Structural characterization and mass spectrometry fragmentation signatures of macrocyclic alkanes isolated from a Sydney Basin torbanite, Australia
2023-06-28XianxinMengHongLuZhirongZhangPinganPengJohnVolkman
Xianxin Meng • Hong Lu• Zhirong Zhang • Ping’an Peng •John K. Volkman
Abstract Individual hydrocarbons identified to be macrocyclic alkanes in a torbanite from the Sydney Basin(Australia) were successfully isolated from its extracts using preparative gas chromatography and analyzed by NMR.Saturated cyclic structures were confirmed by single peaks in the NMR 1H and 13C spectra indicating single forms of H and C atoms exist in these biomarker molecules. This is consistent with the methylene unit in a ring system assignment of the macrocyclic alkanes and accounts for a formula of (CH2)n. The unusual molecular structures of these compounds are consistent with those that were identified from previous GC retention index data and co-injection with a standard supports the previous research. The mass spectral fragmentation behaviors of representative cyclic alkanes were further investigated by comparing them with the mass spectra of isolated individual macrocyclic alkanes. The characteristic fragment ions in the macrocyclic alkanes of(M–28)+and base peaks of m/z 97, 111, 125, etc., can be assigned as being generated by simple α-/i-cleavage and hydrogen rearrangement.These fragmentation pathways combined with retention indices should assist in differentiating these compounds from monounsaturated alkenes and alkylated monocyclics having similar mass spectral characteristics in other geological samples.
Keywords Macrocyclic alkanes ∙Torbanite ∙NMR ∙Preparative GC ∙MS fragmentation
1 Introduction
The widely distributed green microalgaBotryococcus braunii(B.braunii)accounts for a significant proportion of organic matter in various sediments (e.g., Largeau et al.1984, 1986; Brassell et al. 1986; Dubreuil et al. 1989;Volkman 2014). Despite their morphological uniformity,B. brauniican be chemically classified into three main races (A, B, and L) determined by the specific lipids they produce, i.e.,n-alkadienes (Metzger et al. 1991), botryococcenes (Maxwell et al. 1968; Moldowan and Seifert 1980), lycopadienes (Metzger et al. 1991) and cyclobotryococcenes (Huang et al. 1995; Summons and Powell 1986, 1987), and the co-occurrence of multiple races in the same sediments is now documented (Grice et al. 1988; Zhang et al. 2007; Lu et al. 2018). Additional series of novel macrocyclic alkanes (C15–C34) and corresponding monomethylated counterparts (C17–C26) have been reported in Torbanites (Audino et al. 2001; Grice et al.2001;Zhang and Volkman 2020),petroleum(Audino et al. 2002). These unusual saturated cyclic alkanes were identified based on co-injection with a single synthetic standard of cyclopentadecane and GC retention index plots(Audino et al. 2001).
Early studies had noted these compounds, but assumed that they were monounsaturated alkenes(Allan et al.1980).Indeed, macrocyclic alkanes are not easily distinguished from ubiquitously distributedn-alkylcyclohexanes (Fowler et al.1986)and monounsaturated alkenes(Alexander et al.1992)in sediments based solely on mass spectra since each hydrocarbon group shows effectively one double bond equivalent within an aliphatic structure.
A fraction enriched in macrocyclic alkanes can be prepared from the saturated fraction of solvent extracts using molecular sieves (e.g., included by X13 and excluded by ZSM-5, Audino et al. 2004; Zhang and Volkman 2020)thus allowing further study. The recent report (Zhang and Volkman 2020) on this same sample, showed that these alkanes had enriched carbon isotope signatures that are typical of lipids derived fromB. brauniiRace A (Grice et al. 2001) and are probably generated from the algaenan or its polyaldehyde precursor (Audino et al. 2002).
In the present study, individual macrocyclic alkanes were successfully separated and collected by preparative gas chromatography(pGC)after which NMR analysis was carried out for more reliable confirmation of their molecular structures, and contrast with that identified by Audino et al. (2001, 2002). Mass spectral fragmentation behaviors are also discussed.
2 Materials and methods
An organic-rich and low-maturity torbanite collected from the Sydney Basin, Australia was previously found to contain abundant macrocyclic alkanes based on mass spectral analysis(Zhang and Volkman 2020).The same sample was finely ground and Soxhlet extracted for 72 h with DCM from which a saturated fraction was eluted with 80 ml ofnhexane by open column separation(0.3 m × 1 cm,packed with alumina pre-activated at 450 °C for 4 h and silica preactivated at 150 °C for one day, Lu et al. 2018). Ureaadduction (Liao 2021) was then applied to removenalkanes and the resultant branched/cyclic alkane fraction was used to isolate individual compounds collected by preparative gas chromatography (pGC).
GC–MS was performed on a Trace Ultra GC interfaced to a Thermo DSQ-II MSD operating with a full scan mode(m/z= 50–600).ADB-5MScapillarycolumn(60 m × 0.32 mm × 0.25 μm film thickness)was used for compounds separation with GC oven temperature ramped from 60 °C(hold for 1 min)to 300 °C(30 min isothermal)at a rate of 4 °C/min. To accurately determine the MW of macrocyclic alkanes, the saturated fraction was also analyzed by GC–FIMS(field ionization mass spectrometry,Beckey 1997) composed with an Agilent 8890 GC equipped with a PW-5 MS Ultra (30 m × 0.25 mm × 0.25 μm film thickness) column and interfaced to an AccuTOF GCx-plus MSD. The GC oven was programmed from 40 °C (held for 2 min) to 300 °C (held for 15 min) with a rate of 40 °C/min.
The pGC analysis followed the method introduced by Liao et al. (2018). Briefly, the branched/cyclic alkane fraction was injected into an Agilent 7890A GC interfaced to a Gerstel-preparative fraction collector (PFC) system with He as carrier gas at a constant flow rate of 3.0 mL/min. A DB-5 capillary column (60 m × 0.53 mm × 1.5 μm) was used to separate compounds with the GC oven temperature initially held at 80 °C for 2 min, then ramped to 300 °C (held for 40 min) at a rate of 10 °C/min.
Six compounds were successfully collected and two were in sufficient amounts for NMR analysis to assess their structures, in order to confirm the structures provided by(Audino et al. 2001).1H and13C NMR spectral analysis was conducted on a Bruker AVANCE III 600 MHz NMR spectrometer (operating at 600.19 MHz for1H NMR and 150.93 MHz for13C NMR). Spectra were recorded in deuterated chloroform (CDCl3) solution with TMS (tetramethylsilane) as the internal standard.1H NMR chemical shifts were referenced relative to the residual proton signal (7.28 ppm) and13C NMR chemical shifts were referenced to the13C multiple (centered at 77.02 ppm) of CDCl3. 2D experiments of1H–1H COSY, HSQC, and HMBC were not further carried out on these two purified compounds since only a single peak occurred in the1H(1.31 ppm) and13C (28.39 ppm and 28.31 ppm, respectively) spectra.
3 Results and discussion
The total ion chromatogram(TIC)of the saturated fraction of torbanite is shown in Fig. 1A. Macrocyclic alkanes(C15–C31) are more abundant than then-alkanes between C16and C24with hopanes dominated by C29and C30αβhomologs extending to C34(Fig. 1B).Mass spectra of pure C21and C22macrocyclic alkanes are shown in Fig. 1C, D.Same as the usually saturated cycloalkanes the major fragment ions are even electron ions (Friedman and Long 1953;McLafferty 1959).The characteristic ions ofm/z69,83,97 and 111 indicate one double bond equivalent(DBE),as well as the assumed molecular ions ofm/z294 and 308,which are highly comparable with previous results(Audino et al. 2001). Although the macrocyclic alkanes can be identified by GC retention index data(Audino et al.2001),the GC–FIMS can provide a more reliable molecular weight (MW) for these compounds.
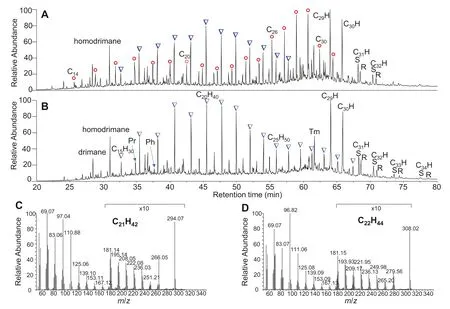
Fig.1 The TIC of the saturated hydrocarbon (A)and the urea-excluded branched/cyclic alkane fraction(B)of torbanite extracts,and the mass spectra of pGC-purified macrocyclic alkanes (C21H42 and C22H44, C and D). Red circles: n-Alkanes; inverted triangles: macrocyclic alkanes;CnH = hopanes; Tm = 17α(H)—22, 29, 30—trisnorhopane; × 10 refer to local magnification ten times of mass spectra
Partial TIC and selected mass chromatograms encompassing the region ofn-C19ton-C25alkanes are shown in Fig. 2. From C18to C23, the macrocyclic alkanes can be revealed by their MW and are consistent with previously assumed MW of these compounds (Audino et al. 2001). It is notable that macrocyclic alkanes elute just after thenalkanes with one more carbon number, however, the elution distance is a bit longer in the GC–FIMS analysis probably due to the different GC conditions (see Sect. 2).
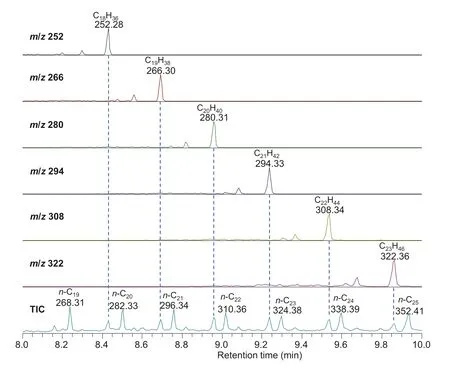
Fig. 2 Partial TIC and diagnostic mass chromatograms of GC–FIMS analysis of the saturated fraction.CnH2n = macrocyclic alkanes;n-Cn = n-alkanes;numbers refer to MW
The two macrocyclic alkanes (C21H42and C22H44)collected by pGC were subjected to NMR analysis(Fig. 3).Unusual single peaks were found in the1H and13C spectra of cycloheneicosane (1.31, 28.39 ppm) and cyclodocosane(1.31, 28.31 ppm), respectively. These rarely reported spectra in two compounds both confirm that single types of H and C atoms exist in their molecular skeletons and that the compounds contain repeated methylene units. The NMR results of these two compounds are highly comparable to that of synthetic cycloheptadecane and cycloheneicosane (Riache et al. 2014) as well as cyclodotriacontane isolated from plants (Red’kina et al.1989). The possibilities that these macrocyclic alkanes could be monounsaturated alkenes or alkyl cyclohexanes(both of which commonly exist in sediments) can now be ruled out since they would have an anisotropic distribution of C and H atoms. The only possibility for these compounds is to be macrocyclic alkanes as proposed previously(Audino et al. 2001). It’s worth noting that there were the same chemical shifts in the1H spectra of the two compounds, which may be due to the influence of adjacent water peaks.
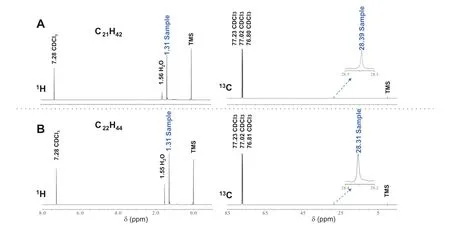
Fig. 3 1H and 13C NMR spectra of two selected macrocyclic alkanes (C21H42 and C22H44); TMS = tetramethylsilane (internal standard)

Fig.4 Fragmentation pathways of cyclohexane(A)to cyclodocosane(B)explaining the source of[M–28]+∙and abundant odd fragment ions and neighboring even-numbered ions in Fig. 1C, D. γH gamma hydrogen rearrangement, rH hydrogen rearrangement
Since the structure of the macrocyclic alkanes is now confirmed by NMR,an understanding of their mass spectra can consequently be useful for further identifying these biomarkers in other studies. We can compare their mass spectra with the characteristic ions of the well-characterized mass spectra of common cyclic alkanes such as cyclopentane, cyclohexane, and cyclooctane. It was very early proposed that these common cyclic alkanes mainly experience simple-bond cleavage during electron ionization with the corresponding fragment ions formed along with proton transfer (Abramson and Futrell 1967;McLafferty 1959; Stevenson 1958), however, it’s worth noting that molecular ion of cycloalkanes produces fragment ions should involve two bond cleavages (McLafferty 1959). Through statistical analysis of the abundance of fragment ions from cyclopentane to cyclooctane in the mass spectra (McLafferty 1963), a common single cleavage (α- or i-cleavage) or cyclization-displacement mechanism both after hydrogen rearrangement was proposed for the fragmentation pathway of the cyclic alkanes(Wang and Xiong 2005). For the latter, the cyclizationdisplacement mechanism was usually described for evenelectron ions bearing heteroatoms to format stable cations(e.g., oxonium, ammonium ions) in 4–7 membered rings(McLafferty 1980) or even larger cyclic intermediates(Dias and Djerassi 1972;Weinkam 1974),which was more easily observed in chemical ionization (CI) rather than electron ionization (EI) mass spectra (Lias et al. 1974;McLafferty 1980).Thus,single cleavage(α-or i-cleavage)is more suitable for the fragmentation of cyclic alkanes,for example, molecular ions of cyclohexane undergoing αcleavage (McLafferty 1963) or i-cleavage (McLafferty 1966; McLafferty and Turecek 1993; Wang and Xiong 2005) of C–C bond after ring opening will produce characteristic fragment ions of [M–C2H4]+∙in an odd-electron ion series, which could lose a substituent (e.g., ∙H) to further produce an even electron (i.e., C4H7+, McLafferty 1959).As a consequence,the characteristic fragment ion of[M–28]+∙such asm/z56 (C4H8)+∙for cyclohexane(Fig. 4A) can be well explained by the above mechanism.
Based on the above mechanisms, for the macrocyclic alkanes in this study,the σ bond fracture occurs when it is bombarded by electrons, and the corresponding open ring molecular ions would produce accordingly. The β-γ bond cleavage(α or i),fragment ions[M–28]+∙will be produced as[M–C2H4]+∙ions(Friedman and Long 1953;McLafferty 1959), which could undergo two pathways to continuously generate fragments following processes comprising α and i cleavages (Fig. 4B-I and II) with hydrogen rearrangement(McLafferty 1963) leading to the abundant characteristic ions (m/z69, 83, 97, 111, 125…) in the mass spectra(Fig. 1C, D). In the pathway I (Fig. 4B-I), the fragment ions [M–28]+∙could produce [M–CnH2n+1]+(n = 5, 7,9…) via α and i cleavages with hydrogen rearrangement.The[M–CnH2n+1]+(n = 4,6,8…)would be produced by i cleavages after that loss of substituent(∙H)from the radical cation [M–28]+∙(Fig. 4B-II1), or loss of alkyl radicals(e.g., CH2CH3) with even carbon number after hydrogen rearrangement (Fig. 4B-II2). Above pathways I and II would lead to the abundant characteristic ions (m/z69, 83,97, 111, 125…) in the mass spectra (Fig. 1C, D).
Unique triple-neighboring fragments(e.g.,m/z208,209,210) can also be noticed in the mass spectra of our macrocyclic alkanes (Fig. 1C, D). We consider that these[M–CnH2n]+∙(n = 4, 6, 8…) with odd-electron ions are produced by the α cleavage of [M–C2H4]+∙(Fig. 4B-III2),and the series of even ions([M–CnH2n+2]+∙,n = 4,6,8…)would be produced by single hydrogen rearrangements(Fig. 4B-IV2). With respect to [M–CnH2n]+∙(n = 5, 7,9…),they were probably related to losing neutral molecule(CH3CH = CH2)from the molecular ions(Fig. 4B-III1and IV1). Above pathways III and IV could also explain the even fragment ions likem/z194, 222, 236, 250. Overall,the abundance of fragment ions is dominated by [M–Cn-H2n+1]+,because hydrogen rearrangement reactions would take place more easily than direct α cleavage in macrocyclic alkanes.Thus,in the three types fragment ions in the mass spectra (Fig. 1C, D), the fragment pathway of [M–CnH2n+1]+cations was obviously associated with i-cleavage after hydrogen rearrangement, and the even radical cations of [M–CnH2n]+∙and [M–CnH2n+2]+∙were mainly attributed to the α-cleavage.
4 Conclusions
A series of apparently monounsaturated compounds have been isolated from the saturated fraction of solvent-extracts of a Sydney Basin torbanite.NMR analysis of two purified individual compounds showed single peaks in their1H and13C spectra indicating single types of H and C atoms in these molecules confirming them to be macrocyclic alkanes. Based on the investigation of their mass spectral fragment mechanisms, the production of characteristic fragment ions for macrocyclic alkanes can be well explained by simple cleavage (α- or i-cleavage) directly after hydrogen rearrangement,in which the i-cleavage was usually associated with the odd fragment pathway of [M–CnH2n+1]+cations while the α-cleavage was related to the even fragment ions with higher carbon numbers.
AcknowledgementsHong Lu acknowledges the financial support from The Strategic Priority Research Program of the Chinese Academy of Sciences (XDA14010102), and Chinese National Science Foundation grants (41973069; 41673045).
Declarations
Conflict of interestWe declare no conflicts of interest in this study.
杂志排行
Acta Geochimica的其它文章
- Coupling between the Cenozoic west Pacific subduction initiation and decreases of atmospheric carbon dioxides
- Geochronology and origin of Paleoproterozoic charnockites with old crustal signature in the Haisyn block of the Ukrainian shield
- A review of GEMC method and its improved algorithms
- Trace-element geochemistry and S–O isotopes in the fluoritebarite mineralization of Merguechoum, Moroccan eastern Meseta: insights into ore genesis to the Pangea rifting
- Textural and compositional zoning in plagioclase phenocrysts:implications for magma chamber processes in the Emeishan large Igneous Province, SW China
- Petrogenesis of the Neoarchean zincian chromite within ultramafic xenoliths, Bastar Craton, India
