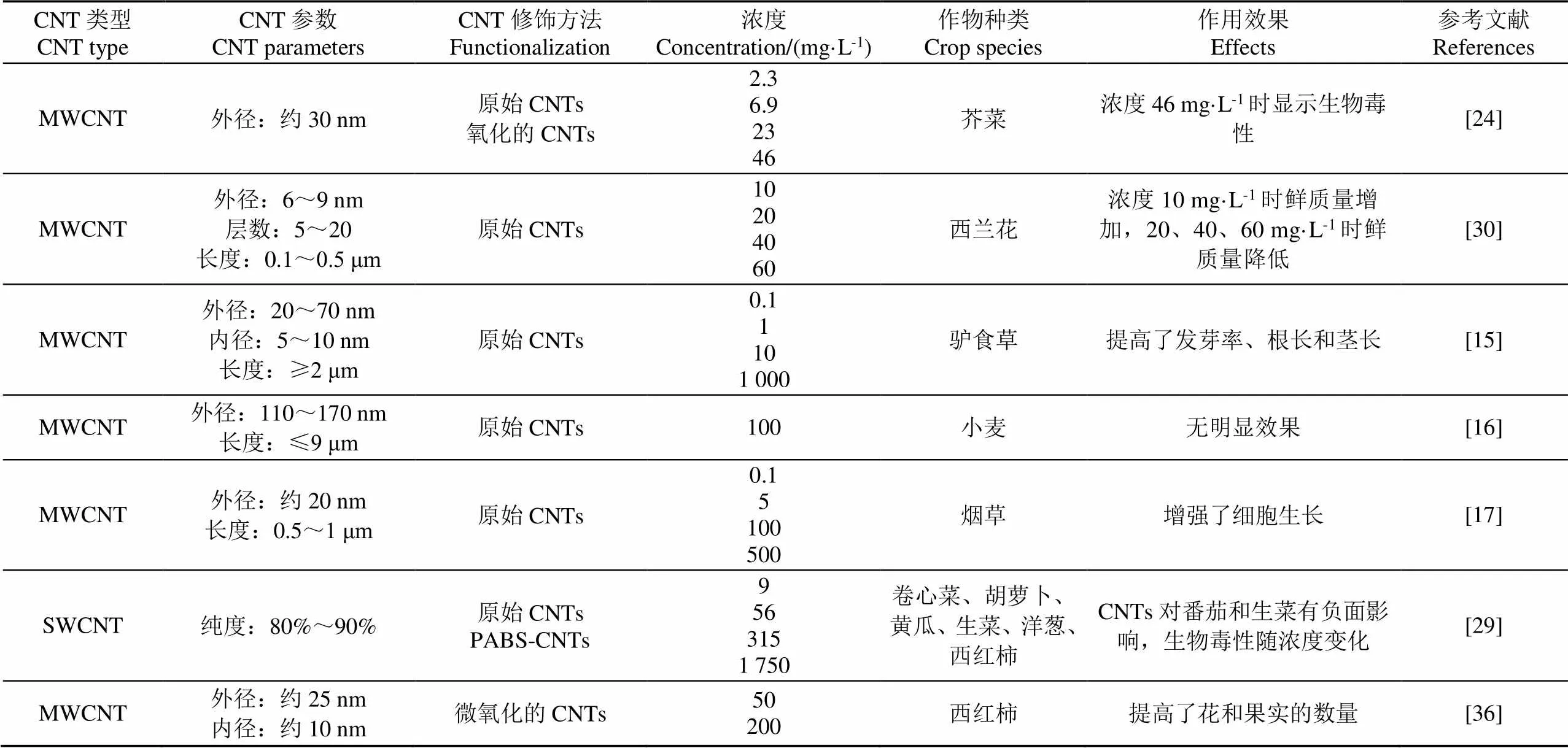
表1 作物培育研究中的CNTs参数
注:SWCNT:单壁碳纳米管;MWCNT:多壁碳纳米管。
Note: SWCNT: Single-walled carbon nanotube; MWCNT: Multi-walled carbon nanotube.
2.3 碳纳米管在作物监测中的应用
核酸适配体、蛋白质或特殊ssDNA片段修饰的SWCNTs具有很好的近红外荧光特性,已经被广泛用来研制各种生物传感器[44],基于荧光强度变化的传感器工作原理如图2a所示,当SWCNTs周围的化学环境发生变化时,其荧光强度或荧光中心波长会发生变化。例如,Lambert等利用ssDNA缠绕的SWCNTs高选择性地检测玉米黄曲霉毒素B1(aflatoxin B1,AFB1),而不受伏马菌素(fumonisin B1,FB1)等毒素的影响[45],另外,基于CNTs的电化学传感器也可以实现对真菌毒素的检测[46]。Wu等报道了利用对H2O2敏感的DNA适配体修饰的SWCNTs来检测H2O2,实现通过SWCNTs荧光的强弱变化实时反映植物体内H2O2的含量变化,原理如图 2b所示[47],有望实现对胁迫下植物体内ROS变化(时间点和位置)的监测。Lew等利用ssDNA修饰的SWCNTs实现了对莴苣、芥菜、菠菜、大头菜、酸模和拟南芥中创伤叶片处H2O2含量的实时在线测量[48]。
虽然SWCNTs表面可以较为容易地修饰多种生物大分子,但是修饰过程会改变适配体或DNA的构象,这可能会影响其与靶标分子的特异性结合,而这种结合是否具有可逆性的荧光响应也是需要解决的问题。
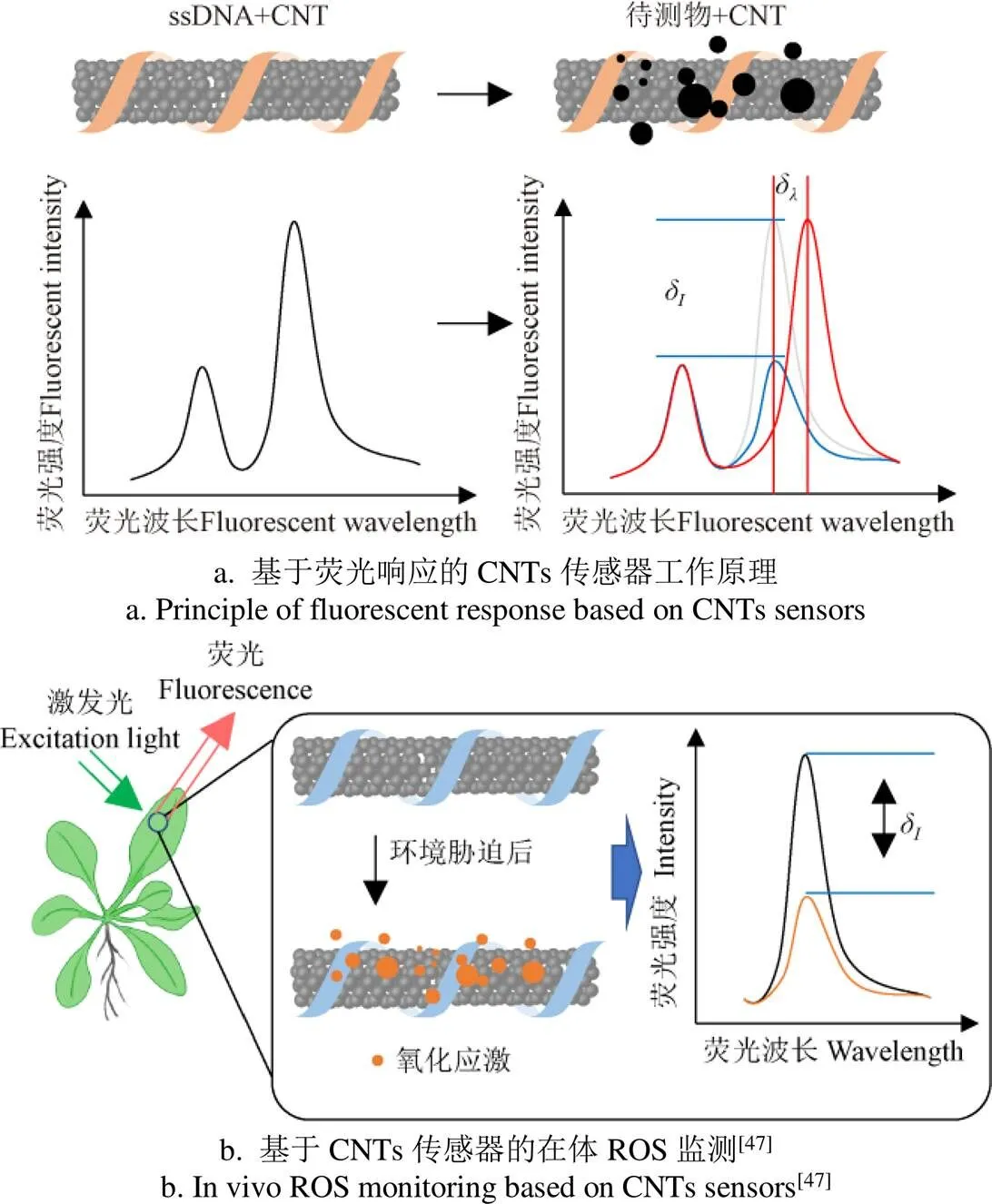
注:当基于荧光响应的CNTs传感器与待测物结合后,其荧光的光强或中心波长会发生变化。δI为光强的变化量,δλ为中心波长的变化量。
2.4 碳纳米管在DNA/RNA递送中的应用
SWCNTs的直径很小,表面修饰DNA后的复合物直径仍然小于植物细胞壁孔隙尺寸(例如,原始SWCNTs直径1.3 nm,聚合物修饰后的直径为8.1 nm,再经DNA培育后的直径变为16.3 nm[49]),能够穿透植物细胞壁[50]。不过,SWCNTs的长度可能会影响其穿过细胞壁,因此一般需要将SWCNTs变短。
与传统植物DNA递送和小干扰RNA(small interfering RNA,siRNA)施用方法不同,CNTs介导的DNA/RNA递送具有瞬时转化率高的特点[51-52],而且CNTs还可以保护多核苷酸避免被核酸酶降解[53]。目前,SWCNTs被认为是基因递送的理想载体[54-55],例如:Kwak等利用壳聚糖修饰的SWCNTs选择性地将质粒DNA递送至叶绿体,在成熟的芥菜、金莲花、烟草和菠菜植物以及分离的拟南芥叶肉原生质体中实现了叶绿体靶向转基因递送和瞬时表达,转化率达到88%[56]。Demirer等通过共价修饰和非共价修饰的SWCNTs实现了在本氏烟草、芝麻菜、小麦、棉叶和芝麻菜原生质体上的高效DNA递送和强蛋白表达[49,57]。
Apartsin等详述了siRNA绑定CNTs形成复合物的方法、靶细胞如何吸收siRNA-CNTs复合物以及细胞内siRNA如何解绑等[58]。Edwards等将双链DNA(double-stranded DNA,dsDNA)连接到聚酰胺-胺聚合物(PAMAM)修饰的MWCNTs上形成的PAMAM- CNT- dsRNA复合物注射到赤拟谷盗幼虫体内时,发现PAMAM-CNT在细胞液泡和细胞核中可见,而且两个靶基因α-微管蛋白和线粒体RNA聚合酶的敲除水平显著增加。与单独注射dsRNA(靶基因α-微管蛋白)相比,注射PAMAM-CNT-dsRNA的幼虫发现疱翅表型的发生率更多,这表明,使用功能化的CNTs进行dsRNA递送可以提高RNA干扰对害虫物种的功效[59]。
碳纳米管用于DNA/RNA递送的关键是基因的“装载”。碳纳米管的结构决定了dsRNA和dsRNA很难直接缠绕到CNTs上[60],文献中主要有两种形式实现dsDNA/dsRNA递送:1)先将两条互补的单链DNA(single-stranded DNA,ssDNA)/ssRNA分别缠绕到CNTs表面,当上述混合物进入细胞后,ssDNA或ssRNA与CNTs解吸附,再形成dsDNA或dsRNA,原理如图3a所示[50];2)先使用聚合物修饰CNTs,然后通过电荷吸附作用或形成络合物等方式将dsDNA或dsRNA固定在CNTs表面,原理如图3b所示[59]。另外,还有一种方法可以尝试:原理如图3c所示[61-62],将dsDNA或dsRNA与一段ssDNA相连,ssDNA通过π-π堆积缠绕到CNTs表面后,即可将dsDNA或dsRNA固定在CNTs上。
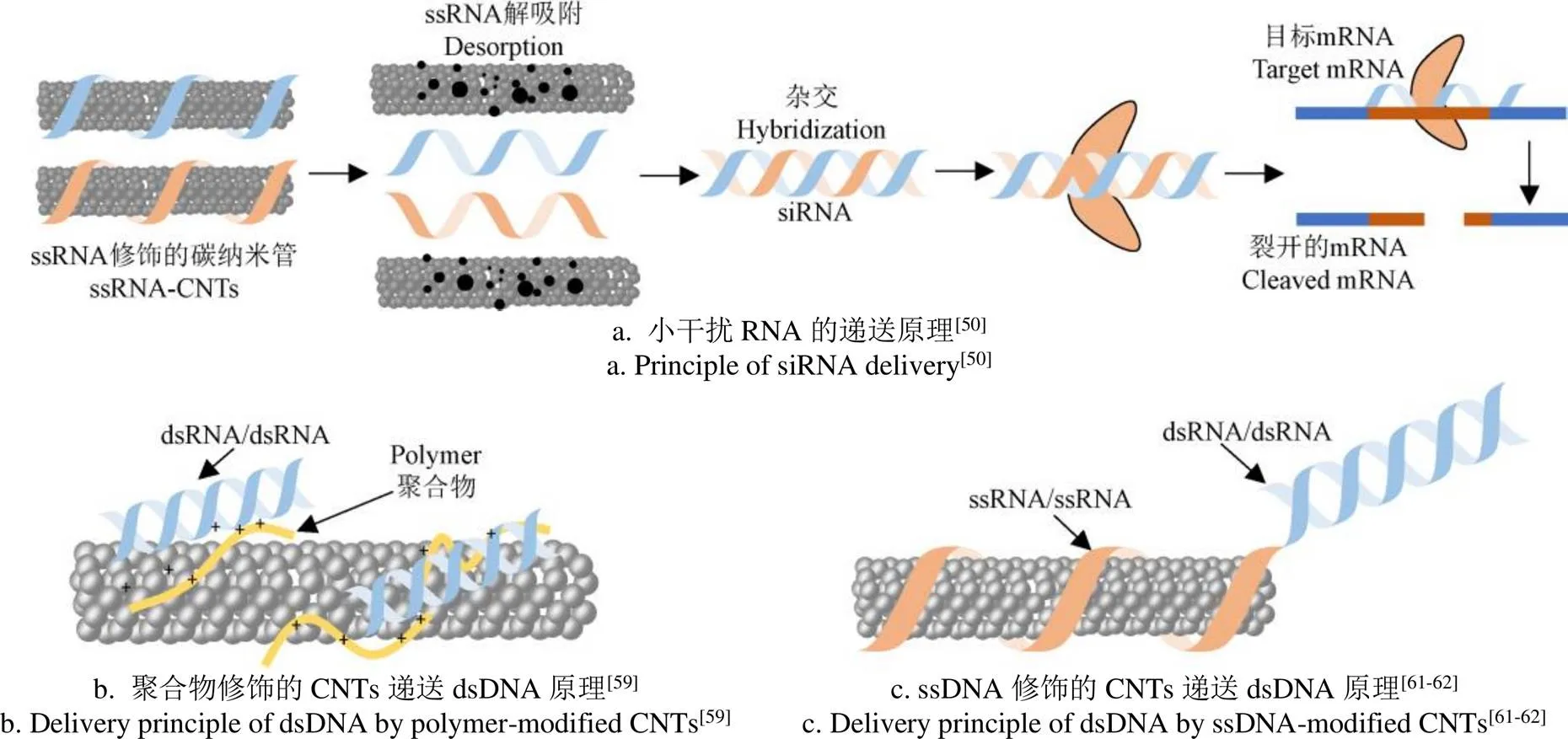
图3 DNA/RNA递送的操作方法
3 作物研究中碳纳米管的使用问题分析
3.1 碳纳米管培养基的有效浓度问题
浓度是研究CNTs影响作物生长发育必须衡量的参数,虽然文献中都给出了所使用的CNTs培养基浓度,但实际能够进入植物细胞的有效CNTs浓度是不确定的[63]。这是因为一部分文献中采用超声直接分散的原始未修饰的CNTs,分散效果较差,CNTs聚集团较多(如图4a所示[16],箭头处为聚集团,Copyright 2009 American Chemical Society);另一部分文献采用超声分散的表面羧基化的CNTs,由于羧基与CNTs的质量比和体积比均过低,其在一定时间后仍然会产生聚集、沉淀。
尽管文献中的透射电子显微镜或荧光图像显示植物细胞中的CNTs往往是小的聚集团[64](如图4b所示[65],Copyright 2021 American Chemical Society),尺寸在数μm甚至百μm量级[53],但目前还没有直接证据证明CNTs是以聚集团的形式进入的细胞。与之相反的是,Husen等论证了当CNTs通过毛细作用被输送到通道狭窄的点时,CNTs会积聚并阻塞营养物质进一步流动的通道[66];Martínez等使用超声分散的未修饰MWCNTs-霍格兰溶液(直径6~9 nm,长度0.1~0.5 μm)水培西兰花,发现在植株根部和茎部的CNTs都是以单根的形式存在[30]。综上分析,CNTs应该是以单根形式穿过细胞壁,并会在细胞质中的聚集,而CNTs培养基中的聚集团是不太可能整体进入植物细胞的。因此,研究者应当注意测量CNTs的有效浓度。
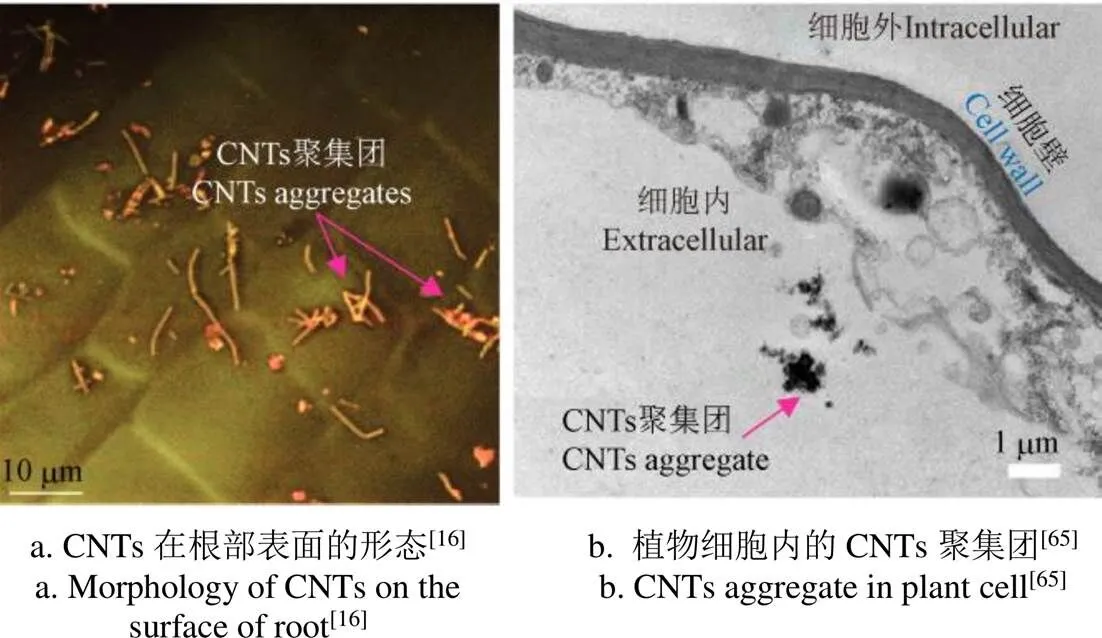
图4 试验中CNTs的典型形态
3.2 碳纳米管的特异性作用问题
试验中可采用的CNTs商品较少,只有表面氨基化、羧基化或羟基化的几类。由于并未在表面修饰特殊功能的材料,因此在研究作物植株受CNTs影响时,CNTs往往是被当作一种纳米尺寸的材料使用,试验结果也与大部分的NPs(如CeO2、TiO2、ZnO等)对作物培育的影响相同:都可以对种子引发、种子萌发、幼苗生长过程、对抗胁迫压力产生有益影响,从而提高种子发芽率,增大芽长和根长[18,67-69]。究其原因,基本共识是由于NPs可以导致细胞壁穿孔,增强了水的吸收、改变了种子的抗氧化系统等[19],亦即在促进作物生长方面,与其他NPs相比,无修饰或非选择性修饰的CNTs并未显示具有独特的作用效果。
3.3 碳纳米管带来的食品安全问题
无论是被用于人类/动物疾病治疗、植入式传感,还是被用于农业,CNTs的生物毒性一直是被讨论的问题。相比于人类/动物领域主要研究CNTs的直接毒性,农业中还需要考虑其间接毒性问题[70]。
3.3.1 碳纳米管的直接毒性
美国国家职业安全卫生研究所在职业性癌症致癌物清单中并未将CNTs列为致癌物[71],但其一项研究表明,接触CNTs的工人离体全血样本对两种微生物刺激剂(脂多糖和葡萄球菌肠毒素)功能性免疫反应较低[72]。
REZAEI等创建了用于全面调查番茄植株器官中积累的MWCNTs可能带来的健康风险评估平台,用CNTs污染植株结出的西红柿喂养小鼠,结果显示所有参与评估的动物器官和生理参数都没有显示出毒性反应,表明由于根部施用导致在植物不同部位积累的CNTs数量不足以对小鼠产生毒性[73]。
3.3.2 碳纳米管的间接毒性
CNTs中的碳原子主要以sp2杂化形式存在,管壁碳原子的P电子形成大范围的未配对的离域π电子云,因此可以通过π-π堆积(π-stacking)与其他含有碳环状结构的物质(如芳香族化合物)结合[74-77]。另外,表面修饰物也会与离子结合,如吸附水中重金属离子(镉、稀土、铅、铜离子等)[78-80]。上述吸附作用或离子结合作用使得CNTs可以作为载体将外源污染物运输到农作物的多个部位,造成外源有毒物质在植株内的积累,例如:
1)Chen等研究发现,在超声分散的MWCNTs与污染物(六氯苯HCB、滴滴涕p-p′ DDT)的同时作用下,污染物在芥菜植株内的富集,并给出了几种污染物的动力学积累过程[81]。WILD等发现超声分散MWCNTs穿透过的小麦根部细胞壁更加容易使大分子菲进入该细胞[16],但是没说明菲是由于π-π堆积作用附着在碳管壁一起进入的还是由被碳管扩大的细胞壁孔洞进入的细胞。
2)Wang等的研究表明,羧化的MWCNTs与Pb+Cd的组合导致蚕豆幼苗营养元素不平衡,叶片中Pb和Cd的富集引起叶片氧化胁迫和损伤,而单独的MWCNTs-COOH或Pb+Cd作用却没有上述效果[82]。
相较而言,含有CNTs的作物食用安全问题,可以直接参考动物或人类直接CNTs暴露的毒性研究结论。但是,在含有污染物的植株生长环境中,CNTs运载外源毒性物质进入植株带来的间接毒性问题需要更深入的研究。
4 作物研究中碳纳米管试验方案建议
4.1 碳纳米管选型建议
SWCNTs与MWCNTs具有同类型的特性,都具有很高的比表面积和长径比、高的化学反应活性等[83-84],但SWCNT在几何尺寸、荧光特性和生物毒性方面具有明显优势。通过综合分析文献可知:
1)大直径的MWCNTs对作物培育的负面影响较少。
2)考虑到细胞壁的通过性,直径越小的CNT越容易穿过细胞壁,或允许“装载”更大的分子。
3)CNT是一维纳米材料,长度也会影响植物细胞的吸收。文献中尺寸短的CNTs对作物的影响更显著。
4)虽然有文献指出共价修饰后的MWCNTs可以产生荧光(激发光中心波长350 nm,荧光中心波长为418 nm)[85-86],但其不是常用的荧光物质。而非共价修饰的SWCNTs具有非常优秀的荧光特性[87],其典型手性CNTs的荧光发射峰在近红外Ⅱ区,远离植物色素的自发荧光波段,避免植物组织自发荧光的干扰,在表征方面具有优势。
5)世界卫生组织国际癌症研究机构公布的致癌物清单中,MWCNTs被认定为2B类致癌物,而SWCNT被认定为3类致癌物[88]。SWCNT的直接生物毒性比MWCNT小很多。
因此,本文建议在作物培育的研究中使用短型大直径多壁碳纳米管作为试验材料;而在以递送和传感为主的作物研究中,使用短型单壁碳纳米管作为试验材料。
4.2 碳纳米管培养基制备方法建议
由于碳纳米管疏水性强、易聚集,若采用超声分散未修饰的或表面羧基化的CNTs来制备溶液培养基,或采用将CNTs粉末与琼脂直接搅拌混合的方法制备琼脂培养基[34,89],不同的研究者很难获得相同的有效浓度。
为了保证浓度对作物影响结果的可复现性,本文建议采用表面修饰的碳纳米管进行试验研究,修饰物可以为在植株体内可降解的有机分子如ssDNA、吡唑醚菌酯等[90-91],由于营养液中的阳离子可能与CNTs表面修饰物发生反应,因此不建议用营养液来分散CNTs。
CNTs培养基储备液的制备流程建议为CNTs和修饰物的水溶液混合后在水浴杯中进行超声分散,将分散液高速离心4 h后取80%~90%的上层溶液,利用分光光度计测量CNTs浓度。
由于制备琼脂时需要将水加热至接近沸腾,而修饰物在高温下,构象可能会发生改变甚至解析附,故制备CNTs琼脂培养基的流程建议为琼脂粉与少量高纯水混合后加热至全部溶解,再加入CNTs储备液搅拌均匀并定容。
基于上述两种培养基,可以方便地利用水培法和琼脂培育法探索CNTs在作物研究中的各种作用[30]。
4.3 植株内CNTs的表征方法
一般采用透射电子显微镜和扫描电镜对CNTs的形貌[92]或植物组织切片成像[93]。采用电子断层扫描(electron tomography,ET)可以获得细胞的3D图像,在观察CNTs在植物细胞内的体分布方面具有更大的优势[94]。
荧光显微镜也常用于表征CNTs。考虑到显微镜细胞成像系统的通用性,一般农学生物实验室常采用以下3种用法:1)采用对细胞染色的方法对细胞成像,利用CNTs在该激发光波段(如370、488、592 nm等)无荧光的特性,通过研究图像中黑斑来表示其形态和在细胞中的位置[64];2)将荧光团修饰到CNTs表面进行荧光标记,而不对细胞染色,通过融合荧光图像与细胞的明场图像来研究其相对位置(如图5a所示[95],Copyright 2009 American Chemical Society);3)将CNTs作为荧光团的淬灭物质:进入细胞前,连接在探针两端的荧光团和CNTs之间会紧密接触,使得荧光被淬灭。进入细胞后,当探针与目标物结合后,由于构象发生变化,荧光团脱离CNTs表面并发射荧光(如图5b所示[53])。
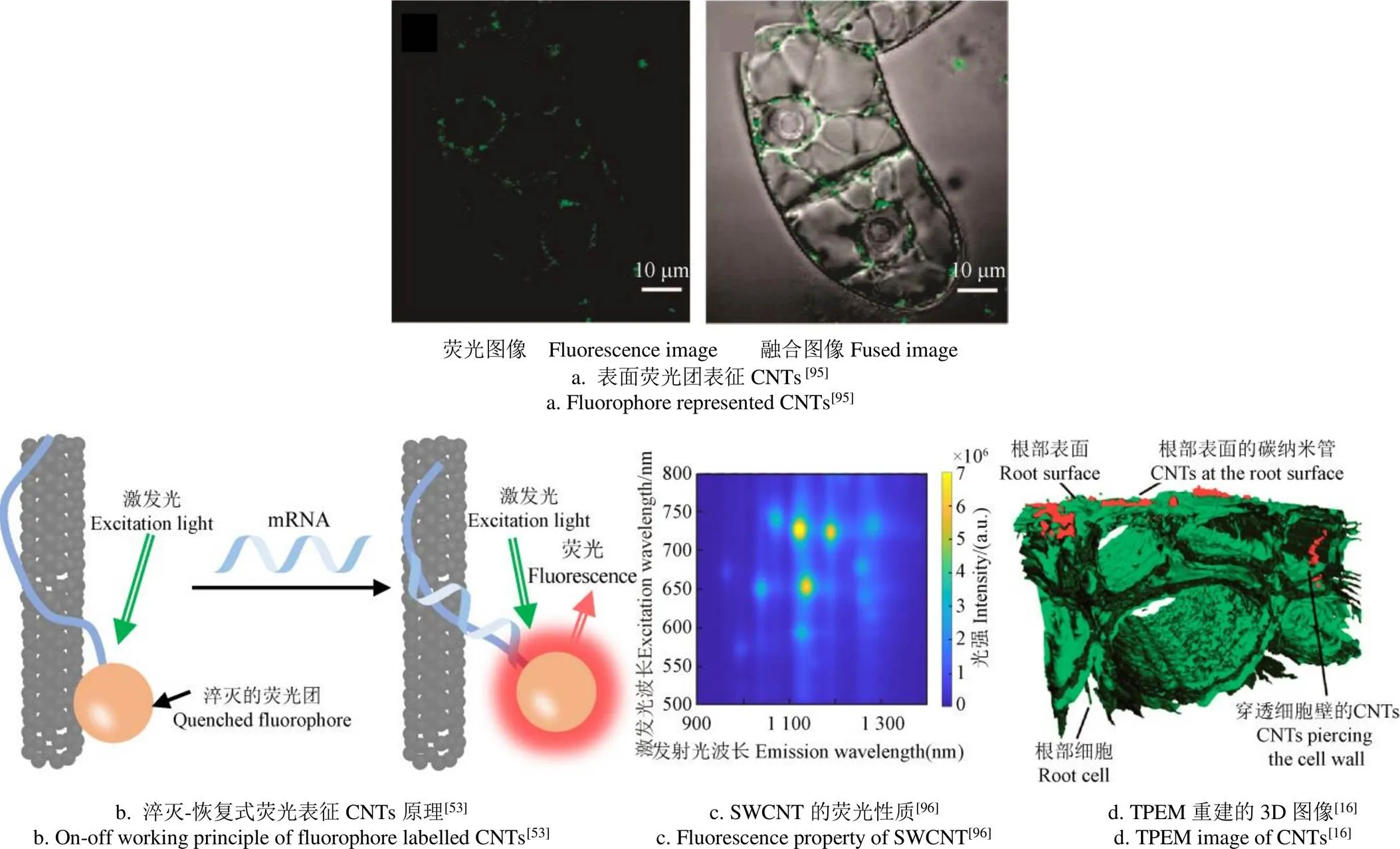
图5 植株内CNTs的不同表征方式
另外,SWCNTs具有非常优秀的荧光特性,其激发光范围为550~800 nm,荧光范围为900~1 400 nm[96](如图5c所示)。近红外荧光显微镜可以被用来检测SWCNTs的荧光,图像包含荧光光强和二维空间位置信息[97],近红外共聚焦荧光显微镜景深小,可以用于确定SWCNTs在细胞内的深度[98]。双光子激发荧光显微镜(two-photon excitation microscopy,TPEM)更适用于检测MWCNTs的荧光(360~530 nm),因为激发光710 nm恰好在植物组织的近红外窗口区,与350 nm的激发光相比优势明显。同时,基于多幅TPEM图像的3D重建技术,可以更清晰地分辨MWCNTs与植物细胞的位置关系(如图5d所示[16],Copyright 2009 American Chemical Society)。
5 结论与展望
碳纳米管在农作物生长发育、抗逆、育种等方面的积极作用已经被证实,应用前景广阔。但也不可避免地存在需要改进的情况,例如,未修饰的和共价修饰的CNTs对作物培育的影响已经有丰富的研究基础,但非共价修饰的CNTs的应用研究相对较少。因此,对CNTs在作物研究中的应用总结与展望如下:
1)CNTs穿透细胞壁的原理、进入细胞后的聚集机制及其在细胞间转运的动力学原理尚缺乏透彻研究,需要设计新颖的试验、采取新的表征手段或采用新的植入式在线传感器进行研究。
2)基于CNTs的植入式在线监测传感器将是CNTs在作物研究中的重要应用方向之一。相对于被用作体外传感器,由于CNTs可以穿透细胞壁,具有优良的近红外荧光特性,适当浓度的CNTs对作物没有生物毒性(甚至有益于作物的生长),因此,非共价修饰的CNTs更适合作为植入式传感器,对植物体内的多种化学物质进行实时在线监测。
3)作为转运载体是CNTs在作物研究中的一个重要应用方向:在siRNA递送方面,siRNA-CNTs复合物在施用周期、植物细胞吸收、害虫细胞吸收、保护RNA被降解方面均有独特的优势,但是,相关文献并没有进行包括siRNA-CNTs复合物的施用、植物细胞吸收、害虫细胞吸收、阻断基因表达、害虫发育停滞或者死亡这一系列完整的试验流程,因此,CNTs介导的RNA干扰抗虫技术还有广阔的研究空间。
4)利用CNTs对有机农药的吸附,实现杀菌剂的精准定点运载及农药缓释,用来提高农药的药效以及延长半衰期也是一个重要的研究方向。
另外,CNTs与动物相互作用(递送、检测、疾病治疗)的研究深度和广度远超其与植物的相互作用研究。近年来,已经有将动物研究方面的技术应用转化到植物方面的例子。在未来,将CNTs在动物应用研究中的经验转化到植物应用研究中将会有效地促进农作物培育的发展。
[1] 乔俊,赵建国,解谦,等. 纳米炭材料对作物生长影响的研究进展[J]. 农业工程学报,2017,33(2):162-170. QIAO Jun, ZHAO Jianguo, XIE Qian, et al. Review of effects of carbon nano-materials on crop growth[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2017, 33(2): 162-170. (in Chinese with English abstract)
[2] 柳馨,铁健,铁生年. 温室多壁碳纳米管芒硝基相变材料储能性能[J]. 农业工程学报,2016,32(6):226-231. LIU Xin, TIE Jian, TIE Shengnian. Energy storage properties of mans nitro phase transition materials of multi-walled carbon nano-tubes of greenhouse[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2016, 32(6): 226-231. (in Chinese with English abstract)
[3] LIU M Q, JOHN M C. Structure of carbon nanotubes studied by HRTEM and nanodiffraction[J]. Ultramicroscopy, 1994, 53(4): 333-342.
[4] ZHANG R F, ZHANG Y Y, WEI F. Controlled synthesis of ultralong carbon nanotubes with perfect structures and extraordinary properties[J]Accounts of Chemical Research, 2017, 50(2): 179-189
[5] CHANG N K, HSU J H, SU C C, et al. Horizontally oriented carbon nanotubes coated with nanocrystalline carbon[J]. Thin Solid Films, 2009, 517(6): 1917-1921.
[6] ZHANG R, NING Z, XU Z, et al. Interwall friction and sliding behavior of centimeters long double-walled carbon nanotubes[J]. Nano Letters, 2016, 16(2): 1367-1374.
[7] KIM H, WANG M, LEE S K, et al. Tensile properties of millimeter-long multi-walled carbon nanotubes[J]. Scientific Reports, 2017, 7(1): 1-7.
[8] CHO W, SCHULZ M, SHANOV V. Growth and characterization of vertically aligned centimeter long CNT arrays[J]. Carbon, 2014, 72: 264-273.
[9] VERMA S K, DAS A K, GANTAIT S, et al. Applications of carbon nanomaterials in the plant system: A perspective view on the pros and cons[J]. Science of the Total Environment, 2019, 667: 485-499.
[10] Safdar M, Kim W, Park S, et al. Engineering plants with carbon nanotubes: A sustainable agriculture approach[J]. Journal of Nanobiotechnology, 2022, 20(1): 1-30.
[11] Mathew S, Tiwari D K, Tripathi D. Interaction of carbon nanotubes with plant system: A review[J]. Carbon Letters, 2021, 31(2): 167-176.
[12] Deinum E E, Mulder B M, Benitez-A Y. From plasmodesma geometry to effective symplasmic permeability through biophysical modelling[J]. Elife, 2019, 8: e49000.
[13] Hughes A, Faulkner C, Morris R J, et al. Intercellular communication as a series of narrow escape problems[J]. IEEE Transactions on Molecular, Biological and Multi-Scale Communications, 2021, 7(2): 89-93.
[14] Faulkner C. Plasmodesmata and the symplast[J]. Current Biology, 2018, 28(24): R1374-R1378.
[15] Smirnova E, Gusev A, Zaytseva O, et al. Uptake and accumulation of multiwalled carbon nanotubes change the morphometric and biochemical characteristics of Onobrychis arenaria seedlings[J]. Frontiers of Chemical Science and Engineering, 2012, 6(2): 132-138.
[16] Wild E, Jones K C. Novel method for the direct visualization of in vivo nanomaterials and chemical interactions in plants[J]. Environmental Science & Technology, 2009, 43(14): 5290-5294.
[17] Khodakovskaya M V, Kanishka d S, Biris A S, et al. Carbon nanotubes induce growth enhancement of tobacco cells[J]. ACS nano, 2012, 6(3): 2128-2135.
[18] Fiol D F, Terrile M C, Frik J, et al. Nanotechnology in plants: Recent advances and challenges[J]. Journal of Chemical Technology & Biotechnology, 2021, 96(8): 2095-2108.
[19] Marslin G, Sheeba C J, Franklin G. Nanoparticles alter secondary metabolism in plants via ROS burst[J]. Frontiers in Plant Science, 2017, 8: 832.
[20] Tenhaken R. Cell wall remodeling under abiotic stress[J]. Frontiers in Plant Science, 2015, 5: 771.
[21] Rossi L, Zhang W, Lombardini L, et al. The impact of cerium oxide nanoparticles on the salt stress responses of Brassica napus[J]. Environmental Pollution, 2016, 219: 28-36.
[22] Rossi L, Zhang W, Schwab A P, et al. Uptake, accumulation, and in planta distribution of coexisting cerium oxide nanoparticles and cadmium in glycine max (.) merr[J]. Environmental Science & Technology, 2017, 51(21): 12815-12824.
[23] Serag M F, Kaji N, Tokeshi M, et al. Introducing carbon nanotubes into living walled plant cells through cellulase-induced nanoholes[J]. RSC Advances, 2012, 2(2): 398-400.
[24] Mondal A, Basu R, Das S, et al. Beneficial role of carbon nanotubes on mustard plant growth: An agricultural prospect[J]. Journal of Nanoparticle Research, 2011, 13(10): 4519-4528.
[25] Gillen A J, Kupis-R J, Gigli C, et al. Xeno nucleic acid nanosensors for enhanced stability against ion-induced perturbations[J]. The Journal of Physical Chemistry Letters, 2018, 9(15): 4336-4343.
[26] Varghese R, Binu N, Dalvi Y B. Biomedical applications of carbon nanotubes[J]. Carbon Nanotubes, 2022: 61-80.
[27] 王丽茹,李浩榛,王茜茜,等. 基于聚苯胺的农用柔性低阻抗pH传感芯片设计与试验[J]. 农业工程学报,2022,38(增刊):184-192. WANG Liru, LI Haozhen, WANG Qianqian, etal. Design and feasibility test of a lower-impedance flexible pH sensor based on polyaniline[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2022, 38(Suppl): 184-192. (in Chinese with English abstract)
[28] Lambert B, Gillen A J, Schuergers N, et al. Directed evolution of the optoelectronic properties of synthetic nanomaterials[J]. Chemical Communications, 2019, 55(22): 3239-3242.
[29] CAÑAS J E, LONG M, NATIONS S, et al. Effects of functionalized and nonfunctionalized single-walled carbon nanotubes on root elongation of select crop species[J]. Environmental Toxicology and Chemistry, 2008, 27(9): 1922-1931.
[30] Martínez B M, Zapata L, Chalbi N, et al. Multiwalled carbon nanotubes enter broccoli cells enhancing growth and water uptake of plants exposed to salinity[J]. Journal of Nanobiotechnology, 2016, 14(1): 1-14.
[31] Rudakiya D, Patel Y, Chhaya U, et al. Carbon Nanotubes in Agriculture: Production, Potential, and Prospects[M]//Nanotechnology for Agriculture. Singapore: Springer, 2019: 121-130.
[32] Patel D K, Kim H B, Dutta S D, et al. Carbon nanotubes-based nanomaterials and their agricultural and biotechnological applications[J]. Materials, 2020, 13(7): 1679.
[33] Haghighi M, Teixeira S J A. The effect of carbon nanotubes on the seed germination and seedling growth of four vegetable species[J]. Journal of Crop Science and Biotechnology, 2014, 17(4): 201-208.
[34] Tiwari D K, Dasgupta-S N, Villaseñor C L M, et al. Interfacing carbon nanotubes (CNT) with plants: Enhancement of growth, water and ionic nutrient uptake in maize (Zea mays) and implications for nanoagriculture[J]. Applied Nanoscience, 2014, 4(5): 577-591.
[35] 赛闹汪青,曹佳鑫,庞海龙,等. 多壁碳纳米管对水稻幼苗的生理学效应及对1, 2, 4-三氯苯毒性的缓解[J]. 应用与环境生物学报,2020,26(3):534-542. Sainao Wangqing, Cao Jiaxin, Pang Hailong, et al. Multi-walled carbon nanotubes: Their effects on the physiological responses ofL. seedlings and the toxicity of trichlorobenzene[J]. Chinese Journal of Applied and Environmental Biology, 2020, 26(3): 534-542. (in Chinese with English abstract)
[36] Pandey K, Anas M, Hicks V K, et al. Improvement of commercially valuable traits of industrial crops by application of carbon-based nanomaterials[J]. Scientific Reports, 2019, 9(1): 1-14.
[37] Khodakovskaya M V, Kim B S, Kim J N, et al. Carbon nanotubes as plant growth regulators: Effects on tomato growth, reproductive system, and soil microbial community[J]. Small, 2013, 9(1): 115-123.
[38] González-G Y, Cadenas-P G, Alpuche-S Á G, et al. Carbon nanotubes decrease the negative impact of Alternaria solani in tomato crop[J]. Nanomaterials, 2021, 11(5): 1080.
[39] 包兴成. 多壁碳纳米管与KH2PO4配施对夏黑葡萄生长及果实品质的影响[D]. 石河子:石河子大学,2021. BAO Xingcheng. Effects of Multi Walled Carbon Nanotubes Combined with KH2PO4on Growth and Fruit Quality of Summer Black Grape[D]. Shihezi: Shihezi University, 2021. (in Chinese with English abstract)
[40] Tan X, Lin C, Fugetsu B. Studies on toxicity of multi-walled carbon nanotubes on suspension rice cells[J]. Carbon, 2009, 47(15): 3479-3487.
[41] Ke M, Ye Y, Zhang Z, et al. Synergistic effects of glyphosate and multiwall carbon nanotubes on Arabidopsis thaliana physiology and metabolism[J]. Science of the Total Environment, 2021, 769: 145156.
[42] Woo H, Kim I, Park S. Estimating the reducing power of carbon nanotubes and granular activated carbon using various compounds[J]. Water, 2021, 13(14): 1959.
[43] 贺君杰. 多壁碳纳米管与氢化镁缓解紫花苜蓿铜胁迫的分子机理[D]. 南京:南京农业大学,2020. HE Junjie. Molecular Mechanism Underlying Multi-Walled Carbon Nanotubes and Magnesium Hydride-Alleviated Copper Toxicity in Alfalfa[D]. Nanjing: Nanjing Agricultural University, 2020. (in Chinese with English abstract)
[44] Lambert B P, Gillen A J, Boghossian A A. Synthetic biology: A solution for tackling nanomaterial challenges[J]. The Journal of Physical Chemistry Letters, 2020, 11(12): 4791-4802.
[45] Lambert B P. Directed Evolution of DNA-wrapped Single-walled Carbon Nanotube Complexes for Optical Sensing[D]. Lausanne: EPFL, 2021.
[46] 朱成喜,刘东,李玉叶,等. 比率电化学传感技术在农产品真菌毒素检测中的研究进展[J]. 农业工程学报,2022,38(5):259-268. ZHU Chengxi, LIU Dong, LI Yuye, et al. Research progress on ratiometric electrochemical sensors for the detection of mycotoxins in agricultural products[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2022, 38(5): 259-268. (in Chinese with English abstract)
[47] Wu H, Nißler R, Morris V, et al. Monitoring plant health with near-infrared fluorescent H2O2nanosensors[J]. Nano Letters, 2020, 20(4): 2432-2442.
[48] Lew T T S, Koman V B, Silmore K S, et al. Real-time detection of wound-induced H2O2signalling waves in plants with optical nanosensors[J]. Nature Plants, 2020, 6(4): 404-415.
[49] Demirer G S, Zhang H, Matos J L, et al. High aspect ratio nanomaterials enable delivery of functional genetic material without DNA integration in mature plants[J]. Nature Nanotechnology, 2019, 14(5): 456-464.
[50] Demirer G S, Zhang H, Goh N S, et al. Carbon nanocarriers deliver siRNA to intact plant cells for efficient gene knockdown[J]. Science Advances, 2020, 6(26): eaaz0495.
[51] Lv Z, Jiang R, Chen J, et al. Nanoparticle‐mediated gene transformation strategies for plant genetic engineering[J]. The Plant Journal, 2020, 104(4): 880-891.
[52] Zhao Y, Xu R, Hua X, et al. Multi-walled carbon nanotubes induce transgenerational toxicity associated with activation of germline long non-coding RNA linc-7 in C. elegans[J]. Chemosphere, 2022, 301: 134687.
[53] Wu Y, Phillips J A, Liu H, et al. Carbon nanotubes protect DNA strands during cellular delivery[J]. ACS Nano, 2008, 2(10): 2023-2028.
[54] Wang J W, Grandio E G, Newkirk G M, et al. Nanoparticle-mediated genetic engineering of plants[J]. Molecular Plant, 2019, 12(8): 1037-1040.
[55] Kumar S, Nehra M, Dilbaghi N, et al. Nanovehicles for plant modifications towards pest-and disease-resistance traits[J]. Trends in Plant Science, 2020, 25(2): 198-212.
[56] Kwak S Y, Lew T S, Sweeney C J, et al. Chloroplast-selective gene delivery and expression in planta using chitosan-complexed single-walled carbon nanotube carriers[J]. Nature Nanotechnology, 2019, 14(5): 447-455.
[57] Demirer G S, Zhang H, Goh N S, et al. Carbon nanotube–mediated DNA delivery without transgene integration in intact plants[J]. Nature Protocols, 2019, 14(10): 2954-2971.
[58] Apartsin E K, Buyanova M Y, Novopashina D S, et al. Hybrids of siRNA with Carbon Nanotubes as RNA Interference Instruments[M]. Singapore: Pan Stanford Publishing, 2016: 33-58.
[59] Edwards C H, Christie C R, Masotti A, et al. Dendrimer-coated carbon nanotubes deliver dsRNA and increase the efficacy of gene knockdown in the red flour beetle Tribolium castaneum[J]. Scientific Reports, 2020, 10(1): 1-11.
[60] Cao C, Kim J H, Yoon D, et al. Optical detection of DNA hybridization using absorption spectra of single-walled carbon nanotubes[J]. Materials Chemistry and Physics, 2008, 112(3): 738-741.
[61] Wu S J, Schuergers N, Gillen A J, et al. Characterization of double-stranded DNA (dsDNA) on single-walled carbon nanotubes (SWCNTs)[C]//ECS Meeting Abstracts. Bristol: IOP Publishing, 2018: 680.
[62] Wu S J, Schuergers N, Lin K H, et al. Restriction enzyme analysis of double-stranded DNA on pristine single-walled carbon nanotubes[J]. ACS Applied Materials & Interfaces, 2018, 10(43): 37386-37395.
[63] 闫素英,张田歌,袁雪,等. 基于响应面法的碳管纳米流体稳定性[J]. 农业工程学报,2022,38(21):261-267. YAN Suying, ZHANG Tiange, YUAN Xue, et al. Stability of carbon tube nanofluid based on response surface methodology[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2022, 38(21): 261-267. (in Chinese with English abstract)
[64] Gohari G, Safai F, Panahirad S, et al. Modified multiwall carbon nanotubes display either phytotoxic or growth promoting and stress protecting activity in. in a concentration-dependent manner[J]. Chemosphere, 2020, 249: 126171.
[65] YANG Jingxu, YAN Zhengjuan, XU Dehua, et al. Enhanced growth of broad beans (.) through separating antagonistic nutrients using nitrogen-doped carbon nanotubes[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(48): 16437-16449.
[66] Husen A, Siddiqi K S. Carbon and fullerene nanomaterials in plant system[J]. Journal of Nanobiotechnology, 2014, 12(1): 1-10.
[67] Masoomeh A K, Mehdi M, Asgari L B, et al. Influence of nano-priming on seed germination and plant growth of forage and medicinal plants[J]. Plant Growth Regulation, 2021, 93(1): 13-28.
[68] Zhao L, Peralta-V J R, Varela-R A, et al. Effect of surface coating and organic matter on the uptake of CeO2NPs by corn plants grown in soil: Insight into the uptake mechanism[J]. Journal of Hazardous Materials, 2012, 225: 131-138.
[69] Rossi L, Zhang W, Lombardini L, et al. The impact of cerium oxide nanoparticles on the salt stress responses of[J]. Environmental Pollution, 2016, 219: 28-36.
[70] 罗斌. 碳纳米管干扰对植物多样性与生态系统功能关系的影响[D]. 杭州:浙江大学,2020. LUO Bin. Effects of Carbon Nanotube Disturbance on Biodiversity-ecosystem Functioning Relationships[D]. Hangzhou: Zhejiang University, 2020. (in Chinese with English abstract)
[71] CENTERS for DISEASE CONTROL and PREVENTION. Occupational cancer-carcinogen list[EB/OL]. 2012-05-02 [2023-03-03]. https://www.cdc.gov/niosh/topics/cancer/ npotocca. html.
[72] Mary K, Matthew M, Christine A. et al. Association of occupational exposures with ex vivo functional immune response in workers handling carbon nanotubes and nanofibers[J]. Nanotoxicology, 2020, 14(3): 404-419.
[73] Rezaei C S, Anas M, Liu S, et al. Comprehensive risk assessment of carbon nanotubes used for agricultural applications[J]. ACS Nano, 2022, 16(8): 12061-12072.
[74] Zheng Y, Bachilo S M, Weisman R B. Photoexcited aromatic reactants give multicolor carbon nanotube fluorescence from quantum defects[J]. ACS Nano, 2019, 14(1): 715-723.
[75] Liu S, Zha Y, Wang Y, et al. Characteristics of aniline and nitrobenzene adsorption on single-walled, multi-walled and graphitized multi-walled carbon nanotubes[J]. Current Science, 2019, 117: 683-689.
[76] Li X, Chen Y, Chen Z, et al. The recent progress on gaseous chlorinated aromatics removal for environmental applications[J]. Separation and Purification Technology, 2022, 296(9): 121364.
[77] Li P, Guo H, Xu H. Environmentally friendly ionic side chain organic small molecule/single-walled carbon nanotube composites have high TE performance[J]. Journal of Materials Science, 2022, 57(39): 18524-18534.
[78] Li Y H, Wang S, Luan Z, et al. Adsorption of cadmium (II) from aqueous solution by surface oxidized carbon nanotubes[J]. Carbon, 2003, 41(5): 1057-1062.
[79] Peng X, Luan Z, Di Z, et al. Carbon nanotubes-iron oxides magnetic composites as adsorbent for removal of Pb (II) and Cu (II) from water[J]. Carbon, 2005, 43(4): 880-883.
[80] Liang P, Liu Y, Guo L. Determination of trace rare earth elements by inductively coupled plasma atomic emission spectrometry after preconcentration with multiwalled carbon nanotubes[J]. Spectrochimica Acta Part B: Atomic Spectroscopy, 2005, 60(1): 125-129.
[81] Chen G, Qiu J, Liu Y, et al. Carbon nanotubes act as contaminant carriers and translocate within plants[J]. Scientific Reports, 2015, 5(1): 1-9.
[82] Wang C, Liu H, Chen J, et al. Carboxylated multi-walled carbon nanotubes aggravated biochemical and subcellular damages in leaves of broad bean (Vicia faba.) seedlings under combined stress of lead and cadmium[J]. Journal of Hazardous Materials, 2014, 274: 404-412.
[83] Vander W R L, Berger G M, Ticich T M. Carbon nanotube synthesis in a flame using laser ablation for in situ catalyst generation[J]. Applied Physics A, 2003, 77(7): 885-889.
[84] Eatemadi A, Daraee H, Karimkhanloo H, et al. Carbon nanotubes: Properties, synthesis, purification, and medical applications[J]. Nanoscale Research Letters, 2014, 9(1): 1-13.
[85] Minati L, Speranza G, Bernagozzi I, et al. Luminescent short thiol-functionalized multi-wall carbon nanotubes[J]. Diamond and Related Materials, 2011, 20(7): 1046-1049.
[86] Yin Y, Cao G, Fu L, et al. Functionalized multi-walled carbon nanotubes with strong fluorescence emission[J]. Journal of Alloys and Compounds, 2021, 854: 157016.
[87] Ju S Y, Kopcha W P, Papadimitrakopoulos F. Brightly fluorescent single-walled carbon nanotubes via an oxygen-excluding surfactant organization[J]. Science, 2009, 323(5919): 1319-1323.
[88] INTERNATIONAL AGENCY for RESEARCH on CANCER. Agents classified by the IARC monographs, Volumes 1-114[EB/OL]. 2022-09-07[2023-03-03]. https://monographs. iarc. who. int/list-of-classifications.
[89] Samadi S, Saharkhiz M J, Azizi M, et al. Single-wall carbon nano tubes (SWCNTs) penetrate Thymus daenensis Celak. plant cells and increase secondary metabolite accumulation in vitro[J]. Industrial Crops and Products, 2021, 165: 113424.
[90] WANG Y, TIAN J, WANG Z, et al. Crop-safe pyraclostrobin-loaded multiwalled carbon nanotube delivery systems: Higher fungicidal activity and lower acute toxicity[J]. ACS Agricultural Science & Technology, 2022, 2(3): 534-545.
[91] Hu J, Xianyu Y. When nano meets plants: A review on the interplay between nanoparticles and plants[J]. Nano Today, 2021, 38: 101143.
[92] 杨玲梅,吕鹏梅,罗文,等. 碳纳米管掺杂Fe(Ⅱ)-Zn催化剂催化合成生物柴油[J]. 农业工程学报,2011,27(13):129-132. YANG Lingmei, LYÜ Pengmei, LUO Wen, et al. Synthesis of biodiesel by carbon nanotubes doped Fe(Ⅱ)-Zn double metal cyanide catalysts[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2011, 27(13): 129-132. (in Chinese with English abstract)
[93] Talbot M J, White R G. Cell surface and cell outline imaging in plant tissues using the backscattered electron detector in a variable pressure scanning electron microscope[J]. Plant Methods, 2013, 9(1): 1-16.
[94] Pelz P M, Griffin S, Stonemeyer S, et al. Solving complex nanostructures with ptychographic atomic electron tomography[EB/OL]. 2022-06-17[2023-03-03]. https://arxiv. org/abs/2206.08958
[95] Liu Q, Chen B, Wang Q, et al. Carbon nanotubes as molecular transporters for walled plant cells[J]. Nano Letters, 2009, 9(3): 1007-1010.
[96] Zubkovs V, Wang H, Schuergers N, et al. Bioengineering a glucose oxidase nanosensor for near-infrared continuous glucose monitoring[J]. Nanoscale Advances, 2022, 4(11): 2420-2427.
[97] Nißler R, Ackermann J, Ma C, et al. Prospects of fluorescent single-chirality carbon nanotube-based biosensors[J]. Analytical Chemistry, 2022, 94(28): 9941-9951.
[98] Antonucci A, Reggente M, Roullier C, et al. Carbon nanotube uptake in cyanobacteria for near-infrared imaging and enhanced bioelectricity generation in living photovoltaics[J]. Nature Nanotechnology, 2022, 17(10): 1111-1119.
Progress and analysis of carbon nanotube applications in crop research
LIU Xuewen1, GAO Juntao2, LI He1, YANG Zishang1, CHEN Jing1※
(1.,,,450002,2.,,450047,)
The promotion and biotoxic effects of carbon nanotubes (CNTs) on crop cultivation have drawn much attention over the last decade. The studied species have covered common crops, such as rice, corn, wheat, tomato, and grapes. There was great inconsistency in the previous experiments, including the various species, growth stages, and cultivation methods of crops as well as the various types, dispersion, application, and concentrations of CNTs, resulting in the diversification of test results in the literature. To establish a relatively unified experiment framework for carbon nanotubes, this study first reviewed the progress of the application of carbon nanotubes in following four crop research directions: crop cultivation, stress resistance, physiological index monitoring, and agricultural genetic engineering, and summarized the carbon nanotube parameters used in the literature and their application effects. It was found that the ultrasonic method or carboxylation covalent modification was commonly used to disperse the CNTs for crop cultivation research, which would result that the effective concentration of carbon nanotubes in crop research being unknown. Meanwhile, this kind of non-selectively modified CNTs was used as the type of nano-sized material. There was no significant difference in the effects between NPs and CNTs on the crops. It is still lacking in the unique effects of CNTs on crop growth. Then, the non-covalently modified CNTs were proposed to establish the unique role of CNTs in crop cultivation, for example, they can aggregate at the target sites after CNTs were modified by macromolecules that can be decomposed by special plant enzymes. Biomolecules (e.g., proteins, and nucleic acids), and polymers (e.g., chitosan, PEG, PEI, and PAMAM) can non-covalently be modified on the surface of CNTs, and further used to adsorb the molecules with negative charges, which can be used as carrier or sorbent. In addition, the non-covalent modification caused no defects on the CNT surface, indicating the fluorescence properties of SWCNTs were retained. So, CNTs were often used for DNA/RNA delivery and the development of biosensors. The working principles and application scenarios of selectively modified CNTs were also summarized as: 1) The CNTs were undoubtedly an important research direction for the applications as the carriers of gene transfer. However, the DNAs that were carried by CNTs were difficult to achieve stable gene transformation, in terms of transgenic applications. By contrast, the siRNA-CNTs complexes shared unique advantages, in terms of administration cycle, plant cell uptake, and protection of RNA from degradation during non-transformative gene delivery. One of the future development directions was in the field of agricultural non-transgenic insect-resistant. 2) The surface of SWCNTs was non-covalently modified with a variety of organic substances. The target molecules were then bound to the organic substances. A variety of sensors were obtained using their fluorescence or electrochemical properties. Some of them were more suitable as implantable sensors for the real-time online monitoring of multiple chemicals in plants, compared with the in vitro sensors. The reason was that the CNTs were used to penetrate the cell walls, indicating excellent near-infrared fluorescence properties. There was no biotoxic effect on the crops at appropriate concentrations (and even beneficial to crop growth). Furthermore, the toxicity problem was caused by the accumulation of exogenous toxic substances in the plant, due to the adsorption or ion exchange with the modified CNTs. It was more worthy of investigation than the toxicity problem of CNT itself. Finally, suggestions for the CNTs experiment program in crop research are given, including the selection of carbon nanotubes, the preparation of medium and characterization methods. To sum up, unlike the previous crop cultivation using pristine and covalently modified CNTs, in the future, using non-covalently modified CNTs or non-covalent stacking of CNTs for crop research is an important development direction.
nanometer; crops; carbon nanotube; surface modification; crop cultivation; biotoxicity
2022-12-29
2023-03-02
河南省教育厅国际科技合作项目(222102520017);财政部和农业农村部:国家现代农业产业技术体系项目(CARS-04)
刘学文,博士,高级工程师,研究方向为智能感知技术。Email:liuxuewen1983@hotmail.com
陈静,博士,硕士生导师,研究方向为生物传感器、光电信息处理技术。Email:jingchen1983@outlook.com
10.11975/j.issn.1002-6819.202212182
S-1
A
1002-6819(2023)-08-0001-11
刘学文,高军涛,李赫,等. 碳纳米管在作物研究中的应用进展与分析[J]. 农业工程学报,2023,39(8):1-11. doi:10.11975/j.issn.1002-6819.202212182 http://www.tcsae.org
LIU Xuewen, GAO Juntao, LI He, et al. Progress and analysis of carbon nanotube applications in crop research[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2023, 39(8): 1-11. (in Chinese with English abstract) doi:10.11975/j.issn.1002-6819.202212182 http://www.tcsae.org
