蚕丝基葡萄糖传感器研究进展
2023-07-04王怡汪宇佳陈方春王雅思代方银李智
王怡 汪宇佳 陈方春 王雅思 代方银 李智


摘要: 葡萄糖传感器是糖尿病人监控血糖浓度的必备工具。基于柔性基底开发可用于实时监测的柔性葡萄糖传感器是近年来研究的热点,也是未来应用发展的重要方向。蚕丝具有优异的力学性能、生物相容性及生物可降解性,以蚕丝及蚕丝蛋白与导电活性物质复合开发的柔性葡萄糖传感器展现出优异的传感性能及出色的长期稳定性。本文通过对基于丝蛋白、丝纤维、蚕丝织物开发的蚕丝基葡萄糖传感器的研究进展进行综述,对比所开发的蚕丝基葡萄糖传感器的传感性能,分析其特点及作用机制,并展望蚕丝基葡萄糖传感器在柔性可穿戴传感器领域的发展前景。
关键词: 蚕丝;丝素蛋白;蚕丝织物;葡萄糖传感器;葡萄糖氧化酶;柔性电子器件
中图分类号: TS141.8
文献标志码: A
文章编号: 1001-7003(2023)03-0008-08
引用页码:
031102
DOI: 10.3969/j.issn.1001-7003.2023.03.002(篇序)
糖尿病是由于体内胰岛素分泌缺陷等原因所引起的慢性代谢疾病,其主要特征是血液或代谢液中的葡萄糖浓度较高,当空腹血糖超过7 mM即可诊断为糖尿病。国际糖尿病联合会2021年发布数据显示,全球糖尿病患者总数为5.37亿,约占总数的10.5%。其中,中国糖尿病的患者人数约占1/4(数据来自IDF Diabetes Atlas)。患者长期处于高血糖状态易引起一系列的急性并发症,严重情况下甚至会诱发器官衰竭而导致死亡。作为一种慢性疾病,糖尿病尚无有效治愈方法,目前主要治疗手段是通过血糖监测,及时给药或注射胰岛素以达到降血糖的目的。因此,实时准确血糖监测手段对于糖尿病诊断及治疗至关重要。
葡萄糖传感器可快速监测血液和汗液中葡萄糖的指数,是糖尿病患者日常监测血糖的必备工具。葡萄糖传感器分为酶式葡萄糖传感器和非酶葡萄糖传感器两大类。酶式葡萄糖传感器开发最早,其中应用最广泛的酶是葡萄糖氧化酶
(GOD),通过利用GOD对葡萄糖的特异性结合,催化葡萄糖氧化为葡萄糖酸内酯和过氧化氢,从而实现对葡萄糖的高选择性检测。早在1967年,Updike等[1]率先开发出基于GOD的酶电极,随后在不断增长的医疗保健需求推动下,基于GOD的葡萄糖传感器得以快速发展,目前商业上应用广泛的指刺式血糖仪便为其代表。非酶葡萄糖传感器则是利用葡萄糖与酶以外的活性物质发生化学反应,使电位发生变化从而实现对葡萄糖的定量检测。常用的活性物质有Pt[2-5]、Au[6]、Pd[7]、Ni[8]、Cu[9-11]、Co[12]、Mn[13]、Fe[14-16]等金属及其氧化物,碳纳米管[17-19]、石墨烯[20-21]等碳活性材料。
基于传感机制的变化,酶式葡萄糖传感器的开发主要经历三代的发展[22],如图1所示。第一代葡萄糖生物传感器[23]通常依赖于GOD催化的葡萄糖由O2天然底物氧化,并测量酶产生的过氧化氢产物或O2辅因子的消耗;第二代葡萄糖生物传感器中用其他电子受体取代O2,利用各种有机和无机化合物作为电子穿梭媒介,其中二茂铁衍生物和铁氰化物[24]络合物由于具有较低的操作电位和高化学稳定性,应用较为普遍;第三代葡萄糖生物传感器在电极和葡萄糖氧化酶之间直接进行电子转移,是一种理想化的传感器,无需外加氧气等媒介体,极大地降低了干扰,具有更快的电子传输速率[25],但这类葡萄糖传感器仍处于研究阶段。
近年来,随着智能可穿戴器件的快速发展,为生物传感器的开发及应用提出新的要求及高度,柔性可穿戴、可实时监测和高稳定性成为葡萄糖传感器应用发展的重要方向,成为近年来研究的热点。纺织材料由于其广泛的来源,良好的柔韧性及可加工性,是柔性智能可穿戴电子器件开发的理想载体。其中,具有悠久纺织应用历史的蚕丝,其优异的力学性能、生物相容性、可降解性及易于加工特性而获得广泛的认可,被应用到柔性葡萄糖传感器的开发及应用研究[26-27]。
本文旨在總结和展望蚕丝材料在葡萄糖传感器领域的应用,从蚕丝在传感器领域的主要应用形态出发,重点讨论蚕丝基葡萄糖传感器的研究进展,以蚕丝织物和丝素蛋白两种主要形式将蚕丝基葡萄糖传感器进行分类。分别讨论了天然蚕丝纤维、蚕丝织物、碳化蚕丝织物及丝素膜、丝素纺丝纤维材料在葡萄糖传感器领域的应用,并对它们的制备方法、传感性能进行简要分析。同时,对近年来利用蚕丝基制备的葡萄糖传感器的相关性能进行比较。最后,对蚕丝基葡萄糖传感器在柔性可穿戴领域的挑战及未来发展方向进行了展望,以期能为设计和研究更多类似的开发高性能蚕丝基柔性葡萄糖传感器提供参考。
1 蚕丝性能
蚕丝是由蚕丝腺合成的丝蛋白液通过吐丝结茧凝固而形成的连续长纤维,具有轻、柔、韧、洁、光的特点,享有“纤维皇后”的美誉。蚕丝内部大量的β-折叠构象及其高取向度赋予其优异的力学性能,其良好的生物相容性和生物可降解性能更是其从传统纺织领域拓展到生物医学领域研究及应用的关键。蚕丝纤维表面丰富的胺基(—CHNH)、氨基(—NH2)、羧基(—COOH)等活性基团可用作制备复合材料的桥梁[28]。通过浸轧、抽滤或直接碳化的方法可以在蚕丝纤维及织物基础上制备出具有导电性能的蚕丝基材料,组装出电极、压力传感器及葡萄糖传感器等柔性传感器件[29]。此外,提取蚕丝中的丝胶和丝素成分,通过与导电物质复合纺丝、成膜、成胶等方法,可制备出具有电化学性能的蚕丝蛋白基复合材料,开发出蚕丝基柔性传感器件[30]。
2 蚕丝基葡萄糖传感器的开发及应用
2.1 基于蚕丝纤维、蚕丝丝织物的葡萄糖传感器
天然蚕丝不具导电性,对不同外界刺激的敏感性、响应性和对温湿度的自适应较弱。为构建蚕丝传感器,需要通过表面涂层或自身碳化处理两种方式制备成导电复合物,再通过负载酶或其他葡萄糖敏感材料,如金属及其氧化物等,制备成葡萄糖传感器(图2)。赋予天然蚕丝导电性能通常有三个途径:在蚕丝纤维或纱线表面涂覆导电物质;在蚕丝织物表面涂层或印刷导电物质;通过碳化蚕丝织物直接获得导电性。
在蚕丝纤维或纱线上涂覆导电物质,再通过编织等方式可制备成柔性传感器件。Choudhary等[31]首先将碳墨与铁氰化钾混合涂布到蚕丝纱线上,烘干后再涂覆GOD,形成导电性能良好每厘米电阻仅100 Ω的工作电极,再将制备的多个电极编织成织物,形成传感器阵列。通过在经纱方向上使用脱胶(亲水)和非脱胶真丝(疏水)的组合,可对单个传感器中液体流动路径进行控制,实现对血液中葡萄糖和血红蛋白的有效检测。由于蚕丝表面光滑,直接在蚕丝纤维或纱线上涂覆导电物质会有黏附性差而导致其结合效果不佳等的问题。为提高蚕丝表面与导电材料的相互作用,可通过对蚕丝表面进行前处理,以提高蚕丝和导电物质的结合效果。Ye等[32]利用特制的六氟异丙醇蚀刻蚕丝纤维表面而不破坏纤维的内部结构,再将碳纳米管涂覆到刻蚀后的蚕丝表面,制备成具有高机械性能、超疏水性、耐溶剂性和热敏感性的复合纤维,并进一步通过编织制备成对力、应变、温度等敏感的蚕丝传感器。通过制备导电蚕丝纤维或纱线,再编织成传感器的方法通常具有较好的柔性及传感性能[33-34],但工艺较复杂,且在编织的过程中容易导致导电涂层的脱落,影响传感器的性能。通过单根纱线形成微流体通道进行液体传输,利用纱线将待测物转移到检测区,使显色剂与葡萄糖发生比色反应显示不同颜色,进而实现不同浓度葡萄糖的响应也是一种高效直观的葡萄糖检测方法,但目前针对织物类比色葡萄糖传感器的研究还集中在棉纱及棉织物上[35-38],对于蚕丝方面还有待开发。尽管基于蚕丝纤维或纱线已开发出压力、温度、湿度等多种传感器,但相应的葡萄糖传感器还较少,如何在导电蚕丝纤维或纱线上进一步负载酶或葡萄糖响应材料从而开发出葡萄糖传感器还有待于更深入的探索及研究。
蚕丝织物表面进行涂层或印刷导电活性物质如碳基材料和导电聚合物等能获得手感好、耐用性高、导电性能佳的传感元件,再通过电化学沉积等方法负载葡萄糖响应材料可开发出柔性葡萄糖传感器。图2表示了基于蚕丝纤维、织物开发的葡萄糖传感器的制备方法。Cai等[39-40]利用三元溶剂对蚕丝织物进行前处理,再将其浸渍于聚苯胺或还原氧化石墨烯分散液中,通过多次循环干燥复合,制备出具有超高电容保留率的多功能柔性蚕丝织物电极(图2(a))。在此基础上,再通过电化学沉积铜纳米粒子制备成非酶葡萄糖传感器,其灵敏度可达199.8 μA/mM(图2(b),数据未发表)。研究表明,基于导电涂层的蚕丝织物开发的柔性葡萄糖传感器具有较好的传感性能及应用前景。
高温碳化丝织物可使其热分解转化为碳材料,赋予其良好的导电性能,再通过负载葡萄糖敏感元件可开发出具有高灵敏度的葡萄糖传感器。He等[41]利用不同碳化温度下得到的蚕丝织物作为工作电极和对电极,负载GOD后制备为葡萄糖传感器贴片,用于实时和多路汗液中的葡萄糖监测分析,其灵敏度为6.3 μA/mM,检测下限低至5 μM(图2(c))。当温度足够高时,蚕丝内部的β-微晶结构可转化为纳米级石墨化晶区,同时,蚕丝织物转变为掺杂N元素,且具有高导电性及强柔韧性的纺织结构碳纤维制品。Chen等[42]将蚕丝织物浸渍多壁碳纳米管分散液后高温碳化,再通过铂微球进行表面修饰后滴加GOD制备成葡萄糖传感器。添加的碳纳米管具有较大的接触面积,在碳化蚕丝纤维间形成更稳定的连接,使开发的葡萄糖传感器的灵敏度高达288.86 μA/mM,检测下限低至0.05 mM。通过碳化蚕丝织物开发的葡萄糖传感器相比于在蚕丝纤维或织物上涂覆导电物质所开发的葡萄糖传感器具有更优异的传感性能,这可能与导电基底的导电性能有关,良好的导电基底更有利于电子的传输,从而提升葡萄糖传感器的性能[43]。
目前,尽管以蚕丝纤维、纱线或织物为基底开发制备葡萄糖传感器的研究已有一定进展,但总体研究还较少,所使用的方法均较为复杂。制备获得导电蚕丝复合物是开发葡萄糖传感器的关键,也是难点,需要不断探索及研究。此外,如何在导电蚕丝复合物上更稳定地负载GOD及具有葡萄糖响应的活性物质也有待更深入的研究。
2.2 基于丝素蛋白的葡萄糖传感器
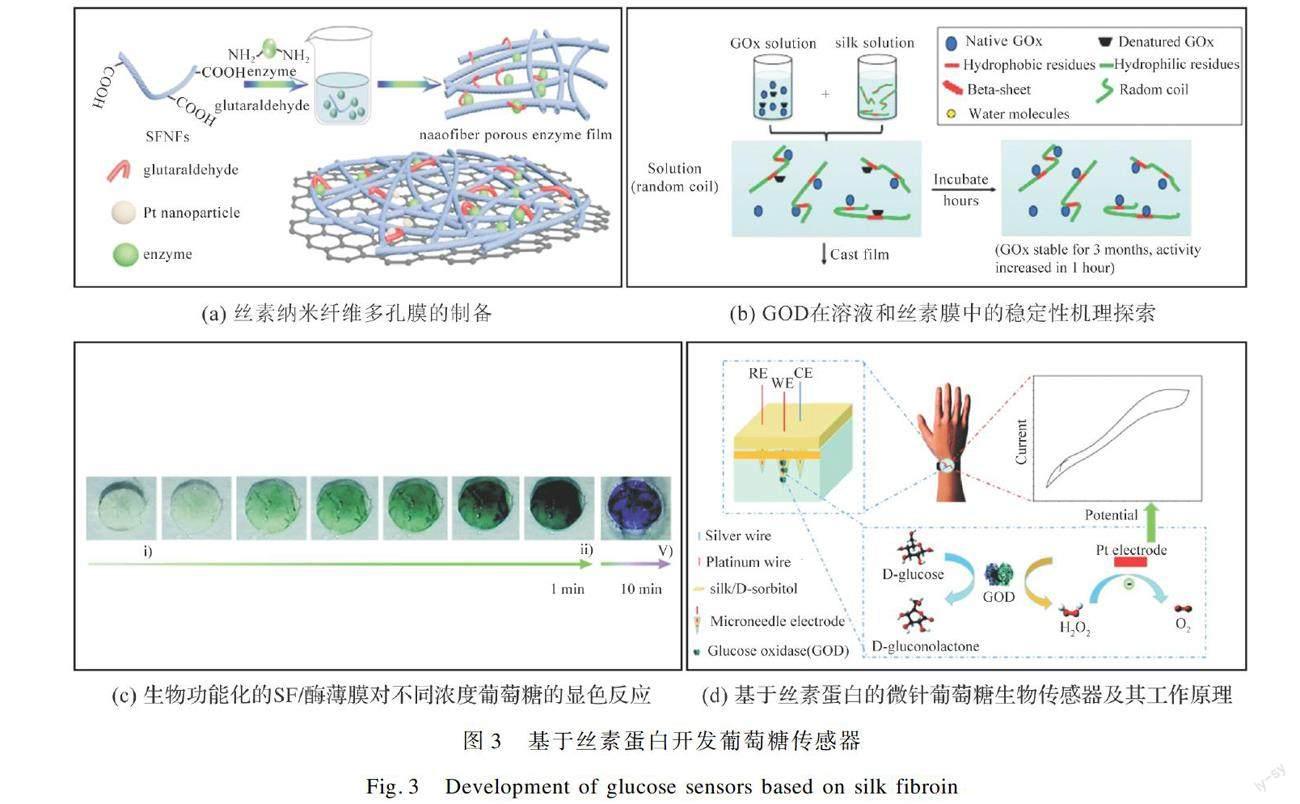
蚕丝主要由丝素蛋白(Silk fibroin,SF)和丝胶蛋白(Silk sericin,SS)组成,其中丝素蛋白约占蚕丝质量的70%~80%。通过脱胶溶解后提取得到的丝素蛋白是优异的天然蛋白材料[44]。丝素蛋白含有大量活性基团,易与葡萄糖氧化酶结合,用于固定及负载葡萄糖氧化酶。此外,基于丝素蛋白极易制备出纤维、膜、水凝胶等不同结构的材料,可开发出具有不同形态和功能的葡萄糖传感器,如图3所示。
Liu等[45]通过将丝素孵育得到丝素纳米纤维(SFNFs),由于SFNFs具有更高结晶度,在纳米纤维表面有大量残基,因此,其具有更好的稳定性和更多的结合位点,有助于提高酶的固定效率。通过戊二醛将酶与SFNFs交联形成多孔酶膜,再将其与纳米铂/石墨烯膜嵌合,制备成葡萄糖传感器(图3(a))。由于纳米铂/石墨烯复合膜表面的羟基和羧基能牢牢地与酶结合,所产生的多孔酶膜能提供更大的比表面积用于与反应物葡萄糖相互作用,该传感器在2 μM~1 mM对葡萄糖具有良好的线性关系,灵敏度为31.02 μA/mM,且具有良好的循环重复性和长期稳定性(25 h)。Lu等[46]研究了包括GOD在内的三种具有不同物理和化学性质的酶在水溶性和不溶性丝素蛋白膜中的长期稳定性,分别从丝素结构的变化、膜中酶的空间分布及酶的变性/复性等方面探讨这些体系中酶稳定性的机理(图3(b)),结果表明丝素膜能在长达10个月的保存时间中保留75%的酶活性,且酶渗漏率仅为0.05%。这是由于丝素蛋白的结晶结构、丰富的氢键交联网络及丝素蛋白链与酶分子之间的疏水作用,可限制GOD链的流动性,从而提高酶的稳定性。研究表明,再生丝素蛋白是GOD的良好载体,是制备酶基葡萄糖传感器的优异材料。Marquez等[47]通过将酶直接溶解在SF的水溶液中,干燥结晶成膜后直接用于葡萄糖的传感测试,结果表明负载GOD的SF膜具备过滤血细胞的能力,通过SF基质与酶反应产生的分子反应可将葡萄糖测定的灵敏度提高2.5倍。其传感机理主要是通过生物功能化的SF薄膜通过酶促反应对葡萄糖作出响应,其中的氧化还原介质与显色物质形成的复合物呈现出不同的颜色,从而测试出不同的葡萄糖浓度(图3(c))。Molinnus等[48]以SF溶液为基底,首先将其滴铸在硅晶片上,利用薄膜技术沉积铂后,滴涂GOD/牛血清白蛋白/戊二醛/甘油的混合溶液形成工作電极,再利用厚膜技术丝网印刷Ag/AgCl糊到丝素基材上制成参比电极,组装形成柔性葡萄糖生物传感器芯片。得益于天然丝素蛋白的特性,该传感器具有优异的生物相容性和可降解性,电极10 d内就可完全降解。为更好地固定GOD,保护GOD活性,提升传感器的性能,还可以在制备过程中加入酶稳定剂、聚合物、有机或无机功能纳米材料。Zhao等[49]通过将SF/D-山梨醇复合材料和铂、银丝结合,再将GOD固定在微针集成铂丝中,制备成新型微针葡萄糖生物传感器系统,用于血糖的微创连续监测(图3(d))。结果表明,GOD被固定在丝素/D-山梨醇基质中,表现出更好的稳定性,制备的传感器具有低糖浓度下响应快、生理条件下易于读数等优点,展示出1.7~10.4 mM葡萄糖的响应范围及31.7 μA/mM的灵敏度。目前为止,基于丝素蛋白的葡萄糖传感器还主要以丝素蛋白膜为主要载体,开发制备的传感器的传感性能与蚕丝织物为载体的传感器的性能相比,无明显差异(表1),导电活性物质的选择及制备方法均会直接影响蚕丝基葡萄糖传感器的传感性能[26]。
現有研究表明,蚕丝纤维中的SF在从溶液到纤维的再折叠过程中,可以在介观尺度上被重构/功能化,即通过添加功能材料参与SF分子的折叠来促进蚕丝功能化。简单来说,就是通过在丝蛋白液中加入功能活性材料,利用养蚕添食法[50]、人工纺丝技术如湿法纺丝、干法纺丝、微流控纺丝、静电纺丝[51-53]等制备出具有特殊尺寸和功能的丝素纤维。通过此方法引入导电活性物质可以开发出具有电化学功能的蚕丝纤维,进而开发出传感器、执行器、光纤、发光纤维和能量收集器传感器件,这也可能成为未来蚕丝基葡萄糖传感器研究的重要方向。
3 结 语
蚕丝由于其出色的性能,可应用于柔性葡萄糖传感器的研究,由简单的蚕丝纤维、纱线到蚕丝织物,再到高性能碳化丝织物,由简单的丝素蛋白到丝素膜,均可作为葡萄糖酶或葡萄糖响应物质的载体,所开发制备的葡萄糖传感器展现出良好的传感性能。但同时,葡萄糖氧化酶的固定率和稳定性不高、蚕丝与导电材料复合后的牢度差、丝素蛋白膜力学性能不佳等问题也直接影响到蚕丝基葡萄糖传感器的综合性能,还有待进一步解决。此外,蚕丝基葡萄糖传感器的成本及维护也是应用发展前不容忽视的因素。目前对于蚕丝基葡萄糖传感器的开发主要集中在对蚕丝蛋白膜和碳化蚕丝织物的整理上,对于以蚕丝纤维、织物本身为基底的研究还较少。通过养蚕添食法及纺丝技术制备的功能化蚕丝,也可以应用到蚕丝基葡萄糖传感器的研究,这些都可能是未来蚕丝基葡萄糖传感器研究的重要方向。总之,尽管蚕丝基葡萄糖传感器的开发研究还处于起步阶段,其应用前景不容忽视,未来发展值得期待。
参考文献:
[1]UPDIKE S J, HICKS G P. Enzyme electrode[J]. Nature, 1967, 214(5092): 986.
[2]ABELLAN-LLOBREGAT A, JEERAPAN I, BANDODKAR A, et al. A stretchable and screen-printed electrochemical sensor for glucose determination in human perspiration[J]. Biosensors & Bioelectronics, 2017, 91: 885-891.
[3]DONG L, LI R Y, WANG L Q, et al. Green synthesis of platinum nanoclusters using lentinan for sensitively colorimetric detection of glucose[J]. International Journal of Biological Macromolecules, 2021, 172: 289-298.
[4]LU Z W, WU L, DAI X X, et al. Novel flexible bifunctional amperometric biosensor based on laser engraved porous graphene array electrodes: Highly sensitive electrochemical determination of hydrogen peroxide and glucose[J]. Journal of Hazardous Materials, 2021, 402: 123774.
[5]XU Y, ZHANG B. Recent advances in porous Pt-based nanostructures: Synthesis and electrochemical applications[J]. Chemical Society Reviews, 2014, 43(8): 2439-2450.
[6]LEE W C, KIM K B, GURUDATT N G, et al. Comparison of enzymatic and non-enzymatic glucose sensors based on hierarchical Au-Ni alloy with conductive polymer[J]. Biosensors & Bioelectronics 2019, 130: 48-54.
[7]KARIMI-MALEH H, CELLAT K, ARIKAN K, et al. Palladium-Nickel nanoparticles decorated on functionalized-MWCNT for high precision non-enzymatic glucose sensing[J]. Materials Chemistry and Physics, 2020, 250: 123042.
[8]JIA H X, SHANG N Z, FENG Y, et al. Facile preparation of Ni nanoparticle embedded on mesoporous carbon nanorods for non-enzymatic glucose detection[J]. Journal of Colloid and Interface Science, 2021, 583: 310-320.
[9]ZHANG Y, LI N, XIANG Y J, et al. A flexible non-enzymatic glucose sensor based on copper nanoparticles anchored on laser-induced graphene[J]. Carbon, 2020, 156: 506-513.
[10]FRANCO F F, HOGG R A, MANJAKKAL L. Cu2O-based electrochemical biosensor for non-invasive and portable glucose detection[J]. Biosensors-Basel, 2022, 12(3): 174.
[11]LIN C H, DU Y, WANG S Q, et al. Glucose oxidase@Cu-hemin metal-organic framework for colorimetric analysis of glucose[J]. Materials Science & Engineering C-Materials for Biological Applications, 2021, 118: 111511.
[12]LI Y, XIE M W, ZHANG X P, et al. Co-MOF nanosheet array: A high-performance electrochemical sensor for non-enzymatic glucose detection[J]. Sensors and Actuators B: Chemical, 2019, 278: 126-132.
[13]GUMILAR G, KANETI Y V, HENZIE J, et al. General synthesis of hierarchical sheet/plate-like M-BDC (M=Cu, Mn, Ni, and Zr) metal-organic frameworks for electrochemical non-enzymatic glucose sensing[J]. Chemical Science, 2020, 11(14): 3644-3655.
[14]RAZA W, AHMAD K. A highly selective Fe@ZnO modified disposable screen printed electrode based non-enzymatic glucose sensor (SPE/Fe@ZnO)[J]. Materials Letters, 2018, 212: 231-234.
[15]LAKHDARI D, GUITTOUM A, BENBRAHIOM N, et al. A novel non-enzymatic glucose sensor based on NiFe(NPs)-polyaniline hybrid materials[J]. Food and Chemical Toxicology, 2021, 151: 112099.
[16]ZHAO W L, ZHANG G P, DU Y, et al. Sensitive colorimetric glucose sensor by iron-based nanozymes with controllable Fe valence[J]. Journal of Materials Chemistry B, 2021, 9(23): 4726-4734.
[17]WANG L Y, XIE S L, WANG Z Y, et al. Functionalized helical fibre bundles of carbon nanotubes as electrochemical sensors for long-term in vivo monitoring of multiple disease biomarkers[J]. Nature Biomedical Engineering, 2020, 4(2): 159-171.
[18]ZHOU Y, FANG Y, RAMASAMY R P. Non-covalent functionalization of carbon nanotubes for electrochemical biosensor development[J]. Sensors, 2019, 19(2): 392.
[19]KARIMI-MALEH H, CELLAT K, ARIKAN K, et al. Palladium-nickel nanoparticles decorated on functionalized-MWCNT for high precision non-enzymatic glucose sensing[J]. Materials Chemistry and Physics, 2020, 250: 123042.
[20]LEE H, CHOI T K, LEE Y B, et al. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy[J]. Nature Nanotechnology, 2016, 6(11): 566-572.
[21]SHAN C S, YANG H F, SONG J F, et al. Direct electrochemistry of glucose oxidase and biosensing for glucose based on graphene[J]. Analytical Chemistry, 2009, 6(81): 2378-2382.
[22]TEYMOURIAN H, BARFIDOKHT A, WANG J. Electrochemical glucose sensors in diabetes management: An updated review (2010-2020)[J]. Chemical Society Reviews, 2020, 49(21): 7671-7709.
[23]FREEMAN M H, HALL J R, LEOPOLD M C. Monolayer-protected nanoparticle doped xerogels as functional components of amperometric glucose biosensors[J]. Analytical Chemistry, 2013, 85(8): 4057-4065.
[24]LIN M J, WU C C, CHANG K S. Effect of poly-l-lysine polycation on the glucose oxidase/ferricyanide composite-based second-generation blood glucose sensors[J]. Sensors, 2019, 19(6): 1448.
[25]MARZO A, MAYORGA-MARTINEZ C C, PUMERA M. 3D-printed graphene direct electron transfer enzyme biosensors[J]. Biosensors & Bioelectronics, 2020, 151: 111980.
[26]張勇, 陆浩杰, 梁晓平, 等. 蚕丝基智能纤维及织物: 潜力、现状与未来展望[J]. 物理化学学报, 2022, 38(9): 64-79.
ZHANG Yong, LU Haojie, LIANG Xiaoping, et al. Silk materials for intelligent fibers and textiles: Potential, progress and future perspective[J]. Acta Physico-Chimica Sinica, 2022, 38(9): 64-79.
[27]李胜优, 刘镓榕, 文豪, 等. 蚕丝基可穿戴传感器的研究进展[J]. 物理学报, 2020, 69(17): 130-142.
LI Shengyou, LIU Jiarong, WEN Hao, et al. Recent advances in silk-based wearable sensors[J]. Acta Physica Sinica, 2020, 69(17): 130-142.
[28]WANG C Y, XIA K L, ZHANG Y Y, et al. Silk-based advanced materials for soft electronics[J]. Accounts of Chemical Research, 2019, 52(10): 2916-2927.
[29]CAI H H, GAO L Z, CHEN L, et al. An effective, low-cost and eco-friendly method for preparing UV resistant silk fabric[J]. Journal of Natural Fibers, 2022, 19(13): 5173-5185.
[30]XU M T, CAI H H, LIU Z L, et al. Skin-friendly corrugated multilayer microspherical sensor fabricated with silk fibroin, poly (lactic-co-glycolic acid), polyaniline, and kappa-carrageenan for wide range pressure detection[J]. International Journal of Biological Macromolecules, 2022, 194: 755-762.
[31]CHOUDHARY T, RAJAMANICKAM G P, DENDUKURI D. Woven electrochemical fabric-based test sensors (WEFTS): A new class of multiplexed electrochemical sensors[J]. Lab on A Chip, 2015, 15(9): 2064-2072.
[32]YE C, REN J, WANG Y L, et al. Design and fabrication of silk templated electronic yarns and applications in multifunctional textiles[J]. Matter, 2019, 1(5): 1411-1425.
[33]WANG L, WANG L Y, ZHANG Y, et al. Weaving sensing fibers into electrochemical fabric for real-time health monitoring[J]. Advanced Functional Material, 2018, 28(42): 1804456.
[34]王晓雷, 缪旭红, 孙婉. 针织间隔导电织物的压力电阻传感性能[J]. 丝绸, 2020, 57(4): 17-21.
WANG Xiaolei, MIAO Xuhong, SUN Wan. Pressure resistance sensing properties of knitted spacer conductive fabrics[J]. Journal of Silk, 2020, 57(4): 17-21.
[35]NILGHAZ A, BAGHERBAIGI S, LAM C L, et al. Multiple semi-quantitative colorimetric assays in compact embeddable microfluidic cloth-based analytical device (mu CAD) for effective point-of-care diagnostic[J]. Microfluidics and Nanofluidics, 2015, 19(2): 317-333.
[36]ZHAO Z Q, LI Q J, DONG Y, et al. Core-shell structured gold nanorods on thread-embroidered fabric-based microfluidic device for Ex Situ detection of glucose and lactate in sweat[J]. Sensors and Actuators B-Chemical, 2022, 353: 131154.
[37]KARAKUS S, BAYTEMIR G, TASALTIN N. Digital colorimetric and non-enzymatic biosensor with nanoarchitectonics of Lepidium meyenii-silver nanoparticles and cotton fabric: Real-time monitoring of milk freshness[J]. Applied Physics A-Materials Science & Processing, 2022, 128(5): 390.
[38]ZHAO Z Q, LI Q J, CHEN L N, et al. A thread/fabric-based band as a flexible and wearable microfluidic device for sweat sensing and monitoring[J]. Lab on A Chip, 2021, 21(5): 916-932.
[39]CAI H H, LIU Z L, XU M T, et al. High performance flexible silk fabric electrodes with antibacterial, flame retardant and UV resistance for supercapacitors and sensors[J]. Electrochimica Acta, 2021, 390: 138895.
[40]CAI H H, WANG Y J, XU M T, et al. Low cost, green and effective preparation of multifunctional flexible silk fabric electrode with ultra-high capacitance retention[J]. Carbon, 2022, 188: 197-208.
[41]HE W Y, WANG C Y, WANG H M, et al. Integrated textile sensor patch for real-time and multiplex sweat analysis[J]. Science Advances, 2019, 5(11): eaax0649.
[42]CHEN C, RAN R, YANG Z Y, et al. An efficient flexible electrochemical glucose sensor based on carbon nanotubes/carbonized silk fabrics decorated with Pt microspheres[J]. Sensors and Actuators B: Chemical, 2018, 256: 63-70.
[43]WANG C Y, LI X, GAO E L, et al. Carbonized silk fabric for ultrastretchable, highly sensitive, and wearable strain sensors[J]. Advanced Materials, 2016, 28(31): 6640-6648.
[44]明津法, 黃晓卫, 宁新, 等. 丝素蛋白材料制备及应用进展[J]. 丝绸, 2021, 58(2): 20-26.
MING Jinfa, HUANG Xiaowei, NING Xin, et al. Preparation and application of silk fibroin materials[J]. Journal of Silk, 2021, 58(2): 20-26.
[45]LIU X, ZHANG W L, LIN Z F, et al. Coupling of silk fibroin nanofibrils enzymatic membrane with ultra-thin PtNPs/graphene film to acquire long and stable on-skin sweat glucose and lactate sensing[J]. Small Methods, 2021, 5(3): 2000926.
[46]LU S Z, WANG X Q, LU Q, et al. Stabilization of enzymes in silk films[J]. Biomacromolecule, 2009, 10(5): 1032-1042.
[47]MARQUEZ A, SANTOS M V, GUIRADO G, et al. Nanoporous silk films with capillary action and size-exclusion capacity for sensitive glucose determination in whole blood[J]. Lab on A Chip, 2021, 21(3): 608-615.
[48]MOLINNUS D, DRINIC A, IKEN H, et al. Towards a flexible electrochemical biosensor fabricated from biocompatible Bombyx mori silk[J]. Biosensors & Bioelectronics, 2021, 183: 113204.
[49]ZHAO L, WEN Z Z, JIANG F J, et al. Silk/polyols/GOD microneedle based electrochemical biosensor for continuous glucose monitoring[J]. RSC Advances, 2020, 10(11): 6163-6171.
[50]蔡海華, 程岚, 李智, 等. 添食法制备改性蚕丝的研究进展[J]. 材料导报, 2020, 34(23): 23190-23198.
CAI Haihua, CHENG Lan, LI Zhi, et al. Research progress on preparation of modified silk by feeding method[J]. Materials Reports, 2020, 34(23): 23190-23198.
[51]ZHAO Z C, LI B T, XU L Q, et al. A sandwich-structured piezoresistive sensor with electrospun nanofiber mats as supporting, sensing, and packaging layers[J]. Polymers, 2018, 10(6): 575.
[52]KHALID A, BAI D B, ABRAHAM A N, et al. Electrospun nanodiamond-silk fibroin membranes: A multifunctional platform for biosensing and wound-healing applications[J]. ACS Applied Materials & Interfaces, 2020, 12(43): 48408-48419.
[53]ZHANG C, FAN S N, SHAO H L, et al. Graphene trapped silk scaffolds integrate high conductivity and stability[J]. Carbon, 2019, 148: 16-27.
[54]LU W D, JIAN M Q, WANG Q, et al. Hollow core-sheath nanocarbon spheres grown on carbonized silk fabrics for self-supported and nonenzymatic glucose sensing[J]. Nanoscale, 2019, 11(24): 11856-11863.
[55]WEI L, LI J H, CHEN C, et al. Ultrasensitive non-enzymatic glucose sensors based on hybrid reduced graphene oxide and carbonized silk fabric electrodes decorated with Cu nanoflowers[J]. Journal of the Electrochmical Society, 2020, 167(12): 127501.
[56]ASAKURA T, KITAGUCHI M, DEMURA M, et al. Immobilization of glucose-oxidase on nonwoven fabrics with bombyx-mori silk fibroin gel[J]. Journal of Applied Polymer Science, 1992, 46(1): 49-53.
[57]KOIKE K, SASAKI T, HIRAKI K, et al. Characteristics of an extended gate field-effect transistor for glucose sensing using an enzyme-containing silk fibroin membrane as the bio-chemical component[J]. Biosensors-Basel, 2020, 10(6): 57.
[58]YOU X Q, PAK J J. Graphene-based field effect transistor enzymatic glucose biosensor using silk protein for enzyme immobilization and device substrate[J]. Sensors and Actuators B: Chemical, 2014, 202: 1357-1365.
Research progress on silk-based glucose sensors
WANG Yi1, WANG Yujia1, CHEN Fangchun1b,c, WANG Yasi1b,c, DAI Fangyin1, LI Zhi1,2
(1a.State Key Laboratory of Silkworm Genome Biology; 1b.College of Sericulture, Textile and Biomass Sciences; 1c.Chongqing EngineeringResearch Center of Biomaterial Fiber and Modern Textile, Southwest University, Chongqing 400715, China; 2.Key Laboratory ofFlexible Devices for Intelligent Textile and Apparel, Soochow University, Suzhou 215123, China)
Abstract:
Glucose sensors, as a tool for diabetics to monitor blood glucose, are of great significance in the diagnosis and treatment of diabetes. With the rapid development of flexible smart wearable devices, the development of glucose sensors based on flexible substrate has also gradually become a research hotspot. Silk has good biocompatibility, degradability and flexibility, its surface is rich in chemical bonds such as carboxyl, hydroxyl and amide bonds which can interact with active substances, and it is a good conductive substrate material. The flexible glucose sensor developed by using silk and silk fibroin as the substrate, and by being compounded with conductive active materials has excellent sensing performance and good long-term stability. This article aims to review the research progress of silk-based glucose sensors developed based on silk fibroin, silk fiber and silk fabric, analyze their characteristics and mechanism, and prospect their development tendency.
In order to fully understand the application of silk in the field of glucose sensors, this paper starts with the development of glucose sensors, and focuses on the research progress of silk-based glucose sensors on basis of two main kinds of silk and silk fibroin. We first introduced the characteristics and properties of silk, and discussed the application of silk yarns, silk fabrics and carbonized silk fabrics in the field of glucose sensors. Then, we summarized the application of fibroin films and spun fibers based on silk fibroin in the field of glucose sensors. By reviewing the preparation methods and sensing performance of various silk-based glucose sensors, we analyzed the role of silk in them, and summarized the effect of the silk substrate on the glucose sensing performance. It is hoped that this paper can provide a reference for the design and development of high-performance silk-based flexible glucose sensors. Glucose sensors based on silk fabrics have better mechanical properties, higher conductivity and sensitivity, while those made of silk fibroin have better biocompatibility and degradability. Finally, we compared and analyzed the sensitivity, response time, detection limit, advantages and disadvantages of the silk-based glucose sensors. In sum, in virtue of its good characteristics, silk has the excellent application value in the field of flexible glucose sensors, and the developed glucose sensors show good sensing performance, which can also keep the original flexibility and biocompatibility of silk and show good conductivity and ultra-high response to glucose molecules. However, the problems such as the poor fixation rate of silk fibroin to glucose oxidase, the poor mechanical properties of silk fibroin films, and the weak binding between silk fabric and conductive materials still need to be solved urgently. In addition, the cost and maintenance of silk-based glucose sensors are also the factors that cannot be ignored.
At present, the development of silk-based glucose sensors is mainly based on the silk fibroin membrane and the carbonized silk fabric, and there are relatively few studies and applications based on the silk fiber and the silk fabric. The functionalized silk produced by the silkworm feeding method and spinning technology can be applied to the research and development of sensors, which would possibly become one of the important research directions of silk-based glucose sensors in the future. Overall, although the development of silk-based glucose sensors is still in its infancy, its application prospects are very broad, and it is worth looking forward to future development.
Key words:
silk; silk fibroin; silk fabric; glucose sensors; glucose oxidase; flexible electronic devices
收稿日期:
2022-08-01;
修回日期:
2023-02-03
基金项目:
重慶市教育委员会科学技术研究项目(KJQN202100203);纺织行业智能纺织服装柔性器件重点实验室开放课题资助项目(SDHY2111);重庆市留创计划创新类资助项目(cx2019090);家蚕基因组生物学国家重点实验室开放课题资助项目(sklsgb-2019KF13)
作者简介:
王怡(1997),女,硕士研究生,研究方向为生物医用纺织品。通信作者:李智,副教授,tclizhi@swu.edu.cn。
