Comparison of efficacy of conbercept, aflibercept, and ranibizumab ophthalmic injection in the treatment of macular edema caused by retinal vein occlusion: a Metaanalysis
2023-07-20QiuXingYaNanDaiXiaoBoHuangLiPeng
Qiu Xing, Ya-Nan Dai, Xiao-Bo Huang, Li Peng
1Department of Ophthalmology, Central South University Xiangya School of Medicine Affiliated Haikou Hospital,Haikou 570000, Hainan Province, China
2Department of Ophthalmology, the Second Xiangya Hospital of Central South University, Changsha 410000, Hunan Province, China
3Department of Ophthalmology, the Changsha Central Affiliated Hospital, Hengyang Medical School, University of South China, Changsha 410004, Hunan Province, China
Abstract● AlM: To evaluate and compare the anatomical and functional outcomes and negative effects of the three anti-vascular endothelial growth factor (VEGF) drugs in the treatment of macular edema (ME) due to retinal vein occlusion (RVO) based on the evidence pooled from current clinical trials and observational studies.
● KEYWORDS: anti-vascular endothelial growth factor;conbercept; aflibercept; ranibizumab; macular edema;retinal vein occlusion
INTRODUCTION
Retinal vein occlusion (RVO) is the second most prevalent retinal vascular disorder characterized by obstruction of the retinal vein which causes macular edema (ME) and retinal and subretinal hemorrhages[1].The incidence is of this disease increases with age and it is linked to hypertension and coagulation abnormalities[2].Currently, the pathogenesis of RVO is not well understood.Oxidative stress has been shown to be a critical factor in RVO pathogenesis[3].ME due to RVO (RVO-ME) is sight-threatening and unlikely to improve without treatment[4], but can be treated by antivascular endothelial growth factor (anti-VEGF) drugs.Recent trials have shown that anti-VEGF therapies (ranibizumab,aflibercept and conbercept) can improve ME in RVO-ME patients[5-7].Ranibizumab was the first and most extensively used anti-VEGF drugs[8].Recently, aflibercept has been use for the treatment of RVO-ME as a fusion protein that binds VEGF and neutralizes its isoforms and has achieved good clinical results in recent clinical trials[9].Conbercept (KH902; Chengdu Kanghong Biotechnologies Company, China), which has a similar structure to aflibercept, is a novel anti-VEGF fusion protein approved by China Food and Drug Administration for the management of retinal conditions[10].Growing evidence has shown that conbercept can effectively treat RVO-ME[11-12].
Previous systematic reviews and Meta-analysis have compared the effectiveness of anti-VEGF drugs in patients with RVO-ME[13-15].Moreover, anti-VEGF therapy improves best corrected visual acuity (BCVA) and reduces central macular thickness (CMT) more effectively and longer than corticosteroid/laser[16], although conbercept was excluded.However, it is still debatable whether anti-VEGF agents involved in recombinant fusion protein are superior to ranibizumab for RVO-related ME.
Thus, we conducted a Meta-analysis using the latest published data to compare the effectiveness and safety of aflibercept and conbercept with ranibizumab in the treatment of RVO-ME patients.
MATERIALS AND METHODS
Search Strategy and Selection CriteriaThis Metaanalysis followed the PRISMA guidelines for Meta-analysis reporting and was registered at the International Prospective Register of Systematic Reviews (PROSPERO, number CRD 42020180797).To identify all of randomized controlled trials(RCTs) and retrospective studies from their establishment until April30, 2022, a comprehensive literature search was conducted on nine online database including EMBASE,PubMed, Cochrane Library, Web of Science, Springer,Clinical Trials.gov, Chinese language search of the Chinese National Knowledge Infrastructure, Wanfang, and Weipu.The following keywords and relative variants were used to identify relevant articles: “retinal vein occlusion”, “macular edema/oedema”, “ranibizumab”or “Lucentis”or “RhuFab V2”or “V2, RhuFab”, “conbercept”or “KH902”or “Lumitin”,“Aflibercept”or “VEGF Trap-regeneron”or “VEGF Trap-Eye, eylea, Zaltrap, ZIV-aflibercept”.The publication language was restricted to English and Chinese languages.To identify additional legible studies, the relevant reference of included studies and systematic reviews lists were also searched.
Inclusion CriteriaThe publications were selected if they met the inclusion criteria.The inclusion criteria in the analysis are listed below.1) Study subjects: patients with RVO-ME before the operation; clinical characteristics and population of research subjects were comparable between groups; 2)Intervention: intravitreal conbercept (IVC)vsintravitreal ranibizumab (IVR), intravitreal aflibercept (IVA)vsIVR; 3)Study design: randomized controlled trial or retrospective study; 4) Outcomes measurement: at least one outcome reported including mean BCVA change ≥15 letter gain, mean CMT change, ocular adverse events (AEs), systemic AEs, and mean number of intravitreal injections; 5) Duration: a followup duration exceeding three months; 6) Number of subjects:more than 20 patients in each study.
Exclusion CriteriaCase reports, conference abstracts, and commentary articles were excluded.In addition, to ensure the accuracy and reliability of the results, studies with less than 10 patients in control or experimental groups or patients with other diseases, such as retinal detachment, age-related macular degeneration and ME in vitrectomy eyes were excluded.Patients with less than three months of follow-up time were also excluded.
Data Extraction and Quality AssessmentData extraction and the literature quality evaluation were conducted by two independent reviewers (Peng L, Xing Q).Microsoft Excel database was used to record all available information of the selected articles, including baseline details, the outcomes(mean BCVA change ≥15 letter gain, mean CMT change,ocular AEs, systemic AEs, mean number of intravitreal injections, follow-up time).To assess the risk of bias and quality of the RCTs and retrospective included articles,Cochrane handbook for systematic reviews of interventions Version 5.3 and the modified Newcastle-Ottawa Scale (NOS)were used, respectively.Any disparities on extracted data were resolved by a third investigator.
Statistical AnalysisFor dichotomous variables, the Odds ratio (OR) was stated at 95% confidence interval (CI) while the weight mean difference (WMD) with 95%CIs was stated for continuous outcomes.To assess the stability of the pooled effect sizes, cumulative Meta-analyses were conducted.Meanwhile, heterogeneity of included studies was evaluated by utilizing the Chi-squared test andI2.WhenI2>50% andP<0.1, heterogeneity was considered significant and thus random-effects model was applied.Otherwise, a fixed-effects model was conducted.Funnel plots and Egger tests was used to detect the possibility of publication bias, with aP-value <0.1 considered significant publication bias.Statistical significance was considered 2-sided for aP-value <0.05.Stata (version 12.0) was used to analyze all retrieved data.
RESULTS
Study SelectionThe online database yielded 452 studiesbased on our search strategy.After screening the literature and removing duplicated records, 364 potentially relevant records were retained.The titles and abstracts were discarded by 2 independent researchers, removing 104 articles.A total of 20 articles were included after a full-text review.Eventually, 11 studies with 1001 eyes compared ranibizumab with conbercept,while 9 studies with 673 eyes compared ranibizumab with aflibercept, respectively[4,11,17-34].

Table 1 Characteristics and quality assessment of the included studies
Baseline CharacteristicsTable 1 and Figure 1 showed a summary of the characteristics of the basic information and the quality assessment of the included studies.Sample sizes ranged from 22 to 384 eyes, with a total of 1674 eyes included, and the follow-up period ranging from 1 to 24mo.The mean age ranged from 51.7±3.2 to 71.1±12.6y.Demographic data and clinical characteristics of the participants were not significantly different.
Data Synthesis and the Meta-analysis
Figure 1 A flow diagram of the eligibility of studies for inclusion in Meta-analysis.
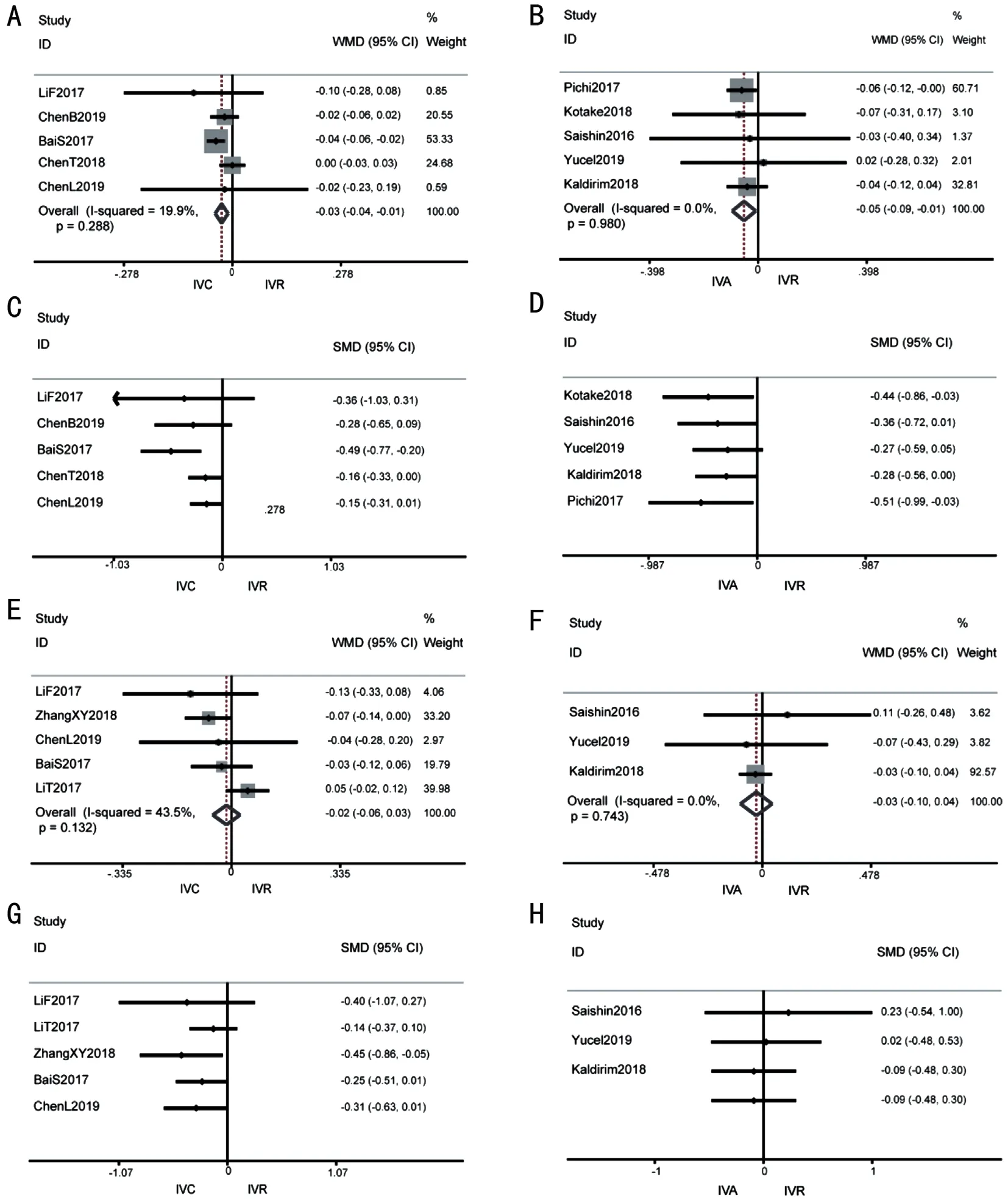
Figure 2 Forest plot of Meta-analysis and cumulative Meta-analysis in mean BCVA change comparing with baseline A: After 1-month treatment of IVC versus IVR; B: After 1-month treatment of IVA versus IVR; C: Cumulative Meta-analysis of IVC versus IVR after 1-month; D:Cumulative Meta-analysis of IVA versus IVR after 1mo; E: After 6-month treatment of IVC versus IVR; F: After 6-month treatment of IVA versus IVR; G: Cumulative Meta-analysis of IVC versus IVR after 6-month; H: Cumulative Meta-analysis of IVA versus IVR after 6-month.BCVA: Best corrected visual acuity; IVC: Intravitreal conbercept; IVR: Intravitreal ranibizumab; IVA: Intravitreal aflibercept.
Mean BCVA change compared with baselineAmong RVO-ME patients, 10 included articles with strong evidence showed that both conbercept and aflibercept had better visual acuity effects than ranibizumab at 1mo [WMD -0.03 logMAR(95%CI -0.04 to -0.01),P=0.001, conberceptvsranibizumab;WMD -0.05 logMAR (95%CI -0.09 to -0.01),P=0.019,afliberceptvsranibizumab].The results showed no significant heterogeneity in the results (I2=19.9%,P=0.288, conberceptvsranibizumab;I2=0,P=0.980, afliberceptvsranibizumab; Figure 2A, 2B).All had similar visual acuity effects at 6mo [WMD-0.02 logMAR (95%CI -0.06 to 0.03),P=0.47, conberceptvsranibizumab; WMD -0.03 (95%CI -0.10 to 0.04),P=0.458,afliberceptvsranibizumab].There was mild heterogeneity in the results (I2=43.5%,P=0.132, conberceptvsranibizumab;I2=0,P=0.743, afliberceptvsranibizumab; Figure 2E, 2F).Similarly, there was no statistically significant difference between aflibercept and ranibizumab in the proportion of patients gaining ≥15 letters [12-24mo OR 1.16 (95%CI 0.81 to 1.66]without evidence of heterogeneity (I2=0,P=0.94).In addition,the cumulative Meta-analysis demonstrated that as the sample size in each study increases, the effect size becoming stable and the 95%CI narrowed (Figure 2C, 2D, 2G, 2H).
Central macular thickness compared with baselineConbercept had significantly higher mean CMT change effects at 1mo [WMD -14.43 (95%CI -25.89 to -2.97),P=0.014]and 6mo [WMD -35.63 (95%CI -49.27 to -21.98),P≤0.001].There was no significant heterogeneity between-study at 1mo(I2=7.8%,P=0.370) and 6mo (I2=45.4%,P=0.066).Aflibercept and ranibizumab had similar mean CMT change effects at 1mo [WMD -10.14 (95%CI -24.62 to 4.34),P=0.170], 6mo[WMD -26.98 (95%CI -62.84 to 8.88),P=0.140] and 12-24mo [WMD -12.34 (95%CI -25.755 to 1.06),P=0.071;Figure 3].There was no significant heterogeneity in the result at 1mo (I2=32.9%,P=0.189) and 12-24mo (I2=0,P=0.606),but significant heterogeneity was detected at 6mo (I2=51.7%,P=0.066; Figure 4A, 4B).
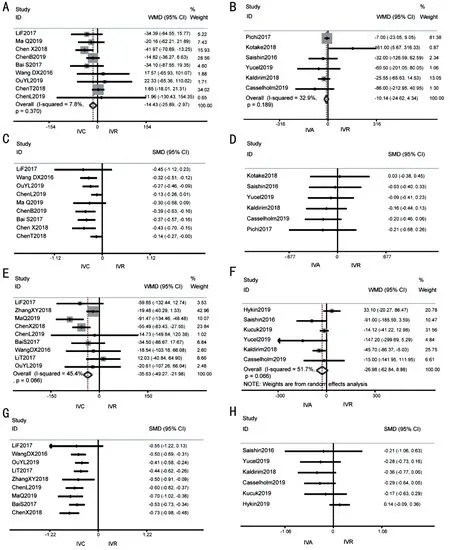
Figure 3 Forest plot of Meta-analysis and cumulative Meta-analysis in mean CMT change comparing with baseline A: After 1-month treatment of IVC versus IVR; B: After 1-month treatment of IVA versus IVR; C: Cumulative Meta-analysis of IVC versus IVR after 1-month; D:Cumulative Meta-analysis of IVA versus IVR after 1-month; E: After 6-month treatment of IVC versus IVR; F: After 6-month treatment of IVA versus IVR; G: Cumulative Meta-analysis of IVC versus IVR after 6-month; H: Cumulative Meta-analysis of IVA versus IVR after 6-month.CMT:Central macular thickness; IVC: Intravitreal conbercept; IVR: Intravitreal ranibizumab; IVA: Intravitreal aflibercept.
Ocular adverse eventsFive studies (n=646 eyes) reported a number of some ocular adverse events, including elevated intraocular pressure, subconjunctival bulbar hemorrhage and postoperative infection.Based on fixed effects model analysis,the occurrence of ocular adverse events was not significantly different between the conbercept and ranibizumab [OR 0.75(95%CI 0.43, 1.30),P=0.305; Figure 4D].Four studies (n=447 eyes) reported that the occurrence of ocular adverse events was not significantly different between the aflibercept and ranibizumab [OR 1.04 (95%CI 0.62, 1.73),P=0.89; Figure 4E].There was no heterogeneity between drugs.In conclusion,the results show that there was no significant differences in adverse reactions in intravitreal injection of the three drugs.
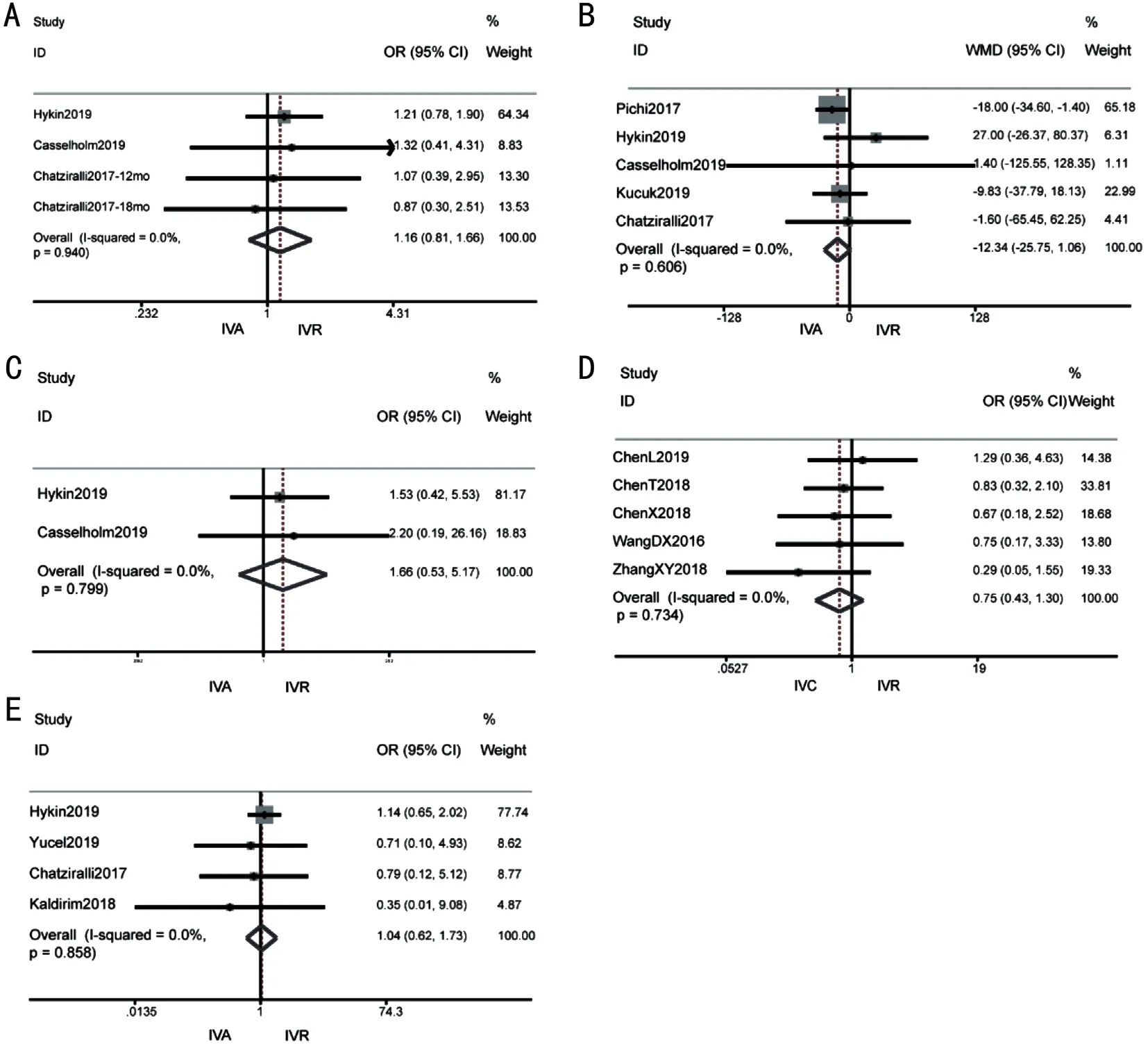
Figure 4 Forest plot of Meta-analysis in other outcomes A: Proportion of patients gaining ≥15 letters after 12-24mo treatment of IVA versus IVR; B: Mean CMT change after 12-24mo treatment of IVA versus IVR; C: Systemic adverse events of IVA versus IVR; D: Ocular adverse events of IVC versus IVR; E: Ocular adverse events of IVA versus IVR.IVC: Intravitreal conbercept; IVR: Intravitreal ranibizumab; IVA: Intravitreal aflibercept.

Table 2 Mean number of intravitreal injections for conbercept and ranibizumab

Table 3 Mean number of intravitreal injections for aflibercept and ranibizumab
Systemic adverse eventsThere was no serious systemic complications related to IVC and IVR in either group during the trial period.Systemic adverse events were observed in IVA versus IVR groups, but at comparable rates between treatment groups.Only two out of the nine trials provided information on the occurrence of systemic adverse events,such as cardiovascular deaths, non-fatal myocardial infarction,and stroke.The data suggested that there was no significant difference in the occurrence of these events between the groups receiving aflibercept and ranibizumab [OR 1.66 (95%CI 0.53,5.17),P=0.385; Figure 3C], with no evidence of heterogeneity(I2=0,P=0.799; Figure 4C).
Mean Number of Intravitreal InjectionsRanibizumab had a higher mean number of intravitreal injections than with conbercept and aflibercept.Tables 2 and 3 shows the average number of intravitreal injections for the three drugs.
Subgroup AnalysisSubgroup analyses were conducted based on the study type [RCTvsRetrospective studies (RES)].Subgroup analysis revealed no statistical significance on mean BCVA change (OR in 15 letters gain, 1.23 letter (95%CI 0.81 to 1.86), afliberceptvsranibizumab), mean CMT change in 12-24mo (WMD -13.79 (95%CI -29.52 to -1.94), afliberceptvsranibizumab) in RCT group with no changed tendency.The mean CMT change results of our subgroup analyses in different follow-up times were also consistent with the overall results.Thus, it is reasonable to believe that the stability of effect sizes increases with the inclusion of more studies.
Publication Bias and Sensitivity AnalysisFunnel plots in the included studies were almost symmetrical.Egger tests revealed that there was no evidence of potential publication bias among included studies.Sensitivity analyses demonstrated that the results were robust.
DISCUSSION
The latest published studies were included in this Metaanalysis to compare the efficacy and safety of ranibizumab,conbercept, and aflibercept for patients with ME due to RVO.Despite the presence of minority non-RCTs, the quality of majority studies was considered to be relatively high based on the result of the quality evaluation of published articles.Furthermore, there was no significant heterogeneity among studies for most outcomes.Sensitivity analysis also indicated the result was stable and not affected by individual studies.Moreover, a meta-analysis showed that well-designed non-RCTs are as good as RCTs based on the evidence level[35]
Sangroongruangsriet al[36]published the latest Meta-analysis in 2018 which included 11 studies with 1830 patients,and compared efficacy of bevacizumab, ranibizumab and aflibercept for treatment of RVO-ME.However, conbercept was not compared with ranibuzumab[36].We first performed an analysis including conbercept.We then performed cumulative Meta-analysis to assess the influence of sample size on the above outcomes.Thus, an independent evaluation of the three drugs for ME due to RVO is urgently needed.
Our study includes 20 published studies with 1674 eyes and demonstrated the most recent intravitreal injection results for the management of ME due to RVO.Conbercept and aflibercept both showed better clinical response in improving vision at 1mo after treatment than ranibizumab, however, no statistically significant difference was found in visual acuity at 6mo or more after therapy.At 6mo of treatment, pooled analysis showed higher decline in CMT in the conbercept than in the ranibizumab group.Conbercept seems to be a more effective treatment of RVO-ME compared with ranibizumab.However, longer-term studies are required for validation.Longer duration of follow-up is highly recommended for confirmation.No statistical difference in mean change of CMT was found at any stage of follow-up in aflibercept.This suggested that conbercept and aflibercept may have sufficient response to initial treatment.This could explain why some previous studies switched from ranibizumab to aflibercept[37-38].IVA seems to be a potential alternative therapy in RVO patients with persistent/refractory ME to ranibizumab[39].A Metaanalysis revealed that switching therapy from bevacizumab or ranibizumab to aflibercept may improve persistent RVOME[40].So, the three anti-VEGF drugs have shown significant visual and anatomical improvements, although there are subtle differences.These may be attributed to the effect associated with its structure and shape.
Aflibercept is a fully human, recombinant fusion protein with a 115-kDa recombinant that acts as a soluble decoy receptor and binds human VEGF-A, VEGF-B, and placental growth factor (PIGF) with high affinity to inhibit downstream signaling mediated.This resents a significantly higher affinity than that of ranibizumab or bevacizumab.Conbercept is also fusion protein with a 143-kDa recombinant harvested from a full human cDNA sequence of Chinese hamster ovary cells.It also acts as a decoy receptor, binding all isoforms of VEGF-A, VEGF-B, VEGF-C, and PIGF with high affinity[41-42].The difference between the two drugs is that the fourth extracellular domain of VEGFR-2 was incorporated into the Fab in conbercept.Despite not being directly involved in ligand-binding, the region promotes receptor dimerization which binds VEGF 100-folds more tightly than the monomeric counterpart.In humans, the intravitreous half-life of aflibercept and conbercept have not been reported.However, animal model studies showed that conbercept lasted 4.2d[43-44], aflibercept lasted 4.8d, and ranibizumab lasted 2.8d[45].All are longer than ranibizumab.Moreover, VEGF Trap keeps significant intravitreal VEGF-binding activity after a single injection with 10-12wk durations, based on a mathematical model.
The most prevalent ocular adverse events are subconjunctival hemorrhage and transient intraocular hypertension.They can be reverted to normal in the short term in both cases.Moreover, no differences was found in ocular adverse events among groups, albeit the confidence of this results is limited as the ocular adverse events rarely occur.Repeated injections carry the risk of adverse outcomes, which has negative effects including endophthalmitis after surgery[46].Currently,the standard treatment for ME is repeated administration of anti-VEGF agents.Nevertheless, some patients develop tachyphylaxis after repeated IVA injections[47].Consequently,the number of injections should be optimized[47].Other studies observed an increased risk of stroke or myocardial infarction after administration of anti-VEGF agents, especially in the patients with a history of vascular infarction-related disease[48].Aflibercept and conbercept required fewer injections than ranibizumab in our Meta-analysis, implying that their use may reduce the risk of tachyphylaxis and other systemic side effects.
Nevertheless, our study had several potential limitations that need to be considered.First, the possibility of selection and publishing bias in nonrandomized comparisons should not be ignored.Second, conbercept has only been used in China for less than 10y, thus the long-term clinical outcomes remain unclear.In 2018, China mandated the use of aflibercept which had the same problem.Third, proactive approaches should be considered.Fourth, the number of included studies and sample size were limited.Only 20 trials comprising 1674 eyes were included.Therefore, more studies with longer follow-up periods are necessary to determine the long-term efficacy of the RVO-ME treatment.
In conclusion, our study was the first to evaluate the efficacy and safety of IVA or IVC with that of IVR for RVO-ME based on nine online databases.In summary, both IVA and IVC are equally effective as IVR in improving vision and reducing CMT.The incidence of adverse events is not significantly different between conbercept and aflibercept compared to ranibizumab, and both conbercept and aflibercept require a lower mean number of injections.Although there is a need for further studies to determine the optimal treatment approach for RVO-ME, our findings demonstrate that conbercept and aflibercept can effectively treat patients with RVO-ME.Moreover, our results indicate that conbercept is a promising anti-VEGF drug for the management of RVO-ME.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge Dr.Ling Gao for statistical help.
Authors’contributions:Peng L conceived and designed the study.Xing Q and Dai YN performed data mining and statistical analyses.Peng L interpreted the results of the statistical analyses.Xing Q and Huang XB prepared the figures and tables.Xing Q drafted the initial manuscript.Peng L made critical comments and revised the initial manuscript.Peng L and Xing Q have primary responsibility for the final content.All authors reviewed and approved the final manuscript.
Foundations:Supported by the Natural Science Foundation of Hainan Province (No.821QN1005); Hainan Provincial Health Commission Project (No.21A200067); Hainan Provincial Classification of Project (No.ZDYF2020110).
Conflicts of Interest: Xing Q,None;Dai YN,None;Huang XB,None;Peng L,None.
杂志排行
International Journal of Ophthalmology的其它文章
- Chickenpox followed streaky multifocal choroiditis with prednison treatment in a girl with asthma
- Pneumonia and ocular disease as the primary presentations of Takayasu arteritis: a case report
- Unilateral blurred vision in pediatric patient associated with cavum velum interpositum cyst
- Highly cited publication performance in the ophthalmology category in the Web of Science database:a bibliometric analysis
- Ocular manifestations and quality of life in patients after hematopoietic stem cell transplantation
- Clinical features, radiological imaging, and treatment strategies of nonmetallic intraorbital foreign bodies: a retrospective analysis
