藏绵羊肺脏结构特点及HIF-1α和AQP1的表达特性
2023-06-27阿依木古丽阿不都热依木张晨蔡勇覃圣罗文学扎西英派
阿依木古丽·阿不都热依木,张晨,蔡勇,覃圣,罗文学,扎西英派
藏绵羊肺脏结构特点及HIF-1α和AQP1的表达特性
阿依木古丽·阿不都热依木1,张晨1,蔡勇2,覃圣1,罗文学3,扎西英派1
1西北民族大学生命科学与工程学院,兰州 730030;2西北民族大学实验教学部,兰州 730030;3天祝藏族自治县畜牧技术推广站,甘肃武威 733200
【背景】低氧诱导因子-1α (hypoxia inducible factor-1α,HIF-1α) 是细胞对缺氧应激做出适应性反应的关键因子之一,主要通过调节基因转录来维持细胞中氧的供需平衡。水通道蛋白( Aquaporins,AQPs) 是疏水性的跨膜转运蛋白,为机体水稳态平衡提供重要的系统调节。肺中,AQP1主要通过调节肺泡、肺间质和毛细血管间水转运从而保持肺内液体平衡。高原环境由于低氧、高寒、大风和强辐射等特点,能够适应并生存的物种相对较少,藏羊作为适应高海拔、高寒气候的特有物种,形成了独特的形态结构和生理功能适应。肺脏作为呼吸主要的执行器官,对低氧或缺氧等极为敏感。【目的】通过研究藏绵羊肺脏结构特点以及HIF-1α与AQP1在不同海拔藏绵羊肺脏中的表达特性,揭示其在藏绵羊高原环境适应性中所起的作用。【方法】选择不同生存海拔藏绵羊和小尾寒羊,断颈致死后快速采集肺组织,H.E染色、PAS染色、Masson染色等观察肺脏显微结构及弹性纤维分布,免疫组织化学SP法及实时荧光定量PCR法检测肺组织中HIF-1α与AQP1蛋白及基因的表达特点。【结果】藏绵羊肺脏被膜厚度为40.28 μm,与小尾寒羊肺脏被膜厚度无显著差异,但其弹性纤维含量显著多于小尾寒羊(<0.05);藏绵羊细支气管黏膜上皮单位面积内杯状细胞数量显著多于小尾寒羊(<0.05),但小支气管及以上分支中两者无显著差异;藏绵羊终末细支气管黏膜上皮仍有少量散在分布的杯状细胞且其终末细支气管平滑肌厚度显著高于小尾寒羊(<0.05);藏绵羊肺内微动脉(直径小于100 μm)平滑肌占血管外径比例显著小于小尾寒羊;藏绵羊呼吸性细支气管平滑肌厚度及单位面积内毛细血管数量显著高于小尾寒羊。肺脏中HIF-1α蛋白主要表达于细支气管黏膜上皮、肺微血管内皮和肺泡隔中,主要表达于细胞质;高海拔藏绵羊和小尾寒羊肺组织中HIF-1α表达均显著高于低海拔绵羊,低海拔藏绵羊肺脏中HIF-1α的表达显著高于低海拔小尾寒羊。AQP1蛋白主要表达于肺泡上皮、肺泡隔及肺微血管内皮、细支气管平滑肌中,主要定位于细胞膜;高海拔藏绵羊肺组织中AQP1表达显著高于低海拔藏绵羊和小尾寒羊(<0.05),但高海拔和低海拔小尾寒羊肺脏中AQP1的表达无显著差异(>0.05)。【结论】藏绵羊肺被膜含有更多的弹性纤维,微血管平滑肌含量较少,但呼吸性细支气管上皮杯状细胞数量及平滑肌含量较多;藏绵羊肺脏中HIF-1α和AQP1的表达均显著高于小尾寒羊,且其表达均随海拔升高而增强。
藏绵羊;HIF-1α;AQP1;肺脏
0 引言
【研究意义】藏绵羊常年生活在高原低温、低氧、干燥环境中,与大多数高原动物一样,经长期自然选择,其肺脏结构和生理功能都出现了适应高原低氧的稳定遗传特性,并形成了特有的适应性结构[1-2]。藏羊养殖作为青藏高原居民重要的经济支柱产业,如何提高其繁殖性能[3],如何增加其经济价值广受关注[4]。笔者前期研究发现[5],藏羊肺脏微细结构与生存海拔相关,但肺脏中HIF-1α和AQP1是否表达,其表达特性与高原低氧环境是否相关,未见相关报道。【前人研究进展】低氧诱导因子(hypoxia inducible factor,HIF)广泛存在于哺乳动物和人体内,作为氧信号转导系统的重要转录因子,在机体氧稳态调节中发挥重要作用。机体缺氧时,肺动脉平滑肌收缩,血管重塑,进而导致肺动脉压升高,极易出现高原相关疾病[6]。研究表明,HIF-1α作为HIF家族蛋白中最重要的转录调节因子[7],广泛参与细胞增殖与凋亡[8]、能量代谢[9]、血管生成[10]和肿瘤发生发展[11]等的调节。水通道蛋白(aquaporins,AQPs)是一组疏水性跨膜转运蛋白,介导机体对体液和渗透压变化的生理反应,在保持内环境稳态中发挥重要作用。根据功能和系统遗传差异,哺乳动物水通道蛋白家族中共有13个成员即AQP0—AQP12[12]。而AQP1是第一个被发现的AQPs家族成员[13],通过气-血屏障转运水分子,从而维持肺内液体平衡[14];有研究发现,AQP介导肺动脉平滑肌增生,在肺水肿、肺部肿瘤等疾病中发挥重要作用[15]。【本研究切入点】缺氧导致AQP1升高并引起肺损伤,而下调AQP1表达可以减轻肺损伤[16]。研究表明,HIF-1α参与调节低氧引起的血管内皮细胞AQP1的升高[17]。【拟解决的关键问题】藏羚羊[18]、牦牛[19]等高原动物肺脏中HIF-1α基因表达高于其他多个组织,但其分布如何,HIF-1α和AQP1在藏绵羊肺脏表达特点如何,与平原绵羊有无差异,未见到相关报道。因此,为探讨藏绵羊肺脏结构特点及HIF-1α和AQP1在肺中的表达特性,本文对藏绵羊和小尾寒羊肺脏组织结构及HIF-1α和AQP1表达进行对比研究,为藏绵羊低氧适应性研究提供理论依据,为HIF-1α和AQP1之间的互作机制研究提供新思路。
1 材料与方法
1.1 试验动物
2018年3—10月期间,于青海省海南州(海拔约为3 500 m)和甘肃省永登县(海拔约2 300 m)分别选取1—2岁、健康雄性藏绵羊与小尾寒羊各6只,共24只,分为以下4组:藏绵羊高海拔(TH)组、藏绵羊低海拔(TL)组、小尾寒羊高海拔(SH)组和小尾寒羊低海拔(SL)组。断颈处死,检查内脏器官,尤其是胸腔,无肉眼可视病变,迅速取出肺脏选取支气管和左肺叶底部约2 cm3的组织块,4%多聚甲醛溶液固定48 h后更换新固定液复固定,常规脱水透明、石蜡包埋、5 μm切片。另取相同部位肺组织,投于RNA保存液中,快速带回实验室备用。
1.2 切片染色
相邻组织切片分别进行常规H.E染色、PAS染色、Masson染色、Gomori醛品红染色及免疫组织化学SP染色。
PAS染色:切片脱蜡复水,按照试剂盒(索莱宝,北京)说明进行染色。5%高碘酸溶液孵育15 min,Schiff雪夫试剂加盖避光孵育20 min,流水冲洗,苏木精染液复染5 min,脱水透明,中性树胶封片。PAS染色后杯状细胞呈红色。
Masson染色:切片脱蜡复水,按照试剂盒(索莱宝,北京)说明进行染色。Regand苏木精孵育15 min,Masson 丽春红复红液孵育10 min,1%磷钼酸分化,苯胺蓝染色5 min,0.2%冰醋酸浸洗,95%酒精分化,脱水透明,中性树胶封片。胶原纤维呈蓝色,弹性纤维呈浅红色,细胞核呈黑色。
Gomori醛品红染色:切片脱蜡复水后,按照试剂盒(索莱宝,北京)说明进行染色。酸性氧化液氧化5 min,酸性漂白液处理1 min,醛品红染色15 min,橙黄G 染色30 s,脱水透明,中性树胶封片。Gomori染色后弹性纤维呈蓝紫色,杯状细胞呈蓝色。
免疫组织化学SP染色:免疫组化染色程序按照SP试剂盒(迈新,福州)说明进行,第一抗体(博士德,武汉)HIF-1α与AQP1多克隆抗体工作浓度分别为1﹕150和1﹕200。同时设阴性对照组,0.01 mol·L-1PBS代替第一抗体,其余步骤相同。硫酸镍胺增强的DAB呈色,梯度酒精脱水,二甲苯透明,中性树胶封片,显微镜观察。阳性产物呈黄褐色,阴性对照组无阳性产物。
1.3 实时荧光定量PCR检测HIF-1α和AQP1基因表达
按照TRIzol试剂总RNA 提取试剂盒(Invitrogen,美国)说明书提取各组肺组织总RNA;按照Super ScriptTM Ⅲ First-Strand Synthesis System for RT-PCR Kit(Invitrogen,美国)说明书反转录合成cDNA。采用qRT-PCR法分别检测HIF-1α和AQP1的表达情况,以β-Actin为内参基因,引物序列见表1。
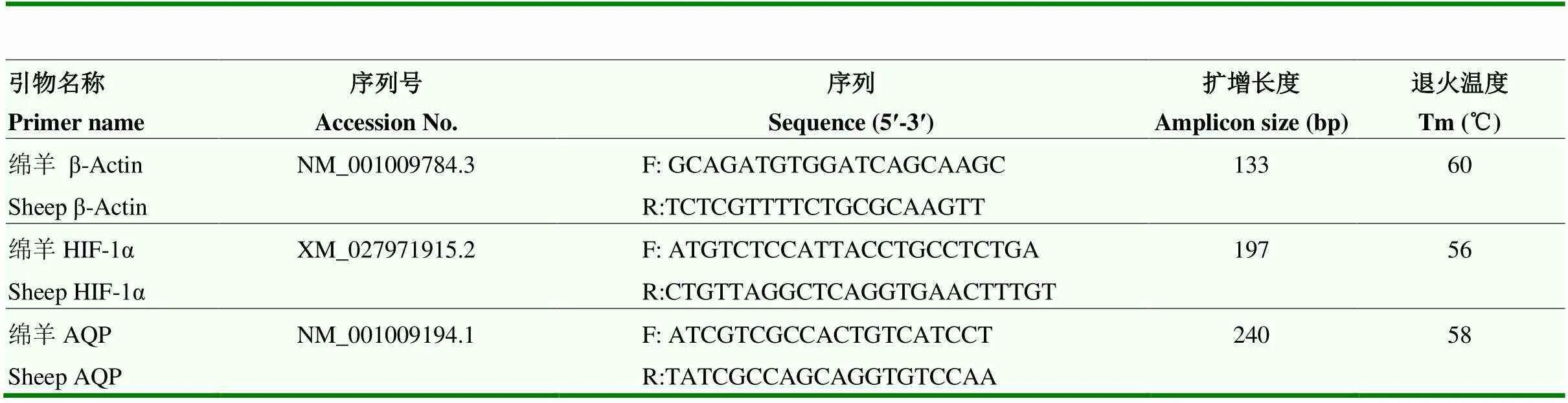
表1 引物序列
1.4 数据与分析
各组染色切片随机取5张,每张切片400倍视野随机取10个不同区域,用Image Proplus6.0软件分析。组织学切片中测量肺被膜厚度、被膜弹性纤维厚度、单位面积内肺泡数量(1 mm2)、肺泡隔中毛细血管数量及各级支气管黏膜上皮单位面积内(1 mm2)杯状细胞数量和管壁平滑肌厚度;测量微动脉外径、中膜平滑肌的厚度并计算肺微动脉中膜肌层厚度占血管外径百分比;计算肺被膜弹性纤维百分比。
免疫组织化学染色切片对免疫阳性产物进行光密度(optical density, OD)值分析。光密度值越大,HIF-1α和AQP1阳性程度越强。实时荧光定量PCR反应结束后,根据标准曲线计算每个样品中目标基因和内参基因含量比值作为目标基因相对表达量。
试验数据均用平均数±标准差(Mean±SD)表示,利用SPSS 22.0软件One-way ANOVA进行方差分析,<0.05为差异显著。
2 结果
2.1 藏绵羊和小尾寒羊肺组织结构特点
藏绵羊和小尾寒羊支气管黏膜上皮均为假复层纤毛柱状上皮,含大量杯状细胞,黏膜下层富含混合腺,由大量浆液腺和少量黏液腺构成;支气管黏膜形成明显皱襞,具有环形平滑肌,外膜透明软骨随管径缩小而逐渐消失。肺脏被膜为浆膜,由间皮和弹性纤维及少量胶原纤维构成;藏绵羊肺脏被膜厚度为40.28 μm,与小尾寒羊肺脏被膜厚度间无显著差异,但其弹性纤维含量显著多于小尾寒羊(<0.05,图1,表2)。

PM:肺浆膜;LA:肺微血管;TB:终末细支气管;HC:透明软骨;MG:混合腺;AD:肺泡管;AS:肺泡囊;PA:肺泡。A:肺被膜(Gomori×100);B:弹性纤维分布(Gomori×100);C:胶原纤维分布(Masson×200);D:细支气管(H.E×40);E:终末细支气管(H.E×100);F:小支气管黏膜及混合腺(PAS×200);G:黏膜上皮杯状细胞(PAS×400);H:混合腺(HE×400);I:呼吸性细支气管(Masson×200);J:肺泡管(Masson×200);K:肺泡及肺泡囊(H.E×400)
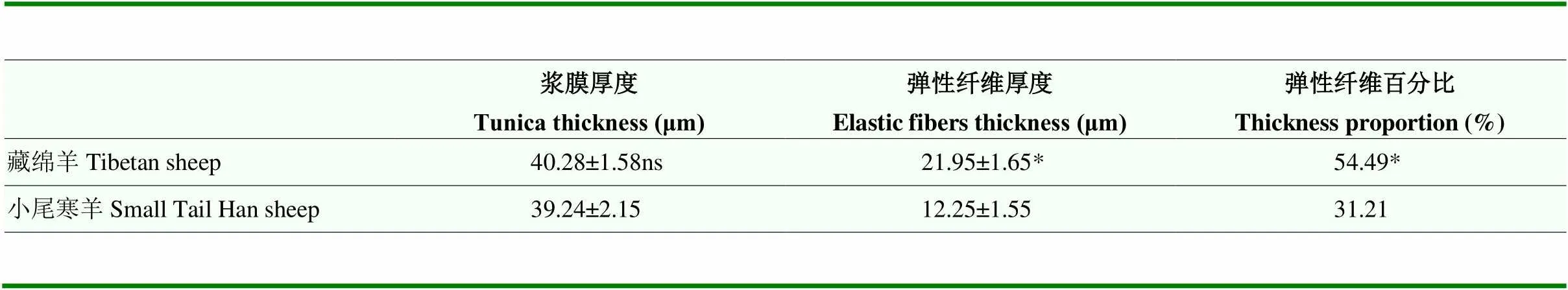
表2 藏绵羊与小尾寒羊肺被膜厚度及弹性纤维占比
与小尾寒羊比,ns 表示差异不显著(>0.05),* 表示差异显著(<0.05)。表3同
Compared with the Small Tail Han sheep, ns indicates no significant difference (>0.05); * indicates significant difference (<0.05). The same as Table 3
藏绵羊呼吸性细支气管平滑肌厚度显著高于小尾寒羊(<0.05),肺泡隔中毛细血管数量也显著多于小尾寒羊(<0.05),其肺泡面积与小尾寒羊对比无显著差异。藏绵羊肺微血管直径小于100 μm时,中膜平滑肌占血管外径比例显著小于小尾寒羊(<0.05);但血管直径大于100 μm时,两者间差异不显著(表3,4)。
藏绵羊细支气管黏膜上皮单位面积内杯状细胞数量显著多于小尾寒羊(<0.05),但上级分支中两者无显著差异。藏绵羊终末细支气管黏膜上皮仍有少量散在分布的杯状细胞,但小尾寒羊中未见杯状细胞。支气管平滑肌厚度也随管径缩小而逐渐减少,段支气管至细支气管平滑肌厚度两者间无显著差异,藏绵羊终末细支气管平滑肌厚度显著高于小尾寒羊(<0.05,表5)。
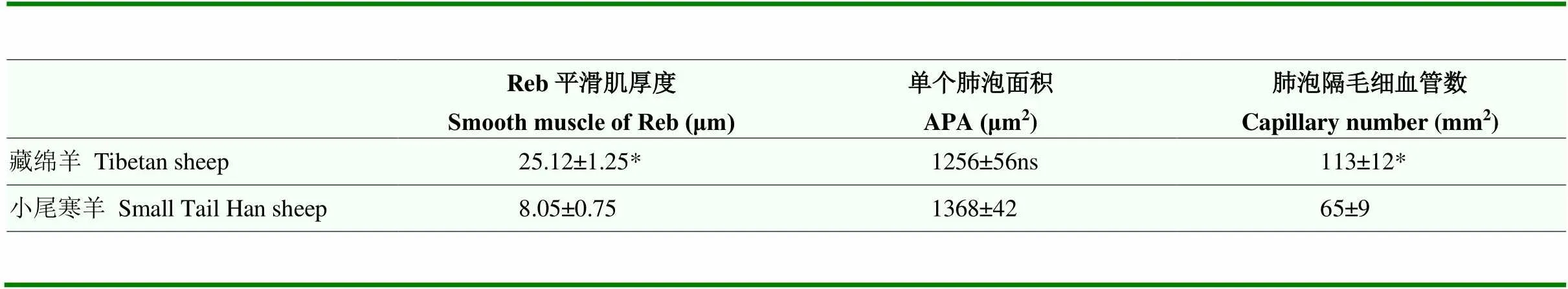
表3 藏绵羊与小尾寒羊肺呼吸部相关数据
Reb为呼吸性细支气管,APA为肺泡面积,NPA为单位面积肺泡数量
Reb: respiratory bronchiole; APA: Area of pulmonary alveolous; NPA: Number of pulmonary alveoli

表4 藏绵羊与小尾寒羊肺微动脉平滑肌占血管外径百分比
相同字母表示差异不显著(>0.05),不同字母表示差异显著(<0.05),ND:未检测到。表5同
Compared with the Small Tail Han sheep, the same letters indicate no significant difference (>0.05), and the different letters indicate significant difference (<0.05). ND: Not detected. The same as Table 5

表5 藏绵羊与小尾寒羊支气管黏膜上皮杯状细胞数量及平滑肌厚度
2.2 肺脏中HIF-1α与AQP1蛋白的分布特征
藏绵羊和小尾寒羊肺组织中HIF-1α蛋白免疫阳性产物广泛分布。主要表达于细支气管黏膜上皮、肺微血管内皮和肺泡隔中,主要定位于细胞质。高海拔藏绵羊和小尾寒羊肺组织中HIF-1α 表达强度均显著高于低海拔绵羊,低海拔藏绵羊肺脏中HIF-1α 显著高于低海拔小尾寒羊(<0.05)。但高海拔藏绵羊肺组织中HIF-1α 表达与高海拔小尾寒羊之间无显著差异(>0.05)。AQP1蛋白在肺脏中分布广泛,主要表达于肺泡上皮、肺泡隔及肺微血管内皮、细支气管黏膜下层和管壁平滑肌中,肺浆膜也有表达,主要定位于细胞膜。高海拔藏绵羊肺组织中AQP1表达显著高于低海拔藏绵羊及高/低海拔小尾寒羊(<0.05),但高海拔和低海拔小尾寒羊肺脏中AQP1的表达无显著差异(>0.05,图2,3)。

a:高海拔藏绵羊(TH)肺脏中HIF-1α蛋白表达;b:低海拔藏绵羊(TL)肺脏中HIF-1α蛋白表达;c:高海拔小尾寒羊(SH)肺脏中HIF-1α蛋白表达;d:低海拔小尾寒羊(SL)肺脏中HIF-1α蛋白表达;e:高海拔藏绵羊(TH)肺脏中AQP1蛋白表达;f:低海拔藏绵羊(TL)肺脏中AQP1蛋白表达;g:高海拔小尾寒羊(SH)肺脏中AQP1蛋白表达;h:低海拔小尾寒羊(SL)肺脏中AQP1蛋白表达
2.3 肺脏中HIF-1α 与AQP1 mRNA的表达特征
高海拔藏绵羊和高海拔小尾寒羊肺组织中HIF-1α mRNA水平显著高于低海拔组,且高海拔藏绵羊肺组织中HIF-1α mRNA水平显著高于高海拔小尾寒羊(<0.05)。藏绵羊肺组织中AQP1 mRNA显著高于小尾寒羊(<0.05),但相同品种高/低海拔绵羊之间差异不显著(>0.05,图3)。
3 讨论
3.1 藏绵羊肺组织结构为适应高原低氧环境出现了显著改变
呼吸系统是开放的系统,极易受到高原低压、低氧环境的影响。研究表明,许多高原动物肺组织结构出现了适应性改变[20-22]。试验结果,藏绵羊肺脏被膜厚度与小尾寒羊无显著差异,但其弹性纤维含量显著多于小尾寒羊。这与陈秋生[23]等在牦牛中研究结果一致。表明,藏绵羊肺脏具有丰富的弹性纤维,保证肺脏具有良好的扩张和回缩力,从而增强其低氧环境中的肺泡有效通气量。同时试验发现,藏绵羊细支气管黏膜上皮单位面积内杯状细胞数量显著多于小尾寒羊,且终末细支气管黏膜上皮中仍有散在分布。推测,足量的杯状细胞分泌大量的黏液,对生活于高原干旱低氧环境中的藏绵羊呼吸道管壁有很好的湿润和保护作用,保证气体运输通畅。且藏绵羊呼吸性细支气管外平滑肌厚度与单位面积内毛细血管数量均显著高于小尾寒羊。表明,高原环境中藏绵羊通过肺组织结构的微细变化来适应低氧干旱的环境。慢性低氧或缺氧均会导致肺血管中膜平滑肌过度增殖,引起血液循环阻力增加,常导致肺动脉高压,减少肺通气效率[24]。但大量研究表明,高原动物如牦牛[23]、高原鼢鼠和高原鼠兔等[22],都没有出现血管平滑肌增厚现象,而是通过肺微血管平滑肌减少来缓解肺血管低氧性收缩。直径较小的肺血管中膜平滑肌减少是高原动物对抗低氧性肺血管收缩反应的组织学基础[25]。本试验发现,藏绵羊肺微血管直径小于100 μm时,血管中膜平滑肌厚度显著低于小尾寒羊,这可能是藏绵羊高原低氧环境中生存但较少发生肺动脉高压的原因。

不同字母表示差异显著(P<0.05),相同字母表示差异不显著(P>0.05)。TH:高海拔藏绵羊;TL:低海拔藏绵羊;SH:高海拔小尾寒羊; SL:低海拔小尾寒羊
3.2 藏绵羊肺脏中HIF-1α和AQP1表达均显著上升
1992年研究者首次提出了低氧诱导因子(hypoxia inducible factor,HIF)的概念,2019年诺贝尔生理学或医学奖的揭示带来了HIF相关研究的新热点,HIF-1α成为HIF家族中最受关注的一员[5]。作为氧信号转导系统最重要的转录因子之一,HIF-1α可调控40多种基因的表达[26]。研究表明,进入高原低氧环境初期,机体HIF-1α、血管内皮生长因子(vascular endothlial growth factor,VEGF)和促红细胞生成素(erythropoietin, EPO)等蛋白表达水平迅速上升,增加外周动脉血管收缩能力[27],提高心输出量并加快组织毛细血管通路的形成等[28],使得动物机体适应低氧环境。研究表明,HIF-1α广泛分布于藏羚羊[18]、牦牛[19]、高原鼠兔[29]等动物体内,且肺脏中表达量最高。杨敏等[30]研究表明,HIF-1α严格受氧浓度的影响,无论高原动物还是平原动物,在富氧条件下HIF-1α少量表达,而在低氧条件时可迅速上升。本研究发现,HIF-1α主要定位于肺泡上皮、细支气管黏膜上皮及血管内皮细胞中,且高海拔地区藏绵羊与小尾寒羊肺脏中HIF-1α蛋白表达无显著差异,但均显著高于低海拔地区绵羊,表明HIF-1α蛋白在肺脏中的表达主要受海拔的影响。AQP1是AQP 家族中第一个被发现,分布于肾中主要调节水的吸收和原尿形成[31]。AQP1也广泛分布于肺部[32],且AQP1过表达促进肺动脉平滑肌细胞增殖与迁移[33]。有报道称,氧化应激致使细胞中AQP1表达显著降低[34]。笔者发现AQP1主要表达于藏绵羊和小尾寒羊肺终末细支气管平滑肌及肺微动脉内皮和肺泡周围毛细血管内皮中,与塔里木兔中研究结果一致[14]。有学者发现,LPS诱导人肺微血管内皮细胞损伤时细胞中AQP1的表达显著下降,而沉默HIF-1α,则细胞损伤及AQP1下降程度显著降低[35];HIF-1α作为转录因子,也可调节塔里木兔肾脏中AQP1的表达,使其适应干旱环境[36]。推测,HIF-1α与AQP1功能呈正相关。本研究可见,高海拔藏绵羊肺脏中HIF-1α和AQP1蛋白表达均显著高于低海拔绵羊,HIF-1α mRNA相对表达量则同时受到绵羊品种及海拔的影响,而AQP1 mRNA之间无显著差异。表明,高海拔对不同品种绵羊肺脏中HIF-1α基因转录水平的影响较为显著,而对肺脏中AQP1表达的影响在其转录或翻译水平影响更显著,其具体机制仍需进一步研究。
4 结论
通过对比研究藏绵羊和小尾寒羊肺脏组织结构,并对比分析在不同海拔生存时,其肺脏中HIF-1α和AQP1的表达特点。发现,藏绵羊肺被膜含有更多的弹性纤维,微血管平滑肌含量较少,但呼吸性细支气管上皮杯状细胞数量及平滑肌含量较多;藏绵羊肺脏中HIF-1α和AQP1的表达均显著高于小尾寒羊,且其表达均随藏绵羊生存的海拔高度升高而增强。
[1] STORZ J F, SCOTT G R, CHEVIRON Z A. Phenotypic plasticity and genetic adaptation to high-altitude hypoxia in vertebrates. The Journal of Experimental Biology, 2010, 213(Pt 24): 4125-4136.
[2] 何建文, 张伟, 刘秀, 李少斌, 王继卿, 胡江, 罗玉柱. 藏绵羊HMOX1基因表达及其多态性与低氧适应性的关联分析. 农业生物技术学报, 2019, 27(3): 441-448.
HE J W, ZHANG W, LIU X, LI S B, WANG J Q, HU J, LUO Y Z.gene expression and association between its polymorphism and hypoxia adaptation in Tibetan sheep (). Journal of Agricultural Biotechnology, 2019, 27(3): 441-448. (in Chinese)
[3] 李讨讨, 王霞, 马友记, 尹德恩, 张勇, 赵兴绪. 藏绵羊BOLL的分子特征及其在睾丸中的表达调控与功能分析. 中国农业科学, 2020, 53(20): 4297-4312.
LI T T, WANG X, MA Y J, YIN D E, ZHANG Y, ZHAO X X. Molecular characterization of Tibetan sheep BOLL and its expression regulation and functional analysis in testis. Scientia Agricultura Sinica, 2020, 53(20): 4297-4312. (in Chinese)
[4] 吴震洋, 唐晓惠, 付玉华, 王昇, 章程, 李京津, 余梅, 杜小勇. 藏绵羊不同毛色皮肤组织miRNA表达谱及靶基因分析. 中国农业科学, 2018, 51(2): 351-362.
WU Z Y, TANG X H, FU Y H, WANG S, ZHANG C, LI J J, YU M, DU X Y. Profiles of miRNAs and target gene analysis with white and black skin tissues of the Tibetan sheep. Scientia Agricultura Sinica, 2018, 51(2): 351-362. (in Chinese)
[5] 张晨, 刘斯汝, 蔡勇, 李囿蓉, 何灵芝, 蔡建亮, 罗文学, 阿依木古丽. 藏山羊与藏绵羊肺组织结构的对比研究. 动物学杂志, 2019, 54(5): 687-692.
ZHANG C, LIU S R, CAI Y, LI Y R, HE L Z, CAI J L, LUO W X, AYIMUGULI. Comparison of lung microstructure between Tibetan goat and sheep. Chinese Journal of Zoology, 2019, 54(5): 687-692. (in Chinese)
[6] HOWELL K, PRESTON R J, MCLOUGHLIN P. Chronic hypoxia causes angiogenesis in addition to remodelling in the adult rat pulmonary circulation. The Journal of Physiology, 2003, 547(Pt 1): 133-145.
[7] XU Y R, WANG A L, LI Y Q. Hypoxia-inducible factor 1-alpha is a driving mechanism linking chronic obstructive pulmonary disease to lung cancer. Frontiers in Oncology, 2022, 12: 984525.
[8] LI M Y, MI C L, WANG K S, WANG Z, ZUO H X, PIAO L X, XU G H, LI X Z, MA J, JIN X J. Shikonin suppresses proliferation and induces cell cycle arrest through the inhibition of hypoxia-inducible factor-1α signaling. Chemico-Biological Interactions, 2017, 274: 58-67.
[9] KIM J W, TCHERNYSHYOV I, SEMENZA G L, DANG C V. HIF-1-mediated expression of pyruvate dehydrogenase kinase: A metabolic switch required for cellular adaptation to hypoxia. Cell Metabolism, 2006, 3(3): 177-185.
[10] ZENG M B, SHEN J K, LIU Y Y, LU L Y, DING K, FORTMANN S D, KHAN M, WANG J X, HACKETT S F, SEMENZA G L, CAMPOCHIARO P A. The HIF-1 antagonist acriflavine: visualization in retina and suppression of ocular neovascularization. Journal of Molecular Medicine, 2017, 95(4): 417-429.
[11] ZHAO X R, LIU L D, LI R, WEI X, LUAN W Q, LIU P S, ZHAO J. Hypoxia-inducible factor 1-α (HIF-1α) induces apoptosis of human uterosacral ligament fibroblasts through the death receptor and mitochondrial pathways. Medical Science Monitor, 2018, 24: 8722-8733.
[12] GENA P, PELLEGRINI-CALACE M, BIASCO A, SVELTO M, CALAMITA G. Aquaporin membrane channels: biophysics, classification, functions, and possible biotechnological applications. Food Biophysics, 2011, 6(2): 241-249.
[13] CONNOLLY D L, SHANAHAN C M, WEISSBERG P L. The aquaporins. A family of water channel proteins. The International Journal of Biochemistry & Cell Biology, 1998, 30(2): 169-172.
[14] ZHANG J P, LI S W, LIU J, LI L X, DENG F, BAIKELI B, LI L R, MA X Y, LIU G Q. Higher expression levels of aquaporin (AQP)1and AQP5 in the lungs of arid-desert living. Journal of Animal Physiology and Animal Nutrition, 2020, 104(4): 1186-1195.
[15] YADAV E, YADAV N, HUS A, YADAV J S. Aquaporins in lung health and disease: emerging roles, regulation, and clinical implications. Respiratory Medicine, 2020, 174: 106193.
[16] WANG C, YAN M Y, JIANG H, WANG Q, GUAN X, CHEN J W, WANG C B. Protective effects of puerarin on acute lung and cerebrum injury induced by hypobaric hypoxia via the regulation of aquaporin (AQP) via NF-κB signaling pathway. International Immunopharmacology, 2016, 40: 300-309.
[17] ABREU-RODRÍGUEZ I, SILVA R S, MARTINS A P, SOVERAL G, TOLEDO-ARAL J J, LÓPEZ-BARNEO J, ECHEVARRÍA M. Functional and transcriptional induction of aquaporin-1 gene by hypoxia; analysis of promoter and role of Hif-1α. PLoS ONE, 2011, 6(12): e28385.
[18] 刘芳, 乌仁塔娜, 马兰, 杨应忠, 格日力. 藏羚羊低氧诱导因子1α基因的克隆与组织表达. 生理学报, 2011, 63(6): 565-573.
LIU F, WURENTANA, MA L, YANG Y Z, GERILI. Genetic cloning and expression of hypoxia inducible factor 1 alpha in high altitude hypoxic adaptation species Tibetan antelope (). Acta Physiologica Sinica, 2011, 63(6): 565-573. (in Chinese)
[19] DOLT K S, MISHRA M K, KARAR J, BAIG M A, AHMED Z, QADAR PASHA M A. cDNA cloning, gene organization and variant specific expression of HIF-1α in high altitude yak (). Gene, 2007, 386(1/2): 73-80.
[20] 何俊峰, 余四九, 崔燕. 不同年龄高原牦牛肺脏的组织结构特征. 畜牧兽医学报, 2009, 40(5): 748-755.
HE J F, YU S J, CUI Y. Characteristics of lung structure in different age plateau yak. Chinese Journal of Animal and Veterinary Sciences, 2009, 40(5): 748-755. (in Chinese)
[21] 周大鹏, 刘建国, 王芳, 贾宁. 藏獒肺组织对高原低氧环境的适应特性. 甘肃农业大学学报, 2009, 44(4): 25-28.
ZHOU D P, LIU J G, WANG F, JIA N. Pulmonary tissue adaptation to high altitude of Tibetan Mastiff. Journal of Gansu Agricultural University, 2009, 44(4): 25-28. (in Chinese)
[22] 王晓君, 魏登邦, 魏莲, 齐新章, 朱世海, 饶鑫峰. 高原鼢鼠和高原鼠兔肺细叶的结构特征. 动物学报, 2008, 54(3): 531-539.
WANG X J, WEI D B, WEI L, QI X Z, ZHU S H, RAO X F. Characteristics of pulmonary acinus structure in the plateau zokorand plateau pika. Acta Zoologica Sinica, 2008, 54(3): 531-539. (in Chinese)
[23] 陈秋生, 冯霞, 姜生成. 牦牛肺脏高原适应性的结构研究. 中国农业科学, 2006, 39(10): 2107-2113.
CHEN Q S, FENG X, JIANG S C. Structural study on plateau adaptability of yak lung. Scientia Agricultura Sinica, 2006, 39(10): 2107-2113. (in Chinese)
[24] 张晶晶, CHEN Jun-hao, 赵美平, 武垣伶, 张聪聪, 应磊, 陈锡文, 王万铁. 内质网应激在大鼠低氧高二氧化碳性肺动脉高压中的作用. 中国应用生理学杂志, 2018, 34(4): 327-333, 387.
ZHANG J J, CHEN J H, ZHAO M P, WU Y L, ZHANG C C, YING L, CHEN X W, WANG W T. The role of endoplasmic reticulum stress in pulmonary hypertension in rat induced by chronic hypoxia and hypercapnia. Chinese Journal of Applied Physiology, 2018, 34(4): 327-333, 387. (in Chinese)
[25] 李双, 邹小艳, 付林, 刘忠浩, 白祥慧, 都玉蓉, 郭松长. 高原鼠兔和昆明白小鼠肺组织结构比较. 兽类学报, 2020, 40(2): 162-169.
LI S, ZOU X Y, FU L, LIU Z H, BAI X H, DU Y R, GUO S C. Comparative on pulmonary histochemical characteristics between plateau pika and Kunming mouse. Acta Theriologica Sinica, 2020, 40(2): 162-169. (in Chinese)
[26] LIU J, WANG W, WANG L, CHEN S H, TIAN B, HUANG K W, CORRIGAN C J, YING S, WANG W, WANG C. IL-33 initiates vascular remodelling in hypoxic pulmonary hypertension by up-regulating HIF-1α and VEGF expression in vascular endothelial cells. EBioMedicine, 2018, 33: 196-210.
[27] ZHOU F, DU J, WANG J J. Albendazole inhibits HIF-1α-dependent glycolysis and VEGF expression in non-small cell lung cancer cells. Molecular and Cellular Biochemistry, 2017, 428(1): 171-178.
[28] LUO Y T, TENG X, ZHANG L L, CHEN J N, LIU Z, CHEN X H, ZHAO S, YANG S, FENG J, YAN X Y. CD146-HIF-1α hypoxic reprogramming drives vascular remodeling and pulmonary arterial hypertension. Nature Communications, 2019, 10(1): 1-17.
[29] ZHAO T B, NING H X, ZHU S S, SUN P, XU S X, CHANG Z J, ZHAO X Q. Cloning of hypoxia-inducible factor 1α cDNA from a high hypoxia tolerant mammal—plateau pika (). Biochemical and Biophysical Research Communications, 2004, 316(2): 565-572.
[30] 杨敏, 史兆国, 韩吉龙, 岳耀敬, 郭婷婷, 郭健, 刘建斌, 孙晓萍, 王朝风, 杨博辉. HIF-1α基因G901A多态性与高海拔低氧适应的相关性. 华北农学报, 2013, 28(6): 111-114.
YANG M, SHI Z G, HAN J L, YUE Y J, GUO T T, GUO J, LIU J B, SUN X P, WANG C F, YANG B H. Association between the G901A polymorphism of HIF-1α gene and adaptation to high-altitude hypoxia. Acta Agriculturae Boreali-Sinica, 2013, 28(6): 111-114. (in Chinese)
[31] FU Y, ZHU J J, ZHANG Y L, LIU Z W, SU H, KONG J. Vitamin D regulates the expressions of AQP-1 and AQP-4 in mice kidneys. BioMed Research International, 2019, 2019: 3027036.
[32] VASSILIOU A G, MANITSOPOULOS N, KARDARA M, MANIATIS N A, ORFANOS S E, KOTANIDOU A. Differential expression of aquaporins in experimental models of acute lung injury. In Vivo (Athens, Greece), 2017, 31(5): 885-894.
[33] 赖宁, 钟典, 云昕, 金颖康. AQP1促进肺动脉平滑肌细胞的增殖与迁移. 中国生物化学与分子生物学报, 2017, 33(7): 697-705.
LAI N, ZHONG D, YUN X, JIN Y K. The aquaporin 1 protein promotes migration and proliferation of pulmonary arterial smooth muscle cells. Chinese Journal of Biochemistry and Molecular Biology, 2017, 33(7): 697-705. (in Chinese)
[34] 李璐, 彭旭东, 林静, 赵桂秋. H2O2对人晶状体上皮细胞AQP1和AQP5表达影响及其机制. 青岛大学学报(医学版), 2022, 58(2): 173-177.
LI L, PENG X D, LIN J, ZHAO G Q. Effects of h2o2on expression of aqp1 and aqp5 in human lens epithelial cells and the underlying mechanisms. Journal of Qingdao University (Medical Sciences), 2022, 58(2): 173-177. (in Chinese)
[35] KESKINIDOU C, LOTSIOS N S, VASSILIOU A G, DIMOPOULOU I, KOTANIDOU A, ORFANOS S E. The interplay between aquaporin-1 and the hypoxia-inducible factor 1α in a lipopolysaccharide- induced lung injury model in human pulmonary microvascular endothelial cells. International Journal of Molecular Sciences, 2022, 23(18): 10588.
[36] LUO S J, LI Y L, LI S W, JIANG R J, DENG F, LIU G Q, ZHANG J P. Expression regulation of water reabsorption genes and transcription factors in the kidneys of. Frontiers in Physiology, 2022, 13: 856427.
The Micro-Structure of Tibetan Sheep Lung and Its HIF-1α and AQP1 Expression Characteristics
Ayimuguli Abudureyimu1, ZHANG Chen1, CAI Yong2, QIN Sheng1, LUO WenXue3, ZHAXIyingpai1
1College of Life Science and Engineering, Northwest Minzu University, Lanzhou 730030;2Department of Experimental Teaching, Northwest Minzu University, Lanzhou 730030;3Tianzhu Tibetan Autonomous County Animal Husbandry Technology Station, Wuwei 733200, Gansu
【Background】HIF-1α is one of the key factors for cells to make adaptive response to hypoxia stress. It mainly maintains the balance of oxygen supply by regulating gene transcription. Aquaporins (AQPs) are hydrophobic transmembrane transporters regulating water homeostasis. Among them, AQP-1 mainly regulates the water transport among alveoli, pulmonary interstitium and capillaries, so as to maintain the fluid balance in the lung. The plateau environment is characterized by low oxygen, extremely cold, strong wind and radiation, so there are relatively few species which could adapt and survive. As the unique sheep species adapt to high altitude and high cold climate, Tibetan sheep has formed a special morphological structure and physiological functions adapted to the plateau environment. As the main executive organ of respiration, lung is very sensitive to hypoxia. 【Objective】This study aimed to explore the structural characteristics of Tibetan sheep lung and the expression characteristics of HIF-1α and AQP1, so as to reveal the related roles of HIF-1α and AQP1 in Tibetan sheep high altitude adaption. 【Method】The histochemical HE, PAS and Masson staining, immuno-histochemical SP and real-time fluorescence quantitative were used.【Result】The tunica thickness of lung in Tibetan sheep was 40.28 μm, which showed no significant difference with that of Small Tail Han sheep, but the elastic fiber proportion was significantly higher than that of Small Tail Han sheep (<0.05); the number of goblet cells in bronchiolar epithelium in Tibetan sheep was significantly more than that in Small Tail Han sheep (<0.05), but there was no significant difference in superior branches; some goblet cells were still detected in the epithelium of bronchioli terminales of Tibetan sheep; the thickness of smooth muscle of bronchioli terminales of Tibetan sheep was significantly thicker than that of Small Tail Han sheep (<0.05); the proportion of smooth muscle in the pulmonary arteriole (diameter less than 100 μm) in Tibetan sheep was significantly less than that of Small Tail Han sheep; the thickness of respiratory bronchiole smooth muscle and the number of capillaries in Tibetan sheep were significantly higher than those in Small Tail Han sheep. The HIF-1α protein was mainly expressed in bronchiolar epithelium, pulmonary micro-vascular endothelium and alveolar septum, and mainly detected in cytoplasm; both Tibetan sheep and Small Tail Han sheep, the expression of HIF-1α was significantly higher in high altitude than that of low altitude, and HIF-1α expression in low altitude Tibetan sheep was significantly stronger than that of Small Tail Han sheep living at low altitude. AQP1 protein was mainly expressed in alveolar epithelium, alveolar septum, pulmonary microvascular endothelium, submucosal and smooth muscle of bronchioles, mainly located on cell membrane; the expression of AQP1 in lung of Tibetan sheep living at high altitude was significantly stronger than that of Tibetan sheep from low altitude, and also stronger than that of Small Tail Han sheep either altitude (<0.05), but there was no significant difference between Small Tail Han sheep living at high altitude and low (>0.05).【Conclusion】All those results indicated that the lung capsule of Tibetan sheep contained more elastic fibers and less microvascular smooth muscle than that of Small Tail Han sheep, with more number of goblet cells and smooth muscle in respiratory bronchioles. The expression of HIF-1α and AQP1 in Tibetan Sheep lung were significantly stronger than that of Small Tail Han sheep, and their expression increased with living altitude increasing.
Tibetan sheep; HIF-1α; AQP1; Lung
2021-12-22;
2023-03-23
国家自然科学基金(31760649)、中央高校基本业务费专项资金(31920220070)、甘肃省自然科学基金(20JR10RA122)
阿依木古丽·阿不都热依木,E-mail:Ayimgul80@163.com
10.3864/j.issn.0578-1752.2023.11.013
(责任编辑 林鉴非)
