Study on the occurrence state of indium in sphalerite of Dulong Sn–Zn–In polymetallic deposit, Southwest China
2023-06-28LishengGaoHanjieWenChuanweiZhuXinNieAibingChenGuangshuYang
Lisheng Gao • Hanjie Wen • Chuanwei Zhu • Xin Nie • Aibing Chen•Guangshu Yang
Abstract The Dulong deposit, located in the Laojunshan area of southeastern Yunnan, China, is an important polymetallic deposit due to its high reserves of tin, zinc,and indium. The occurrence state of indium is critical for understanding its supernormal enrichment mechanism.Previous studies investigated the occurrence state of indium (including the valence state) based on the indium content in sphalerite and the correlation between metal concentrations. However, more evidence is needed to better constrain indium occurrence at the micro-, nano-, or even atomic scale. In this study, EPMA-FIB-SEM-TEM and XPS techniques were employed to investigate the indium distribution characteristics and occurrence state in sphalerite from the Dulong Sn–Zn–In polymetallic deposit.The maximum concentration of indium in the indium-rich sphalerite samples is 0.37 %, and the results of the EPMA analysis showed a relatively homogeneous distribution of indium in sphalerite. The FIB-SEM-TEM results demonstrated that the lattice stripes of sphalerite were periodically and continuously distributed at the nanoscale, confirming that sphalerite in the deposit was an excellent single crystal structure,and the peak heights of the various characteristic peaks of indium in the EDX spectra were relatively close to each other, with no distinct peaks of high indium content.In addition, the XPS results indicate that the element valence state of indium in sphalerite is In3+, and it combines with S2- to form a bond. These results indicate that indium in sphalerite of the Dulong deposit is uniformly distributed at both the micro-and nanoscale,and there is no indium-independent mineral. In3+ enters the crystal lattice of sphalerite by replacing Zn2+ in the form of isomorphic substitution.
Keywords Sphalerite ∙Indium ∙Occurrence state ∙Dulong Sn–Zn–In polymetallic deposit
1 Introduction
Indium (In) was first discovered by the German scholars Reich and Richter in 1863 when testing thallium in sphalerite (Schwarz-Schampera and Herzig. 2002). Indium is widely used in aerospace, electronics, solar cells, and semiconductors due to its good light permeability and conductivity (Wen et al. 2019). Since the twenty-first century, indium metal is playing an increasingly important role in developing the world economy. Indium is thus called a ‘‘critical metal’’ or ‘critical mineral resource’’ by many countries because of its importance for the development of defense, modern industry, and high technology(Li et al.2020).Therefore,it is necessary to strengthen the research on indium to ensure the supply of indium resources.
As a dispersed element, mineralogical studies of numerous deposits worldwide in the past few decades have shown that indium exists in several genetic types of deposits (Schwarz-Schampera and Herzig. 2002; Werner et al. 2017). The abundance of indium in the core, lower mantle, upper mantle, terrestrial crust, and oceanic crust is extremely low, with indium concentrations of 0.5 × 10–6,0.01 × 10–6, 0.06 × 10–6, 0.05 × 10–6and 0.072 × 10–6respectively (Taylor and McLennan 1985). Indium is not heterogeneous in ore deposits and rarely occurs as an independent mineral, which is caused by its low concentrations in the crust. Eighteen indium minerals have been identified in nature, which was only observed in a few ore deposits(Wen et al.2019).At present,indium-independent deposits have not been reported, and indium mainly exists in lead–zinc and cassiterite-sulfide deposits as a byproduct.Indium occurs in these deposits mainly as sphalerite,chalcopyrite, stannite, pyrite, and arsenopyrite, among which indium resources, hosted in sphalerite, account for approximately 95% of the global indium resources (Lerouge et al. 2017).
Indium resources in China contain about 18.2% of the identified global reserves (Werner et al. 2017), among which the Dachang, Dulong, and Gejiu deposits are the most important indium-bearing deposits in China, having approximately 70% of China’s identified indium reserves(Li et al. 2020). As the second largest indium-bearing deposit in China,the Dulong deposit has associated indium reserves of ~6000 t. At present, research on the distribution characteristics, occurrence state, and substitution mechanism of indium is not well-constrained and further studies are needed to better understand its geochemical behaviors in such hydrothermal systems. Specifically,whether there are some indium-independent minerals and how indium is enriched in sphalerite in the Dulong deposit need further constraints. Previous studies indicated that indium is present in sphalerite lattice as In+3based on in situ trace element analyses, suggesting that indium requires either coupling substitution or coupling vacancies to achieve charge compensation due to Zn occurring as Zn2+in sphalerite,with indium substitution mechanisms as following: Cu++ In3+↔2Zn2+(Bauer et al. 2017;Cook et al. 2012), In3++ Sn3++ (vacancy) ↔3Zn2(Belissont et al. 2014), In3++ (Cu, Ag)++ Sn2+↔3Zn2+(Frenzel et al. 2016), and In3++ Sn4++ (Cu,Ag)++ (vacancy) ↔4Zn2+(Frenzel et al. 2016). However, it is still unclear whether indium-independent minerals exist in sphalerite at the nanoscale.Moreover,indium has two main valence states in nature (In+, In3+) (Gunn.2014), and its valence state in sphalerite is less well-constrained. The valence state of indium in indium-bearing sphalerite is essential to establish a substitution mechanism.
Here,sphalerite from the Dulong Sn–Zn–In polymetallic deposit was selected as the research object. Through analyses of electron probe microanalyzer(EPMA),focused ion beam combined scanning electron microscopy (FIBSEM), and transmission electron microscopy (TEM), the distribution and occurrence of indium in sphalerite were studied from microscale to nanoscale.The valence state of elements on the surface of sphalerite samples was studied by X-ray photoelectron spectroscopy (XPS).
2 Geological setting
The Laojunshan area in southeastern Yunnan is a critical polymetallic resource base in China and worldwide. The area is tectonically located at the junction of the Cathaysian, Yangtze, and Indochina blocks (Fig. 1a) (Cheng et al.2013;Hu et al.2017;Xu et al.2015;Yan et al.2006).Advantaged metallogenic conditions and complex geological environments result in a series of large-scale ore deposits, with several polymetallic deposits from west to east,such as the Gejiu,Bainiuchang,Dulong,Nanyangtian and other polymetallic deposits (Liu et al. 2007). The outcropping strata in the Laojunshan area are complicated,with partial outcrops of strata from the Proterozoic to the Cenozoic. The fault structures in the Laojunshan area are developed,and the magmatic activity and mineralization in the area are affected by the intersecting faults in different directions, with the main faults including the Maguan-Dulong, Nanwenhe, and Wenshan-Malipo faults (Fig. 1b).The Dulong deposit is distributed nearly north–south,with a broad area of more than 10 km2. From north to south, it is mainly composed of Tongjie, Manjiazhai,Lazizhai, Jinshipo, and other ore segments (Fig. 2a),among which the Manjiazhai ore segment is the largest one.The strata outcropping in the mining area is mainly the Neoproterozoic Xinzhaiyan Formation, the Middle Cambrian Tianpeng Formation, and the Longha Formation,among which the Xinzhaiyan Formation is composed of schist and some marble,and is the most crucial ore-bearing stratum in Dulong mining area. The Middle Cambrian Tianpeng Formation is distributed in the western part of the mining area. Influenced by metamorphism, the Tianpeng Formation is mainly composed of metamorphic rocks, and the main rock lithologies include schist,marble,and gneiss.Three sets of roughly parallel NS-trending faults(F0,F1,F2) are the main ore-controlling structures of the deposit,which are of great significance to the formation of tin,zinc,and other polymetallic ores. The F1fault crops out in the central part of the mine area, and the hanging wall and footwall of the fault are all strata of the Xinzhaiyan Formation. Mineralization in the vicinity of the F1fault is widespread and intense, with tin-zinc polymetallicmineralization characteristic of the ore section from Tongjie to Lazizhai (Fig. 2a) (Tao et al. 2016; Liu et al.2022). Recent geochemical and geochronological data suggest that the main magmatic activity in the mining area is the Laojunshan composite granite formed by the intrusion of the late Yanshanian period (Liu et al. 2007; Lan et al. 2016). Previous studies indicated that the only granitic rocks outcropping in the mining area are those from the third phase (75-87 Ma) of the Laojunshan composite granite, which is small in scale and occurs as narrow dikes or apophyses, and the lithology is granite porphyry(Liu et al.2007;Xu et al.2015;Zhao et al.2018).
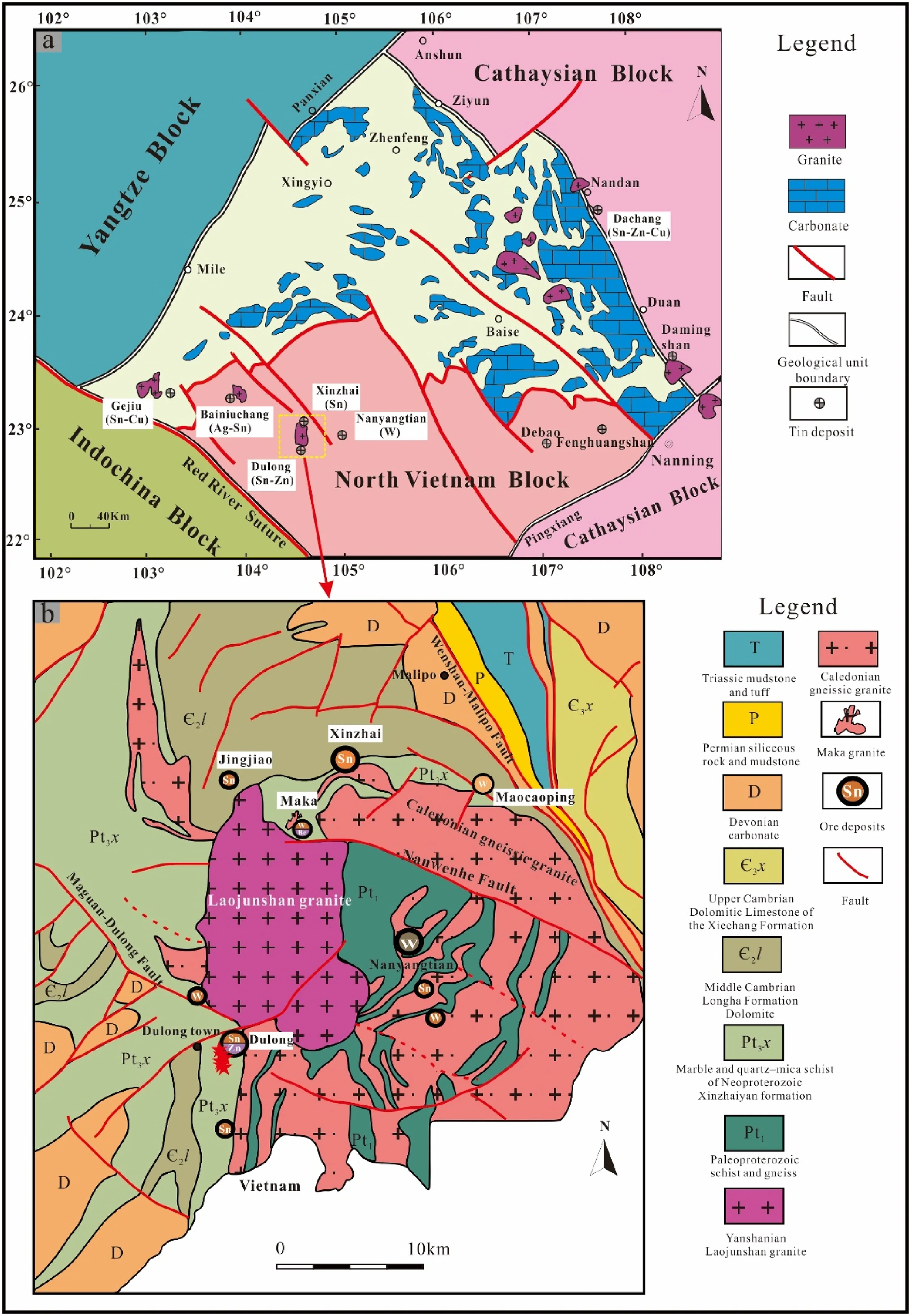
Fig. 1 a Simplified structural map showing the location of the study area (Xu et al. 2015).b Geological map of the Laojunshan ore district, Yunnan Province, China (Liu et al.2006)
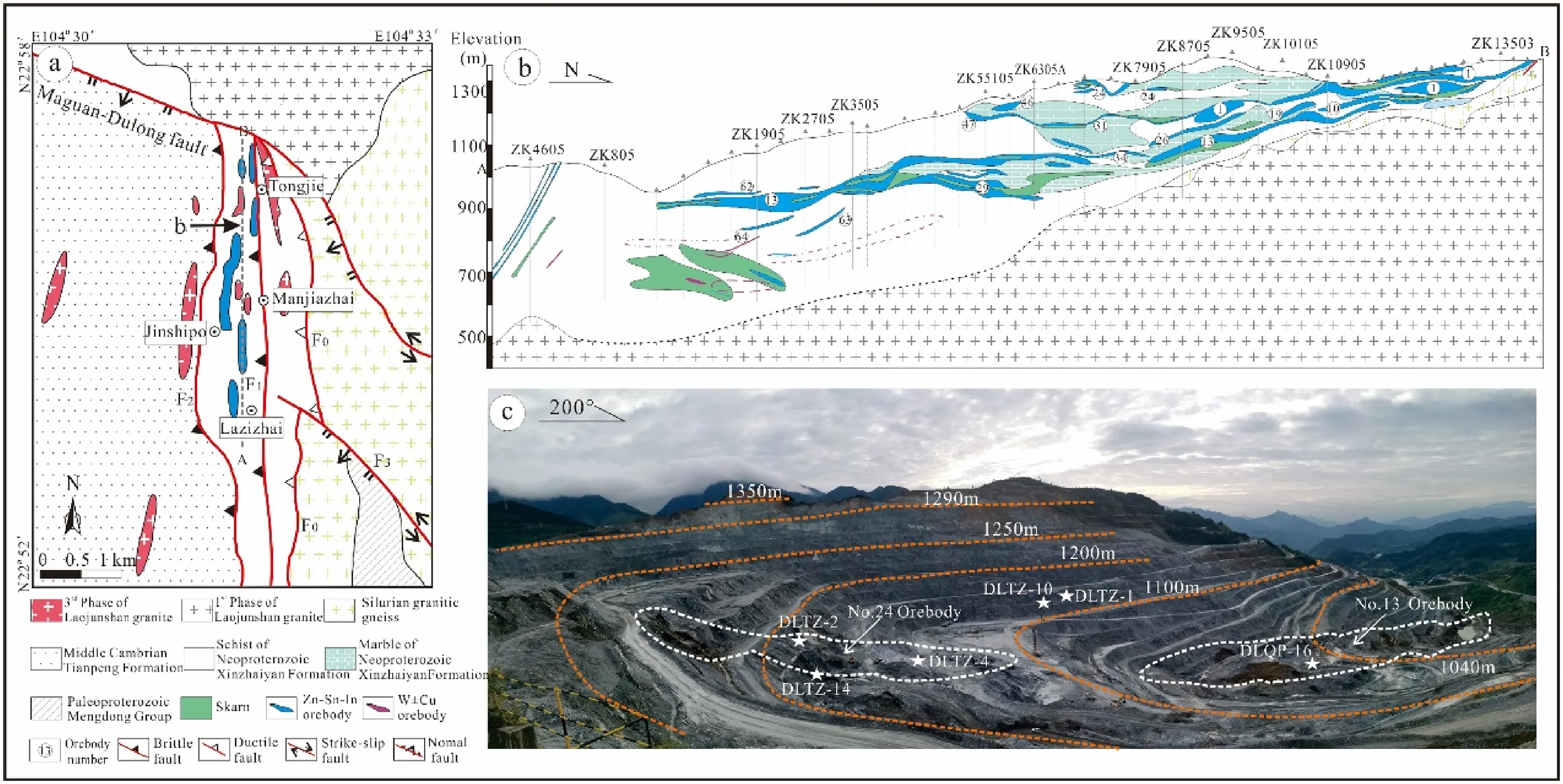
Fig. 2 a Geological map of the Dulong Sn–Zn–In polymetallic deposit (Liu et al. 2007).b Sectional view of the main ore blocks in the mining area [from the Lazizhai ore block(S)to the Tongjie ore block(N)](Xu et al.2020).c Photo of open pit of the Manjiazhai ore block (the samples were mainly collected at orebodies No.13 and No.24)
3 Methods
Sulfide samples were collected from orebodies 13 and 24 in the Dulong mining area(Fig. 2c).Representative sphalerite ore samples were selected and processed into thin polished sections, 6 of which were chosen for EPMA analysis to search for indium-rich sphalerite,which was then analyzed by a combined FIB-SEM-TEM. XPS was used to analyze the valence states of indium in sphalerite.
3.1 Electron probe microanalysis (EPMA)
Quantitative mineral compositional data were obtained using a JXA-8230 (JEOL, Japan) electron probe microanalyzer at the State Key Laboratory of Ore Deposit Geochemistry,Institute of Geochemistry,Chinese Academy of Sciences. The specific experimental conditions were: an accelerating voltage of 25.0 kV and an estimated electron beam diameter of 5 μm. The lowest detection limit for the elements analyzed is 0.01%.
3.2 Transmission Electron Microscopy foil preparation
Focused ion beam combined scanning electron microscopy(Scios dual-beam system, FEI, USA) was used for TEM foil preparation of the sphalerite sample with a thickness of approximately 100 nm at the Center for Lunar and Planetary Sciences,Institute of Geochemistry,Chinese Academy of Sciences. The sample preparation process (Fig. 3) is as follows (Ciobanu et al. 2011):

Fig. 3 Flow chart for fabrication of focused ion beam(FIB) cross sections
(1) Using field emission scanning electron microscopy(FESEM), the cut area was selected under the backscattered image (Fig. 3a).
(2) To avoid damage to the target area during ion sputtering, a Pt protective layer with a certain width was deposited in this area (Figs. 3b, c).
(3) A focused high-energy Ga ion beam was used to etch the area in front of and behind the Pt protective layer, and then the sample under the Pt protective layer was gradually thinned (Fig. 3d).
(4) The thinned sample was excised by an ion beam from the interface between the bottom and the matrix(Fig. 3e);
(5) The sample was removed from the excavation site and welded on the copper carrier net using an operating manipulator (Figs. 3f, g);
(6) The sample was further thinned and polished using a low-energy ion beam, resulting in a sample size of approximately 10 μm × 10 μm and a sample thickness of approximately 100 nm (Figs. 3h, i).
3.3 Transmission Electron Microscopy analysis(TEM)
The micron structure and composition of each specimen were examined by Transmission electron microscopy(TEM) using a Tecnai G2 F20 S-Twin instrument (FEI,USA) at the State Key Laboratory of Environmental Geochemistry, Institute of Geochemistry, Chinese Academy of Sciences. The structural data of the sample was obtained by selected area electron diffraction (SAED) and fast Fourier transform (FFT) images in high-resolution transmission electron microscopy (HRTEM) mode. In addition, the elemental composition information of the samples was obtained from energy-dispersive X-ray spectrometry (EDX).
3.4 X-ray Photoelectron Spectroscopy analysis(XPS)
X-ray photoelectron spectroscopy is a highly sensitive surface analysis technique with a sample analysis depth of approximately 2 nm. Before the XPS analysis, the sphalerite sample was polished to a size of about 10 × 10 × 3 mm. However, in the sample preparation process, complete elimination of surface contamination is not possible as any sample can get contaminated by moisture and adventitious carbon even through a short exposure to the atmosphere.Hence,the sample needs to be dried at 30 °C in a vacuum oven and then stored in an anaerobic environment. The elemental composition andvalence states of sphalerite samples were characterized by EscaLab 250Xi X-ray photoelectron spectroscopy(Thermo Fisher, USA) at the Analysis and Test Center, Guangdong University of Technology. Using monochromated Al Kα(1486.6 eV) source operated at 110 W,and the C 1 s peak at 284.8 eV was used to calibrate the XPS spectra.
4 Results and discussion
4.1 Micron-scale studies of indium-rich sphalerite
To satisfy the requirements of TEM,the indium-rich zones were selected based on the results of EPMA, the data of which are listed in Table 1. The results show a high iron content in the sphalerite samples, with iron concentrations ranging from 8.84 to 13.84 %,indicating that marmatite is the primary type of sphalerite in the Dulong deposit.Cadmium concentrations are relatively high, ranging from 0.12 to 0.22 %; by contrast, tin concentrations are generally lower than the detection limit of EPMA. All samples,except for DLQP-16, were near or below the detection limit for indium. DLQP-16 had a maximum indium concentration of 0.37 %,and this sample could be selected for FIB preparation.In addition,EPMA mapping scan analysis was carried out on DLQP-16 (Fig. 4) to investigate the elemental distribution of indium, cadmium, and other related elements at the micron scale. The results show that the distribution of indium and cadmium in the samples was relatively homogenous,and no abnormal enrichment points(areas) of these two elements were found in the EPMA element distribution maps.
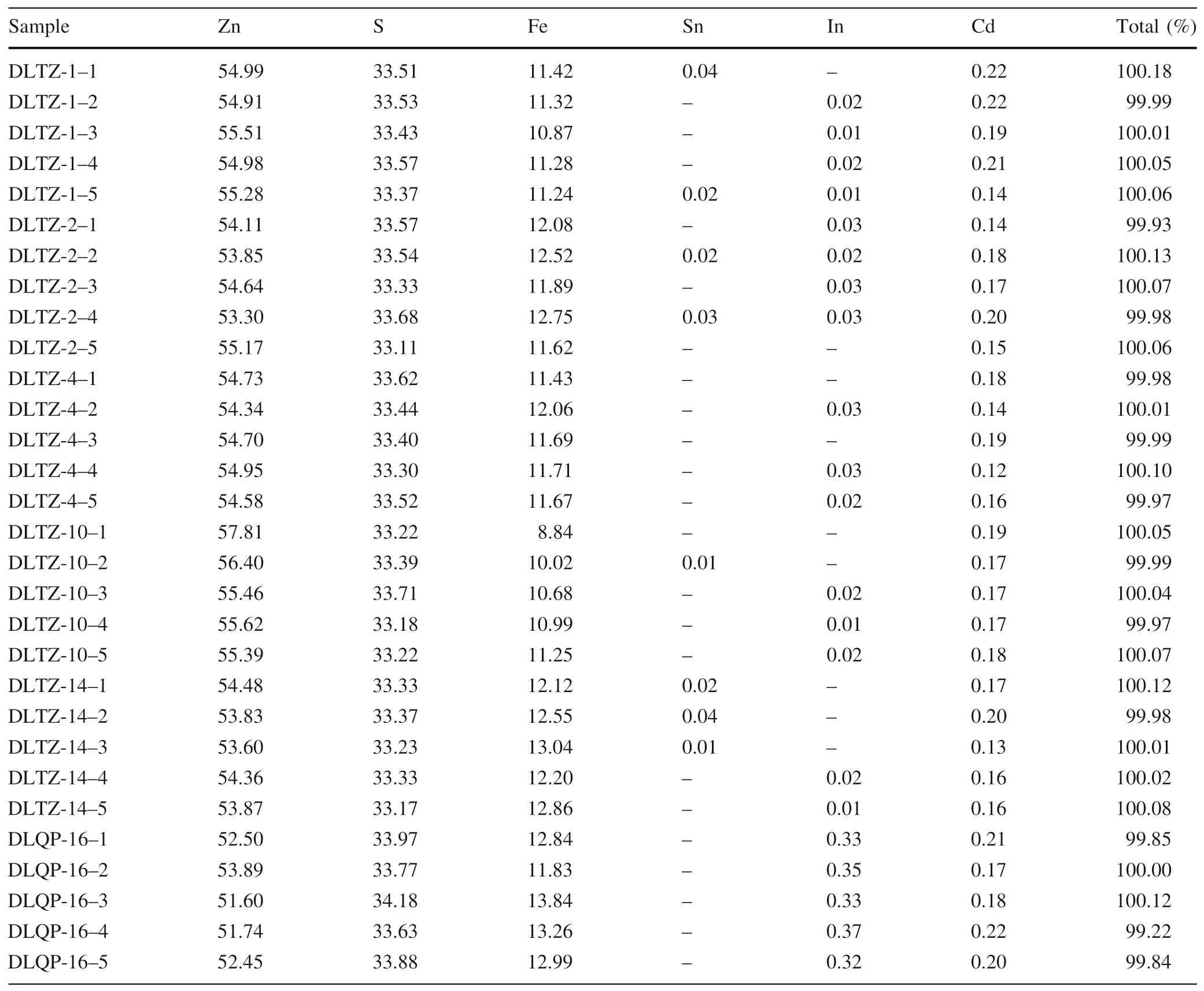
Table 1 EPMA analytical results of sphalerite in the Dulong deposit (%)

Fig. 4 EPMA element distribution maps of sphalerite showing the distribution characteristics of Zn, S, In, Fe and Cd

Fig.5 TEM analysis of the first cross-section. a low magnification TEM image,b high magnification TEM(HRTEM) image, c fast Fourier transformed (FFT) pattern of lattice fringing pattern,d results of the energy spectral composition of the first crosssection

Fig. 6 TEM analysis of the second cross-section. a low magnification TEM image,b high magnification TEM(HRTEM) image, c fast Fourier transformed (FFT) pattern of lattice fringing pattern,d results of the energy spectral composition of the second cross-section
4.2 Nanoscale studies of indium-rich sphalerite
The indium-rich sphalerite sample prepared by FIB-SEM was analyzed using the TEM technique. The HRTEM images (Figs. 5b, 6b) were recorded from the red circled areas in Figs. 5a and 6a indicate that both study areas are well crystallized with lattice fringe spacings of approximately 0.3121 nm and 0.3136 nm, respectively, which are assigned to the(111)crystallographic interplanar distances of sphalerite (Dong et al. 2018; Zhu et al. 2022). The continuous lattice fringes in the whole region reveal that each sample has an excellent single crystalline structure,which is consistent with the two-dimensional spot array in the FFT pattern(Figs.5c,6c)of its corresponding HRTEM image (Madkhli and Shirbeeny. 2020). In addition, chemical elemental analysis of the selected area was carried out by an EDX spectrometer.The EDX results are illustrated in Figs.5d and 6d,which show the characteristic peaks of Zn,S, Fe, Cu, Mn, In, and Cd of the two selected areas. The contents of indium and cadmium in the two selected areas indicate that the distribution of these two elements at the nanoscale in the sphalerite sample is homogeneous, and there are no anomalously enriched regions. Moreover, no indium/cadmium-independent minerals or nanoparticles were observed at the nanoscale,indicating that indium and cadmium mainly enter the lattice of sphalerite as isomorphism.
Among the indium-independent minerals identified thus far,CuInS2,the most widely distributed one,often requires the participation of large amounts of copper ions. The polymetallic ore concentration area at the junction of the Yangtze, Indochina, and Cathaysian blocks, the main indium resource base in China,has thus far not reported the presence of CuInS2and other indium-independent minerals. Previous research has found that the pyrrhotite of the Dulong deposit contains microscale particles of indiumrich sphalerite with indium contents up to 14.75%(Li et al.2020). Although the indium content was so high, no CuInS2was found. Indium is mainly in the form of ion exchange in the sphalerite lattice. It is evident that the output conditions of indium-independent minerals still need further study.
4.3 Elemental valence characteristics of indium-rich sphalerite
The valence states of major and trace elements in sphalerite were measured using XPS spectrometry. The high-resolution XPS spectrum of S, Zn, Fe, In, and Cd are shown in Fig. 7.
Figure 7a shows the XPS spectrometry of S 2p, which can be fitted with the doublets at 161.4 eV and 162.6 eV,corresponding to the peaks of S 2p3/2 and S 2p1/2, indicating that sulfur in sphalerite exists as S2-(Cheng et al.2021; Wu et al. 2018; Zou et al. 2017). In addition, no binding energy of S6+in SO42-was observed, suggesting that sulfur in sphalerite was not oxidized during the processes of sample pretreatment and testing.
As shown in Fig. 7b, the high-resolution Zn 2p energy spectrum can be divided into two characteristic peaks at 1021.82 eV and 1044.86 eV, which were attributed to binding energies of Zn 2p3/2 and Zn 2p1/2(Liu et al.2015;Bhat et al.2017).Other studies have pointed to ZnO being confirmed by two characteristic peaks of Zn 2p3/2(1022.25 eV)and Zn 2p1/2(1045.35 eV)(Abdelkrim et al.2022). However, this phenomenon was not found in Fig. 7b, indicating that Zn–O is not present on the surface of the sample. Therefore, only Zn2+bonded with S2-is present on the surface of the sphalerite sample.
In the Fe 2p spectra (Fig. 7c), the two fitting peaks at 708.44 eV and 722.46 eV are consistent with Fe 2p3/2 and Fe 2p1/2, respectively, which are assigned to Fe2+in sphalerite (Siew et al. 2021). Moreover, the characteristic peaks of Fe 2p3/2 (709.5 eV) and Fe 2p1/2 (723.2 eV)bound to O2-have not been observed (Yamashita and Hayes.2008),indicating that Fe2+only binds to S2-on the surface of sphalerite. Furthermore, a relatively weak peak at 710.33 eV can be indexed as the Fe 2p3/2 of Fe3+(Yang et al.2011), which was likely triggered by exposure of the sample to air during sample preparation. The other weak peaks at 713.87 eV and 727.44 eV could be attributed to the satellite peaks of Fe 2p3/2 and Fe 2p1/2.
The In 3d (Fig. 7d) peaks at 444.88 and 452.47 eV are attributed to In 3d5/2 and In 3d3/2,respectively (Wu et al.2018;Li et al.2020;Liu et al.2021;Yang et al.2021).The peak separations of In 3d are calculated to be 7.59 eV,which is consistent with the characteristic peak of In3+reported in previous studies (Chen et al. 2016; Li et al.2017;Xiao et al.2018;Gultom et al.2021).In addition,the characteristic peaks of In 3d5/2 (441.8 eV) and In 3d3/2(449.4 eV)bound to O2-were not observed in the spectra,indicating that In3+is bonded to S2-on the sample surface(Xiao et al. 2018).
Two noticeable peaks for Cd 3d, located at 404.93 eV and 411.67 eV,could be assigned to Cd 3d5/2 and Cd 3d3/2 in Fig. 7e, which could be identified as the Cd2+chemical state in the Cd–S bond (Chaguetmi et al. 2018;Wang et al. 2019; Pal et al. 2021). Similarly, no evidence shows that Cd is bonded to O2-in the studied samples due to different binding energies between Cd–S and Cd–O(Janani et al.2022),suggesting that Cd2+on the surface of the sphalerite sample existed in Cd–S bonds.
5 Conclusions
(1) EPMA point analysis and mapping scan analysis results show that indium is homogenous in sphalerite from the Dulong deposit, with no abnormal element enrichment point or area. This indicates that indium is distributed uniformly in the form of isomorphism on the micron scale.
(2) Based on in situ sample preparation, detailed TEM results show that indium also exists in sphalerite as isomorphism and is evenly distributed at the nanoscale, and there are no indium-independent minerals at this scale.
(3) XPS analysis indicates that the valence states of Zn,S, Fe, In, and Cd elements in the indium-rich sphalerite of the Dulong deposit are Zn2+,S2-,Fe2+,In3+, and Cd2+, respectively. In3+combines with S2-to form a bond,replacing Zn2+in the sphalerite lattice.
AcknowledgementsThis study was financially supported by the National Nature Science Foundation of China(42072094,42162012).The authors appreciate Xiang Li, Yuanyun Wen, and Kai Yan for their assistance with sample analysis.
Declarations
Conflict of interestWe declare no conflict of interest in this study.
Ethical approvalAll authors have approved the manuscript and agree with its submission.
杂志排行
Acta Geochimica的其它文章
- Coupling between the Cenozoic west Pacific subduction initiation and decreases of atmospheric carbon dioxides
- Geochronology and origin of Paleoproterozoic charnockites with old crustal signature in the Haisyn block of the Ukrainian shield
- A review of GEMC method and its improved algorithms
- Trace-element geochemistry and S–O isotopes in the fluoritebarite mineralization of Merguechoum, Moroccan eastern Meseta: insights into ore genesis to the Pangea rifting
- Textural and compositional zoning in plagioclase phenocrysts:implications for magma chamber processes in the Emeishan large Igneous Province, SW China
- Petrogenesis of the Neoarchean zincian chromite within ultramafic xenoliths, Bastar Craton, India
