Inhibition of pyrrolidine dithiocarbamate on transforming growth factor-beta 2 induced epithelial-mesenchymal transition in human lens epithelial cells
2023-07-11,3
,3
Abstract
•KEYWORDS:pyrrolidine dithiocarbamate; transforming growth factor-beta 2 (TGF-β2); nuclear factor-κB (NF-κB); epithelial-mesenchymal transition; human lens epithelial cells
INTRODUCTION
The most common reason for blindness in the world is cataracts. After cataract surgery, posterior capsular opacification (PCO) is a frequent complication[1]. PCO can affect the recovery of visual function, causing severe visual impairment in the patient. The post-surgical proliferation, emigration, infiltration and epithelial-mesenchymal transition(EMT) transformation by residual lens epithelial cells(LECs) are the main causes of PCO[2]. Currently, there are no effective drugs for the treatment of PCO. Pyrrolidine dithiocarbamate (PDTC) is a recognized nuclear factor-κB (NF-κB) specific inhibitor[3], the primary method of inhibiting NF-κB nuclear displacement was to prevent IκB phosphorylation, thus, NF-κB activity was specifically restrained[4]. Nearly all cells have NF-κB, a transcriptional regulator with a variety of biological functions. When cells are not stimulated, in the cell, the P65 subunits of NF-κB and IκB combine to produce a trimeric complex that is not active and cannot control gene transcription. When cells are stimulated by extracellular signals, NF-κB is activated[5]. Research shows that the transforming growth factor-beta 2(TGF-β2) concentration in a trial water was shown to be dramatically elevated by creating an animal model of PCO in SD rats, the proliferation of LECs and lens clouding are promoted by increased posterior capsule NF-κB and p-NF-κB expression and activation of NF-κB signaling pathways[6]. Recent research has shown that the rat lens after surgery had increased NF-κB expression,moreover, it was consistent with the increase through time and expression of TGF-β in a trial water. It is inferred that after cataract surgery, TGF-β is activated in the a trial fluid of rats, followed by induction of EMT in the lens and activation of the NF-κB signaling pathway, which leads to the development of PCO[7].
According to our studies, PDTC inhibits the NF-κB pathway, which makes it possible to make LECs less hazardous[8]. The antioxidant, anti-inflammatory, and free radical-scavenging properties of ammonium PDTC, due to its precise suppression of the NF-κB signaling pathway and its potent capacity to cause apoptosis, PDTC is currently a hot study subject, numerous prior studies have shown that PDTC can be used in conjunction with a variety of medications to lessen the toxic side effects of one medication while also enhancing its effectiveness[9-10], demonstrates the widespread, security of PDTC applications.
TGF-β is a widely studied growth polypeptide factor. TGF-β2, which is particularly abundant in the lens, is one of three human is of or-ms that it takes, the others are TGF-β1 and TGF-β3[11]. TGF-β2 expression was up-regulated in aqueous humor after surgical trauma or stimulation of the lens[12], it is crucial in controlling how LECs evolve into EMT. Existing research has shown that TGF-β2 is activated in aqueous humor of rats after cataract surgery[13-14]. The AKT/NF-κB signaling pathway is thus activated, which causes EMT[15-16], as a downstream target of TGF-β2/ILK s-signaling, NF-κB takes role in the development of EMT[17]. For example, in the lung cancer study, it was found that inhibition of NF-κB-mediated activation of Snail inhibited TGF-β2 induced EMT[18]. To sum up, TGF-β2 is a critical factor in PCO formation. This study set out to determine the applicability potential of PDTC as a PCO treatment by examining how it affected the growth and mechanism of EMT in lens cells during PCO induced by TGF-β2.
MATERIALS AND METHODS
CultureofCellsandExperimentalGroupWe bought human LECs from human eyes from Mingzhou Biological (Jiangsu, China) and identified by cell STR. The medium required for this cell is MEM medium (MEM, PM150410, Procell Company) containing 1% penicillin-streptomycin#BL505A (purchased from biosharp Company) and 10% fetal bovine serum (FBS#10270-106, Gibco). The cells were raised in a cell incubator at 37℃ and 5% CO2, trypsin#25200056 (Gibco Company) was used to break down the cells without the EDTA, and the cells were passaged when 80% of the cell growth and fusion had occurred. Three categories of LECs were created: 1) Control group: normal culture (n=3); 2) TGF-β2 group: model group, 20 ng/mL TGF-β2 was added at 0h of the experiment to continue the culture (n=3); 3) TGF-β2+PDTC group: experimental group (n=3), various PDTC concentrations (25, 50, 75, and 100 μmol/L) were applied to culture cells during 48h.
LensepithelialcellsproliferativevitalityistestedusingtheCCK-8assayCells are proportionately inoculated in 96-well plates and grown to the required density for dose stimulation. Cells were stimulated for 24, 48h with diluted TGF-β2 at varied concentration (0, 5, 10, 15, 20 ng/mL), following the action time has come to an end, 10 μL of CCK-8 solution were added to each well, and the incubation in the cell incubator continued for an additional 2h. The applicable formula can be used to calculate the activity of the cells.
CellmigrationassayScratch test is applied to epithelial cells to test cell migration, with 5×105cells per well, LECs were planted in 6-well plates, 24h of cell growth and fusion to about 80% for the experiment. First, make a straight vertical line in the middle of each hole with a yellow 200μL gun tip, PBS slowly washes away the scratched cell debris, serum-free medium and various TGF-β2 concentrations (0, 1, 5, 10, 15, 20 ng/mL) were added to treat the cells. The TGF-2 lyophilized powder is redissolved in deionized water and then diluted with alginate to the essential concentration. (TGF-β2 Lot#0321345 purchased from PEPROTECH Company, USA), after 24, 48 and 72h of culture, the same scratch position was fixed and photographed by optical microscope, the migration efficiency of each group was calculated, cell migration rate %=(0h width-post-culture width)/0h×100%).
WesternblotdetectionofproteinexpressionSix-well plates of cells were seeded, and 10 ng/mL TGF-β2 was applied successively. Subsequently, dimethyl sulphoxide (DMSO) is used to dissolve PDTC powder, the optimal PDTC concentrations in the range of 25, 50, 75, and 100 μmol/L were added sequentially as screened in previous experiments. Total protein was extracted from cell lysates containing phosphatase and protease inhibitors (PDTC#S1080 purchased from Beyotime Biotechnology Company) after 48h of treatment, and the BCA (BCA#P1045, Beyotime Biotechnology Company) technique was used to determine protein concentration. 20 μg of total protein was separated by SDS-PAGE gel electrophoresis, after the transmembrane completed, 5% skim milk was refrigerated for 1.5h before being blocked. Overnight at 4℃, antibodies were incubated (β-Actin1: 5000#AF0110; E-cadherin 1:1000#AF1032; αSMA 1:2000#AF0131; p-NF-κB1:1000#AF3390; IκB 1:1000#AF5002; p-IκB 1:1000#AF2002;Iκκ 1:1000#AF6012; p-Iκκ 1:1000#AF3012; BAX 1:1000#AF0120; BCL-2 1:1000#AF6139, all were purchased from Affinity Company; NF-κB 1:1000Lot#8242T and Caspase-3 1:1000#9662S were purchased from CST Company, USA; Cyclin D1 1:1000#A19038 was purchased from ABclonal Company). Wash PVDF membrane 3 times/10min with tris-buffered saline tween (TBST), HRP labeled antibody was incubated for 1h at room temperature (HRP#GB23303 purchased from Wuhan Servicebio Biotechnology Company), wash the film with TBST 3 times, ECL chemiluminescence solution was used for gel imaging (ECL#BL520A purchased from Biosharp Company). Gray analysis was performed using Image J program. The target protein’s relative expression level=target protein/β-Actin protein.
Real-timepolymerasechainreactiontoidentifyassociatedmRNAexpressionAfter the completion of cell stimulus in the 6-well plate, an RNA isolator was added to lyse the cells, add chloroform and mix up and down violently, centrifuge at 12000 rpm/15min at 4℃, take the upper aqueous phase and add the same volume of isopropanol to mix the solution, let stand for 10min and centrifuge at 12000 rpm/10min at 4℃, abandon supernatant. The RNA white precipitate was suspended in 75% ice-cold ethanol, and centrifuged at 7500 rpm/5min at 4℃, and discard the supernatant, dry the white precipitate to a transparent shape. The RNA precipitate was dissolved by the addition of DEPC water, and the concentration and purity of RNA were measured. To make cDNA, reverse transcription of RNA was used. By using the SYBR green fluorescent dye technique, RT-PCR was carried out. (RT-PCR fluorescent dyes were purchased from Servicebio#G3321-05). The primers were created by Wuhan Servicebio Biotechnology Company (Table 1); 2-ΔΔCtresults were utilized to determine the proportional level of each target genes expression.

Table 1 Related primer sequences
StatisticalAnalysisGraphPad Prism 8.0 software was used to statistically analyze the data equally, and one-way analysis of variance (ANOVA) was utilized to compare differences across the groups. Thet-test was utilized for additional two-by-two comparisons where the differences were statistically significant; differences were deemed statistically significant atP<0.05.
RESULTS
EffectofTransformingGrowthFactor-beta2onCellViabilityCell stimulation with different TGF-β2 concentration (1, 5, 10, 15, 20 ng/mL). CCK-8 assay results demonstrated that, in comparison to the control group at 0 ng/mL, the viability of cell proliferation gradually increased with the concentration of TGF-β2, demonstrating concentration-dependent tolerance. According to the experimental results, it is known that the proliferation viability of LECs started to differ at 5 ng/mL of TGF-β2 (P<0.05) and a significant difference at 10 ng/mL (P<0.01). Also, it was observed that the optimal stimulation time at 48h,it is also noteworthy that the proliferative activity of TGF-β2 at 20 ng/mL showed a decreasing trend, which may be related to the high concentration inhibiting cell proliferation.
TransformingGrowthFactor-beta2TherapyImpactonLensEpithelialCellsMigrationTGF-β2 was applied to LECs in various doses (1, 5, 10, 15, 20 ng/mL), the migration distances of cells were recorded at 0, 24, 48, and 72h, respectively. Compared to the untreated TGF-β2 control, TGF-β2 treatment significantly enhanced the migration ability of the cells (Figure 2A). Cells treated with various amounts of TGF-β2 migrated most quickly when the concentration was 10 ng/mL (P<0.01) and the migration efficiency reached the peak at 48h (P<0.05; Figure 2B). As well, increasing the concentration of TGF-β2 to 20 ng/mL inhibited cell migration. The results showed that LECs treated with TGF-β2 increased their migration activity, and the migration effect was the most obvious at the concentration of 10 ng/mL. Therefore, TGF-β2 concentration of 10 ng/mL can be used for subsequent cell modeling experiments.

Figure 1 CCK-8 assay results show that transforming growth factor-beta 2 induces enhanced cell viability in lens epithelial cells. The proliferation viability of the cells in the experimental groups showed enhanced proliferation at 48h of stimulation time in comparison to the group without TGF-β2 treatment (0 ng/mL), and there were significant differences at 10 ng/mL and 15 ng/mL (n=3; P<0.01).
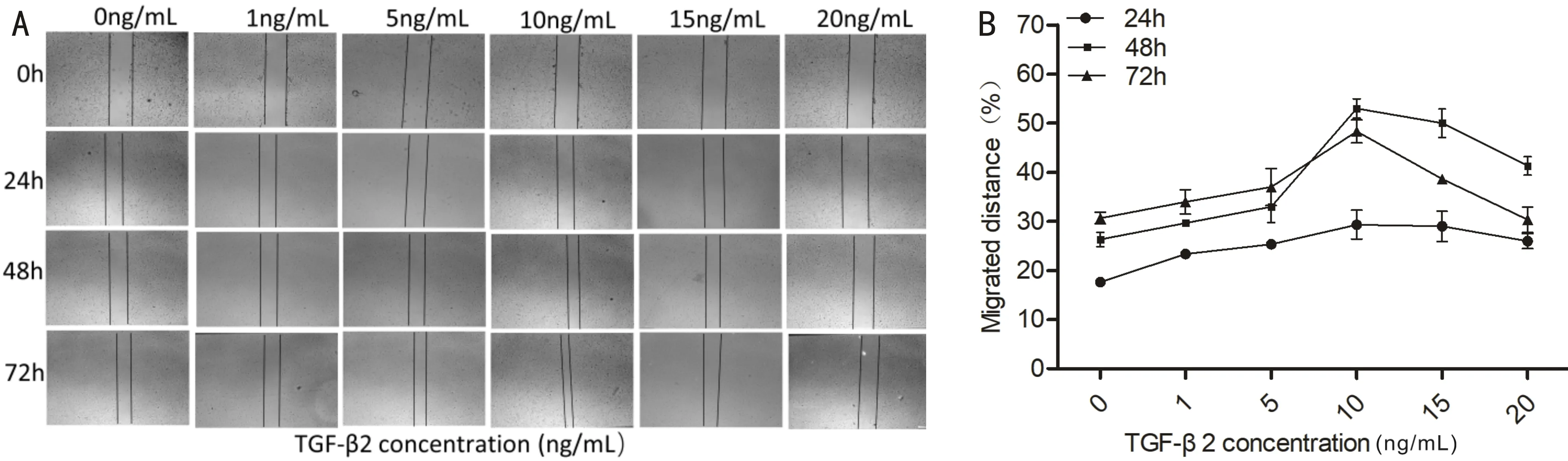
Figure 2 Analysis of the migratory ability of lens epithelial cells in response to different transforming growth factor-beta 2 (Scale bar=100μm). A: TGF-β2 with different concentrations (1, 5, 10, 15, 20 ng/mL). Scratch diagram of cell migration at different time points (0, 24, 48, and 72h) after treatment of cells, as shown in the picture, cells treated with TGF-β2 at a low concentration (1, 5 ng/mL) did not significantly enhance their ability to migrate, migration ability is significantly enhanced at 10, 15ng/mL, cell migration is inhibited by a high concentration of 20 ng/mL; B: Plot of statistical data showing how lens epithelial cells migrate in response to different TGF-β2 concentrations (n=3; P<0.05), representative TGF-β2 10 ng/mL migration rate at 0, 24, 48, and 72h. TGF-β2: Transforming growth factor-β2; LECs: Lens epithelial cells.
DetectionofEpithelial-MesenchymalTransitionMarkersandNuclearfactor-κBPathway-relatedProteinExpressioninLensEpithelialCellsusingWesternBlotAccording to a Western Blot analysis, E-cadherin is a key epithelial marker protein in the onset and progression of EMT when compared to the control group (0 ng/mL), with a rise in TGF-β2 concentration and a longer action period, it gradually lost and reduced, the relative mesenchymal marker α-SMA gradually increased (P<0.05), it reached the peak at the concentration of 10 ng/mL and 48h, it was consistent with results of cell proliferation assay(Figure 3 A-B). These results indicated that TGF-β2 treatment induced LECs cells to complete EMT. Aside from that NF-κB and phosphorylated NF-κB both demonstrated an increasing trend (P<0.05), when TGF-β2 activated the NF-κB signaling pathway (Figure 3 C-D). These results indicate that NF-κB and phosphorylated NF-κB are activated in response to TGF-β2. Therefore, LECs cell proliferation and the EMT process were promoted by TGF-β2 and 10 ng/mL was used as the best simulation condition for PCO modeling in subsequent experiments.
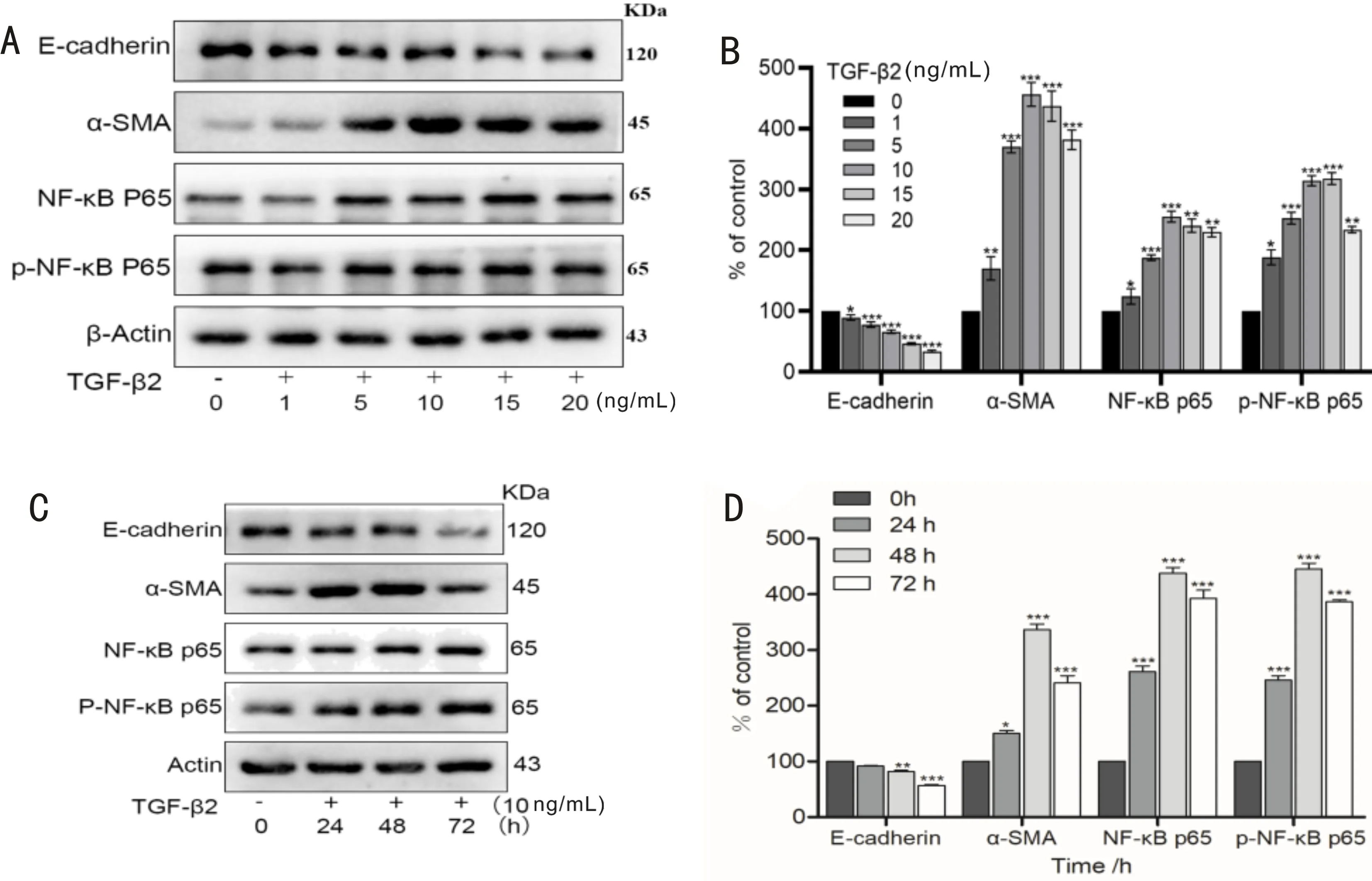
Figure 3 Western blot analysis of epithelial-mesenchymal transition markers and nuclear factor-κB signaling pathway related proteins in transforming growth factor-beta 2-induced lens epithelial cells. A: EMT process-related proteins (expression of α-SMA gradually increased and E-cadherin decreased) in comparison to control; B: TGF-β2 statistical diagram of different concentrations of treated cells (n=3; P<0.05); C: Compared with control, 10 ng/mL TGF-β2-treated cells showed progressive upregulation of α-SMA expression, reduced E-cadherin expression and upregulation of NF-κB signaling pathway protein activation at different time points; D: EMT process related protein and expression strip chart for the NF-κB signaling pathway protein statistical analysis chart, compared with group 0h (n=3; P<0.05). EMT: Epithelial mesenchymal transition; NF-κB: Nuclear factor-κB.
Pyrrolidinedithiocarbamateinhibitstransforminggrowthfactor-beta2toinducenuclearfactor-κBsignalingpathwayfactorsinepithelialmesenchymaltransitioninlensepithelialcellsRelated proteins were detected after PDTC intervention in TGF-β2-induced LECs cells for 48h. The expression of the upstream adaptor proteins IκB-α and in contrast to the TGF-β2 group, the PDTC intervention group had lower levels of p-IκB-α of the NF-κB signaling pathway. While doing so, IκB kinase complex Iκκ activation was inhibited, the protein expressions of Iκκ-α and p-Iκκ-α decreased with the increase of PDTC concentration. NF-κB and p-NF-κB were also gradually down-regulated after inhibition (Figure 4 A-B), it was statistically significant that the TGF-β2 therapy group differed from the other group (P<0.05). These findings imply that PDTC inhibition causes EMT in LECs, which may be mediatedviaNF-κB signaling pathway suppression.

Figure 4 Analysis of Western blot of pyrrolidine dithiocarbamate inhibitory effect on transforming growth factor-beta 2-induced epithelial mesenchymal transition-related proteins. A: As shown in the picture, PDTC inhibits the expression of NF-κB/IκB-α/Iκκ-α during EMT by inhibiting the NF-κB signaling pathway and phosphorylated NF-κB/IκB-α/Iκκ-α also showed inhibitory effects; B: statistical analysis (n=3; P<0.05). PDTC: Pyrrolidine dithiocarbamate; NF-κB: Nuclear factor-κB.
Pyrrolidinedithiocarbamateinhibitionoftransforminggrowthfactor-beta2inducedchangesinapoptoticpathway-relatedfactorsinepithelialmesenchymaltransitioninlensepithelialcellsDifferent concentrations of PDTC (0, 25, 50, 75, 100, 125 μmol/L) are applied to LECs. An analysis for the expression of proteins associated to apoptosis revealed that PDTC significantly elevated BAX and caspase-3, two pro-apoptotic proteins, downregulated the anti-apoptotic protein Bcl-2, and Cyclin D1 expression was downregulated in LECs cells. The variance from the control group was statistically different (P<0.05). These results suggest that PDTC induces apoptosis in LECs cells, which may be mediated by inhibition of apoptosis pathway-related proteins.
EffectofpyrrolidinedithiocarbamateonmRNAexpressionofapoptosisandtransforminggrowthfactor-beta2inducedepithelialmesenchymaltransitionLECs were stimulatedviacell modeling with 10 ng/mL TGF-β2, then PDTC 100 μmol/L was added. TGF-β2 greatly increased the activation of IκB, a crucial protein of the NF-κB pathway, during EMT, according to RT-PCR statistics. Data showed that BAX and Bcl-2, two proteins associated with apoptosis, were slightly altered in the TGF-β2 treatment group as contrasted with the control group. This may be because TGF-β2 has a dual effect on increasing cell proliferation and apoptosis. After PDTC intervention, in contrast to TGF-β2, Bcl-2 mRNA expression dropped while BAX mRNA levels increased. The results were in line with the Western blot methods, which fully demonstrated PDTC capacity to block the NF-κB signaling pathway and induce apoptosis.
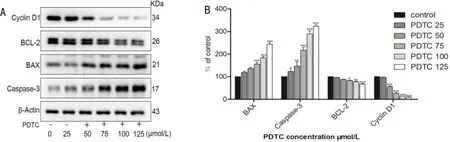
Figure 5 Western blot analysis of pyrrolidine dithiocarbamate on apoptosis-related proteins in lens epithelial cells. A: As shown in the picture, PDTC gradually decreased the expression of the apoptosis-inhibiting proteins Bcl-2 and Cyclin D1, while gradually activating and stimulating the pro-apoptosis related proteins BAX and caspase-3,the effect of PDTC on the change of apoptotic protein at low concentration was not obvious, it had a more significant inhibiting effect at 75-100 μmol/L, further adding the concentration has no apparent effect; B: statistical analysis (n=3; P<0.05). PDTC: Pyrrolidine dithiocarbamate.

Figure 6 Real-time polymerase chain reaction analysis of the inhibitory effect of pyrrolidine dithiocarbamate on transforming growth factor-beta 2-induced epithelial mesenchymal transition and mRNA associated with apoptosis. A: As illustrated in the picture, the TGF-β2 model group NF-κB/I-κB mRNA expression was considerably higher than that of the control group, compared the TGF-β2 model group to the PDTC experimental group, NF-κB/IκB mRNA expression was significantly inhibited (n=3; P<0.05); B: According to the bar chart, when comparing the TGF-β2 model group to the control group, BAX mRNA expression was slightly increased and BCL expression was slightly lowered, compared the TGF-β2 model group to the PDTC experimental group, PDTC significantly decreased BCL expression while increased BAX mRNA expression (n=3; P<0.05). TGF-β2: Transforming growth factor-beta 2; NF-κB: Nuclear factor-κB; PDTC: Pyrrolidine dithiocarbamate.
DISCUSSION
The most frequent side effect following cataract surgery is PCO[19]. Pathogenesis is due to the migration, invasion, and excessive proliferation of residual LECs cells, which lead to PCO. Therefore, it is essential to maintain the normal development and homeostasis of LECs[20]. EMT is essential to the growth of PCO, LECs lose their epithelial morphology and elongate to form mesenchymal cells that secrete extracellular matrix during EMT[21-22]. According to studies, TGF-β2 is viewed as one of the main causes of PCO development, cell viability, migration, and the EMT process in LECs can all be improved by TGF-β2 and activate related signal transduction pathways[23-24], in this way, PCO can be facilitated. In this investigation, we identified PDTC as a potential drug that, by blocking TGF-β2 stimulation of the NF-κB signaling pathway, can inhibit EMT in LECs. Finally, the development of PCO was inhibited. The findings of this investigation demonstrated that TGF-β2 considerably improved LECs cells capacity for migration, α-SMA, a mesenchymal marker, expressed at higher levels, while E-cadherin was down-regulated and activate the NF-κB signaling pathway[25], and this result is consistent with previous studies[26-27]. Studies have shown that cancer cells were effectively stopped from proliferating by PDTC, which also caused them to die[28-29], and inhibited tumorigenesisinvivoandinvitroand PDTC has various biological activities[30-31]. However, its mechanism of action in the management of PCO is still unknown. Our previous studies have confirmed that after pretreatment with PDTC, LECs undergo significant apoptosis. This study shows that the results of the experiment demonstrated that TGF-β2 treatment of LECs for 48h considerably improved the cells ability to migrate. Western blot analysis revealed that the TGF-β2 treated group increased with medication concentration in comparison to the control group, mesenchymal marker α-SMA expression gradually rises whereas epithelial marker E-cadherin expression gradually declines. Therefore, preventing EMT can successfully prevent PCO brought on by aberrant proliferation and migration of LECs, the flatness and regular morphology of the posterior capsule as well as the reduction of posterior capsule clouding can all be achieved by inhibiting the growth of LECs. PDTC can also break DNA in cells, and it can inhibit the migration and proliferation of LECs cells. TGF-β2 was demonstrated in this study induced EMT of LECs by activating NF-κB signal pathway[32].
The activation of NF-κB related pathway is an important pathway for the development of PCO, NF-κB is a regulatory transcription factor with various biological activities[33], when LECs are stimulated with TFG-β2 signaling, IκB is activated and then activates IκB kinase complex Iκκ, which leads to phosphorylation of IκB protein. Degraded IκB participates in physiological and pathological processes such immunity, inflammation, and apoptosis by activating downstream NF-κB[34-35]. By evaluating the effects of NF-κB, IκB-α, and Iκκ-α signaling pathways, we found that phosphorylation of IκB-α and Iκκ-α was highly activated in response to TGF-β2, the presence of PDTC effectively reversed the activation of EMT by TGF-β2, This may be achieved by preventing NF-κB from being activated. The results of this research demonstrated that PDTC intervention greatly increased apoptosis in LECs and caused a gradual decline in cell viability. According to Western blot results, the NF-κB signaling-related pathway, a crucial classical PCO pathway, was inhibited; activity of phosphorylated NF-κB/IκB/Iκκ was considerably lower than that of the group treated with TGF-β2. It is evident from this that demonstrates how PDTC can prevent TGF-β2 induction of EMT by blocking the NF-κB pathway and the apoptotic pathway.
In summary, PDTC suppressed LEC proliferation, migration, and EMTinvitro, and its inhibitory impact might be attributed to the phosphorylation modulation of TGF-β2/NF-κB protein. We suggest that PDTC may be a potential agent to prevent PCO formation after cataract surgery, our results also supported the significance of NF-κB signaling in PCO therapy. However, the concentration of PDTC needs to be determined by additionalinvivotests, the toxic and side effects on important tissues and structures in the eye were tested. We will continue to confirm the efficacy of PDTCinvivoin the future as this study still has flaws.
