Effects of arginine replacement with L‑citrulline on the arginine/nitric oxide metabolism in chickens: An animal model without urea cycle
2023-06-14VictoriaAnthonyUyangaLijingSunYuLiuMeimingZhangJingpengZhaoXiaojuanWangHongchaoJiaoOkanlawonOnagbesanandHaiLin
Victoria Anthony Uyanga, Lijing Sun, Yu Liu, Meiming Zhang, Jingpeng Zhao, Xiaojuan Wang,Hongchao Jiao, Okanlawon M. Onagbesan and Hai Lin*
Abstract Background This study examined the efficacy of L-citrulline supplementation on the arginine/nitric oxide metabolism, and intestinal functions of broilers during arginine deficiency. A total of 288 day-old Arbor Acre broilers were randomly assigned to either an arginine deficient basal diet (NC diet), NC diet + 0.50% L-arginine (PC diet), or NC diet + 0.50% L-citrulline (NCL diet). Production performance was recorded, and at 21 days old, chickens were euthanized for tissue collection.Results The dietary treatments did not affect the growth performance of broilers (P > 0.05), although NC diet increased the plasma alanine aminotransferase, urate, and several amino acids, except arginine (P < 0.05). In contrast,NCL diet elevated the arginine and ornithine concentration higher than NC diet, and it increased the plasma citrulline greater than the PC diet (P < 0.05). The nitric oxide concentration in the kidney and liver tissues, along with the plasma and liver eNOS activities were promoted by NCL diet higher than PC diet (P < 0.05). In the liver, the activities of arginase 1, ASS, and ASL, as well as, the gene expression of iNOS and OTC were induced by PC diet greater than NC diet(P < 0.05). In the kidney, the arginase 1, ASS and ASL enzymes were also increased by PC diet significantly higher than the NC and NCL diets. Comparatively, the kidney had higher abundance of nNOS, ASS, ARG2, and OTC genes than the liver tissue (P < 0.05). In addition, NCL diet upregulated (P < 0.05) the mRNA expression of intestinal nutrient transporters (EAAT3 and PEPT1), tight junction proteins (Claudin 1 and Occludin), and intestinal mucosal defense (MUC2 and pIgR). The intestinal morphology revealed that both PC and NCL diets improved (P < 0.05) the ileal VH/CD ratio and the jejunal VH and VH/CD ratio compared to the NC fed broilers.Conclusion This study revealed that NCL diet supported arginine metabolism, nitric oxide synthesis, and promoted the intestinal function of broilers. Thus, L-citrulline may serve as a partial arginine replacement in broiler’s diet without detrimental impacts on the performance, arginine metabolism and gut health of chickens.
Keywords Amino acids, Arginine, Broiler chicken, Citrulline, Intestinal health, Nutrient transporters, Tight junctions
Introduction
In poultry nutrition, feeding strategies that would promote optimal growth performance, as well as a reduction in feed cost and environmental impact are highly desired. In recent decades, the formulation of reduced crude protein diets along with the utilization of alternative protein sources and the inclusion of non-bound amino acids (AA) have become prevalent. This has facilitated the utilization of commercially available crystalline AA to fortify dietary profiles, replace protein-rich ingredients and support poultry production in an economically feasible approach [1]. These attempts to lower dietary crude proteins have produced several advantages including minimized feed cost, enhanced nutrient utilization, and improved animal welfare [2].Feeding broilers with reduced crude protein diets have the potential to decrease environmental pollution emanating from nitrogen and ammonia emission, improvement in litter quality and welfare of birds, and to a certain extent, it diminishes the dependence on soybean meal to meet the dietary requirements of birds [3].More so, it was reported that decreasing dietary crude protein levels by about 3% did not impede the growth performance and meat quality of chickens [4]. However,a significant decrease in dietary crude protein adversely affects the performance of broilers and increases carcass lipid deposition, thus, discouraging the adoption of reduced crude protein diets [5]. Reduced crude protein diets are typically formulated with increased inclusion of feed grains, reduction in protein-rich feed ingredients, as well as the selected inclusion of non-bound AA to meet the dietary needs of poultry. However, this formulations often increases the dietary starch to protein ratio, which may cause deleterious consequences [3, 5].Therefore, it is imminent to investigate the limitations of AA inclusion and to formulate effective strategies for dietary crude protein reduction.
Asides from their primary role in protein synthesis and accretion, AA also functions to regulate key signaling pathways, causing significant changes in the gene expression, protein turnover, and physiological responses of animals [6, 7]. Studies have shown that dietary supplementation with AA such as arginine, citrulline, glutamine, leucine, and proline can modulate the gene expression and enhance the growth of the small intestine, as well as the skeletal muscle [8]. DietaryL-arginine supplementation promotes enterocyte proliferation, maintains intestinal barrier functions,and ameliorates intestinal inflammation [9, 10]. Thus,along with the conventional essential and non-essential AA, certain functional AA can also act to regulate key metabolic pathways, thus promoting the growth, development, health, and survival of animals [7]. Therefore,supplementation with these AA necessitates continuous assessment to derive optimal inclusions since variabilities can easily arise based on the bird’s strain,environmental conditions, dietary protein status, and production objectives [11].
In poultry nutrition, arginine is an essential AA and its supply is necessary to support protein synthesis, immunity, reproduction, and production performance of birds[12]. Arginine is the fifth limiting AA for broiler chickens,and poultry has high arginine requirements due to their insufficient endogenous arginine synthesis. Also, dietary arginine requirements need to be optimized for modern broiler strains since they undergo rapid growth rate and protein deposition, and they also exhibit arginine–lysine antagonism [1]. Arginine and citrulline are present as metabolic intermediates in the urea cycle, and through enzymatic reactions, citrulline is catalyzed to form arginine, thus serving as a precursor for endogenous arginine synthesis, and consequently nitric oxide (NO) production[13]. Studies have shown that the utilization of commercially available AA such as crystalline arginine and citrulline can promote arginine availability in poultry [14, 15].Citrulline can be used as a precursor for de novo arginine synthesis in the kidney, and various cells, as well as to supply nitrogen for protein homeostasis in peripheral tissues [16]. Importantly, citrulline exhibits a highly specific metabolism, since it can bypass splanchnic extraction, as such, it is not efficiently absorbed in the liver and intestine [16], rather it is transported to the kidney and other extra-renal tissues where the conditions for arginine synthesis are favorable [17].
Based on the demonstrated evidence that citrulline supplementation increased arginine levels in poultry [15],we hypothesized that supplementation ofL-citrulline to an arginine-deficient diet would promote arginine availability, and consequently the physiological responses of broiler chickens. Thus, this study investigated the effects of arginine replacement withL-citrulline on the growth performance, AA profile, NO metabolism, and intestinal functions of broiler chickens.
Materials and methods
Animals and experimental design
A total of 288 1-day-old Arbor Acre chicks were brooded in battery cage units with environmentally controlled systems, continuous lighting, and ad libitum supply of feed and water. The chicks were brooded at 32 ± 1 °C with 55%–60% relative humidity for the first 3 d, then the temperature was gradually reduced until 24 ± 1 °C with 55%–60% relative humidity at 21 days of age. Chicks were weighed and randomly distributed into 3 treatments,8 replicates of 12 chickens each. An arginine deficient basal diet (NC) was designed to contain 20.5% CP, and12.55 MJ/kg ME (Table 1). The basal diet was formulated to meet or exceed the NRC recommendation for broiler chicks, except for arginine [18]. Other dietary treatments were subsequently formulated by supplementing the basal diet with arginine as the positive control(PC: NC diet + 0.50%L-arginine) orL-citrulline (NCL:NC + 0.50%L-Cit) (Table 1). Experimental diets were designed as isonitrogenous using alanine to balance the exclusion of arginine and they were fed to birds from day old until 21 days of age. Bodyweight (BW), bodyweight gain (BWG), feed intake (FI), and feed conversion ratio(FCR) were calculated per replicate of chickens.

Table 1 Composition and nutrient levels of experimental diets for 1—21 d (as-fed basis)
Blood and tissue collection
At 21 days of age, one chicken per replicate was selected for sampling. About 3 mL of blood sample was collected into anti-coagulated tubes and later centrifuged at 4 °C,1500 ×gfor 15 min to obtain the plasma. Plasma samples were stored at - 20 °C until analysis. Broilers were sacrificed via exsanguination and tissue samples were isolated,then snap-frozen in liquid nitrogen. Intestinal sections from the duodenum, jejunum, and ileum were collected as described by Chen et al. [19] and rinsed in saline. A portion of the ileum was opened to scrape the mucosa using glass slides into tubes. Samples collected were snap-frozen in liquid nitrogen and stored at - 80 °C until analysis. The weight of isolated organs was expressed as a percentage of the final body weight of chickens to obtain the relative organ index.
Determination of plasma biochemistry
About 500 μL plasma was used for the determination of metabolites including alanine aminotransferase (ALT),aspartate aminotransferase (AST), urea, urate, creatine kinase (CK), glucose (GLU), triglyceride (TG), total cholesterol (TCHO) and lactate dehydrogenase (LDH). The plasma biochemistry was determined automatically using the Hitachi L-7020 automatic biochemical analyzer(Hitachi High-Technologies Corp., Tokyo, Japan).
Determination of analyzed feed nutrients and plasma AA profile
Feed samples were analyzed for dry matter, crude fat,crude fiber, and ash using standardized methods [20].For AA analysis in experimental diets, feed samples were hydrolyzed with 6 mol/L HCl (Yantai Yuandong Fine Chemical Industry, Shandong, China) at 110 °C for 22 h.After hydrolysis, samples were analyzed by ion exchange chromatography with postcolumn ninhydrin detection using Hitachi L-8900 AA Analyzer (Hitachi High-Technologies Crop., Tokyo, Japan), as previously described [21].
For plasma AA determination, 40 mg salicylic acid(Tianjin Haitong Chemical Industrial Co., Ltd., Tianjin,China) was added to 800 μL plasma for deproteinization [15]. The samples were vortexed and stored at 4 °C overnight. Afterward, the samples were centrifuged at 4 °C, 12,000 r/min for 30 min, and the supernatant was collected. Supernatants were filtered (0.22 μm) into welllabeled tubes and about 500 μL of the filtrate was used for detection of AA contents via ion-exchange chromatography with the Hitachi L-8900 AA Analyzer.
Determination of nitric oxide concentration and total nitric oxide synthase activity
Nitric oxide (NO) and total nitric oxide synthase (tNOS)were determined using commercial test kits (Jiancheng Bioengineering Institute, Jiangsu, China) according to the manufacturer’s instructions. The reaction absorbance for NO concentration was determined at 550 nm using a microplate reader (Elx808, Bio-Tek, Winooski, Vermont,USA), while tNOS was read at 530 nm using a spectrophotometer (Beijing PGeneral, China).
Determination of enzymes associated with arginine metabolism
Enzymes involved in arginine metabolism including endothelial nitric oxide synthase (eNOS), arginase 1,argininosuccinate synthetase (ASS), and argininosuccinate lyase (ASL) were determined using chicken ELISA kits (Shanghai MLBIO Biotechnology Co., Ltd., China).The assay was measured at 450 nm using a microplate reader (Elx808) and the standard curve was used to compute the sample concentration.
Real-time polymerase chain reaction
The total RNA from the kidney and liver tissues were extracted using the NcmZol reagent (NCM Biotech, Shanghai, China), and the RNA concentration and purity were determined using the DS-11 spectrophotometer (Denovix Incorporated, Delaware, United States). Reverse transcription was carried out using the HiFiScript cDNA synthesis kit (CWBIO, Beijing, China) in a 20-μL final reaction volume and the cDNA synthesis reaction was carried out using the Genemate T960 Touch thermocycler (Heal Force Bio-Meditech Holdings Limited, Shanghai, China). The cDNA target sequence was quantified using MagicSYBR mixture(CWBIO, Beijing, China), in a 20-μL volume with appropriate primers (Table 2). The real-time RT-qPCR was performed using ABI QuantStudio5 Real-Time PCR Instrument(Applied Biosystems, ThermoFisher Scientific, Massachusetts, United States). The primers were normalized against β-actin as the housekeeping gene, while the positive control(PC) diet was used as the calibrator. The relative expression of the target genes were analyzed using the 2-ΔΔCTmethod.
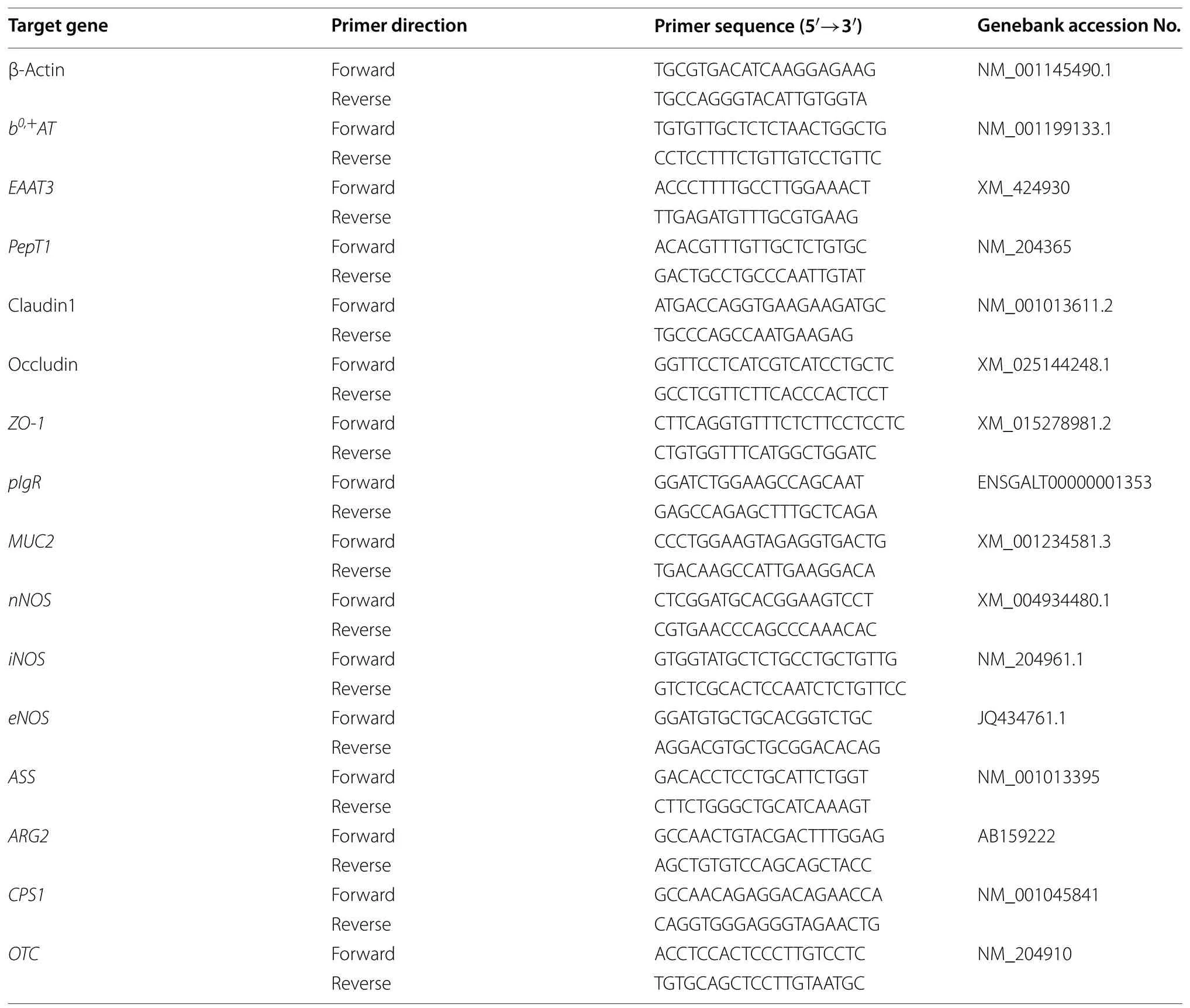
Table 2 List of primers used for real-time PCR analysis
Intestinal histology
About 5 cm section of ileum, duodenum, and jejunum were fixed in 4% paraformaldehyde (Wuhan Servicebio Technology Co., Ltd., Wuhan, China). The intestinal segments were trimmed, processed, and embedded in paraffin wax. 5 μm section of each sample was placed on a glass slide and stained with hematoxylin and eosin for morphometric examination. Slides were visualized using Olympus CX-41 phase contrast microscope (CK-40,Olympus, Tokyo, Japan). The distance between the top of the villus to the villus-crypt junction was measured as villus height (VH), while the distance from the villus-crypt junction down to the bottom of the crypt was measured as crypt depth (CD) [10, 19]. Three measurements were taken per slide and the average was obtained for analysis.The VH to CD ratio was computed per observation.
Statistical analysis
Data collected were analyzed with a one-way analysis of variance using the GLM procedure of SAS (SAS version 8.1;SAS Institute Inc., Cary, North Carolina, USA). The gene expression for arginine metabolizing enzymes was analyzed using two-way ANOVA to evaluate the main effects of tissue (kidney vs. liver), diet (PC vs. NC vs. NCL), and their interaction. Means separation was performed using Duncan’s Multiple Range Test and treatment effects were considered statistically significant at a probability ofP< 0.05.
Results
Production performance and relative organ weights of broilers
The production performance of broilers did not differ among the dietary treatments (Table 3). As such, the BW,BWG, FI, and FCR of broilers fed with either the PC, NC or NCL diets did not vary significantly (P> 0.05). Similarly,the relative organ index of the liver, kidney, spleen, bursa,thymus, ileum, duodenum, and breast muscle of broilers were not affected by dietary treatments as given in Table 4(P> 0.05). However, the relative weight of the jejunum was lowered by the PC diet compared to the NC and NCL diets(P< 0.05). In addition, the thigh muscle was reduced by the NC diet compared to the PC and NCL diets (P< 0.05).
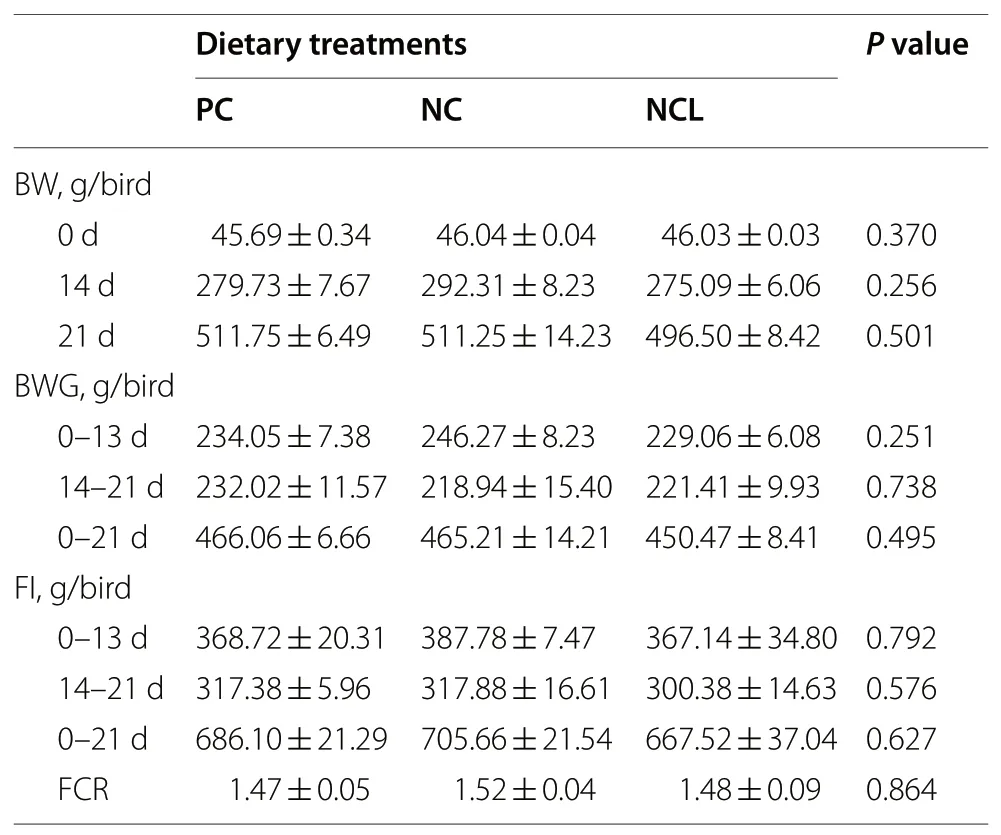
Table 3 Production performance and relative organ weights of broilers fed arginine deficient diets supplemented with arginine or L-citrulline
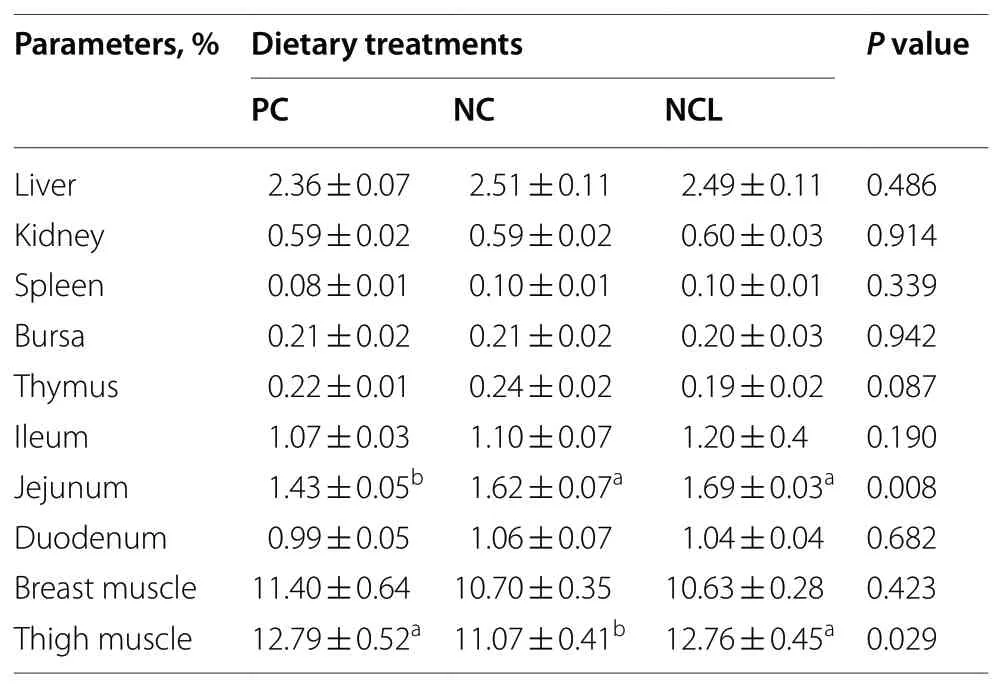
Table 4 Relative organ index of broilers fed arginine deficient diets supplemented with arginine or L-citrulline
Plasma biochemistry and AA profile of broilers
Plasma metabolites such as urea, CK, GLU, TG, TCHO,and LDH were not significantly influenced (P> 0.05) by the dietary treatments (Table 5). The ALT concentration was decreased (P< 0.05) in broilers fed NCL diet compared to the PC and NC diets which were similar.In addition, plasma AST was decreased (P< 0.05) in both the NC and NCL diet groups compared to the PC diet. The urate content was higher in NC fed birds than with the PC and NCL diets (P< 0.05).

Table 5 Plasma biochemistry of broilers fed arginine deficient diets supplemented with arginine or L-citrulline
Among the essential AA shown in Table 6, the plasma concentrations of threonine, leucine, valine, and cysteine were increased by the NC diet significantly higher than both the PC and NCL diets (P< 0.05). However, the leucine content was significantly decreased with the PC diet compared to the NCL diet (P< 0.05). Importantly, the arginine concentration was lowered by the NC diet compared to both the PC and NCL diets (P< 0.05).
Among the non-essential AA and related peptides,the plasma concentrations of aspartate, serine, proline,and alanine were increased by the NC diet (P< 0.05),although they did not differ between the PC and NCL diet. The citrulline content was significantly increased in both the NC and NCL groups compared to the PC diet (P< 0.05). Likewise, NCL diet increased the ornithine levels higher than the NC diet. In contrast, NC diet increased the sarcosine and cystathionine contents higher than the NCL and PC diets, respectively(P< 0.05).
Nitric oxide concentration
The plasma NO was not significantly affected (P> 0.05)by the dietary treatments of PC, NC, and NCL diets(Fig. 1A). However, broilers fed NCL diet exhibited increased kidney NO concentration compared to the broilers fed either NC or PC diets (Fig. 1B;P< 0.05).Also, the NO concentration in the liver was significantly increased by the NCL diet compared to the PC diet (P< 0.05), whereas, NC diet showed a tendency to increase the liver NO content more than the PC diet(Fig. 1C).

Fig. 1 Nitric oxide (NO) concentration in broilers fed arginine deficient diets supplemented with arginine or L-citrulline. (A) Plasma NO (B) Kidney NO (C) Liver NO. PC: Positive control; NC: Negative control; NCL: Negative control + L-citrulline. Data are presented as mean ± SEM (n = 6 to 8). Data are significantly different at *P < 0.05; **P < 0.01
Activities of arginine metabolizing enzymes
The enzyme activities for tNOS and eNOS are presented in Fig. 2. The plasma tNOS did not differ among dietary treatments (Fig. 2A;P> 0.05). However, the plasma eNOS activity was promoted with NCL diet higher than both the PC and NC diets (Fig. 2B;P≤ 0.05). In contrast, the kidney eNOS activity was increased (P< 0.05) in the PC fed group compared to both the NC and NCL diets(Fig. 2C). Also, the liver eNOS activity was increased by NCL diet higher than the PC fed group (Fig. 2D;P< 0.05).

Fig. 2 Enzyme activity of nitric oxide synthase isoforms in broilers fed arginine deficient diets supplemented with arginine or L-citrulline. (A) Plasma tNOS (B) Plasma eNOS (C) Kidney eNOS (D) Liver eNOS. PC: Positive control; NC: Negative control; NCL: Negative control + L-citrulline. Data are presented as mean ± SEM (n = 7 to 8). Data are significantly different at *P ≤ 0.05; **P < 0.01; ***P < 0.001
The plasma arginase 1 activity was significantly increased with the PC diet than the NCL diet (Fig. 3A).Arginase 1 was also higher in the kidney of PC-fed broilers than with the NCL diet (P< 0.05). The NC diet also tended to increase the kidney arginase 1 higher than the NCL diet (Fig. 3B). In the liver, arginase 1 was promoted(P< 0.05) by the PC diet compared to the NC and NCL diets, which were similar (Fig. 3C). Figure 3D shows that the plasma ASS activity was unaffected (P> 0.05) by the dietary treatments, whereas, the kidney ASS activity was decreased (P< 0.05) by NCL diet compared to the PC and NC groups (Fig. 3E). In addition, the liver ASS activity was increased (P≤ 0.05) by the PC diet, since it was relatively higher than the NC diet (Fig. 3F). Furthermore, the plasma ASL activity did not differ between the dietary groups (Fig. 3G), whereas the kidney ASL activity was decreased with NCL diet compared to PC and NC groups (Fig. 3H;P< 0.05). Similarly, ASL activity in the liver was decreased (P< 0.05) by both NC and NCL diets compared to the PC diet (Fig. 3I).
Gene expression of arginine metabolizing enzymes
The relative mRNA expression of the various enzymes involved in arginine metabolism are shown in Fig. 4. In the liver (Fig. 4A), thenNOSandASSexpression were unaffected by dietary treatments (P> 0.05), whereas,theiNOS,ARG2andOTCexpression were significantly upregulated by the PC diet compared to the NCL diet(P< 0.05). Also, PC diet increased the liveriNOSandOTCexpression higher than the NC diet. In addition,ARG2expression was modulated by NC diet compared to the NCL group (P< 0.05). In the kidney (Fig. 4B), theiNOSandASSexpression did not differ among dietary treatments (P> 0.05). However, feeding with PC diet significantly upregulated thenNOS,eNOSandARG2expression higher than the NC diet (P< 0.05). Furthermore, NCL diet also upregulated theeNOS,ARG2, OTC,andCPS 1expression higher than the NC diet (P< 0.05).
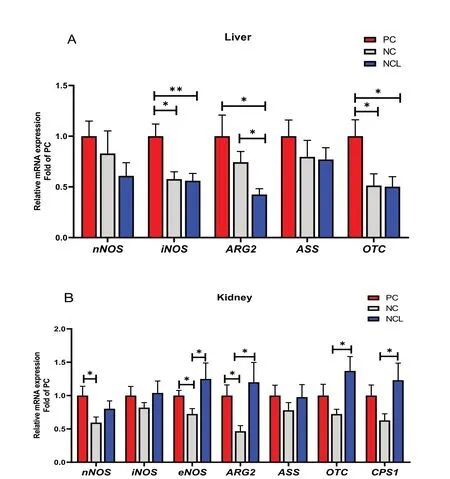
Fig. 4 Gene expression of arginine metabolizing enzymes in the tissues of broilers fed arginine deficient diets supplemented with arginine or L-citrulline. (A) Liver (B) Kidney. PC: Positive control; NC: Negative control; NCL: Negative control + L-citrulline. Data are presented as mean ± SEM(n = 8). Data are significantly different at *P < 0.05; **P < 0.01
It was also observed that the relative abundance of arginine metabolizing genes differed (P< 0.05) among each other (Fig. 5). In the liver,iNOSandOTCgenes had the highest expression, followed byASS, thenARG2and the lowest wasnNOSexpression (Fig. 5A;P< 0.05).In the kidney,ASSwas highly expressed, followed bynNOS,iNOS, andOTCwhich were similar, thenCPS1andARG2,whileeNOShad the lowest gene expression(Fig. 5B;P< 0.05). A comparative assessment of the relative abundance of arginine metabolizing enzymes between the kidney and liver tissue is shown in Fig. 6. It was observed that thenNOS, ASS, ARG2,andOTCwere highly abundant (Ptissue< 0.05) in the kidney compared to their expression in the liver (Fig. 6A–D).
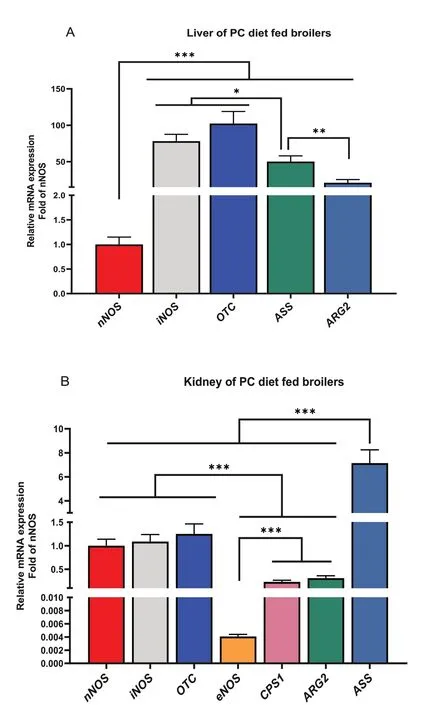
Fig. 5 Tissue expression profile of arginine metabolizing enzymes in broilers fed arginine deficient diets supplemented with arginine (PC diet) (A)Liver (B) Kidney. Data are presented as mean ± SEM (n = 8). Data are significantly different at *P < 0.05; **P < 0.01; ***P < 0.001
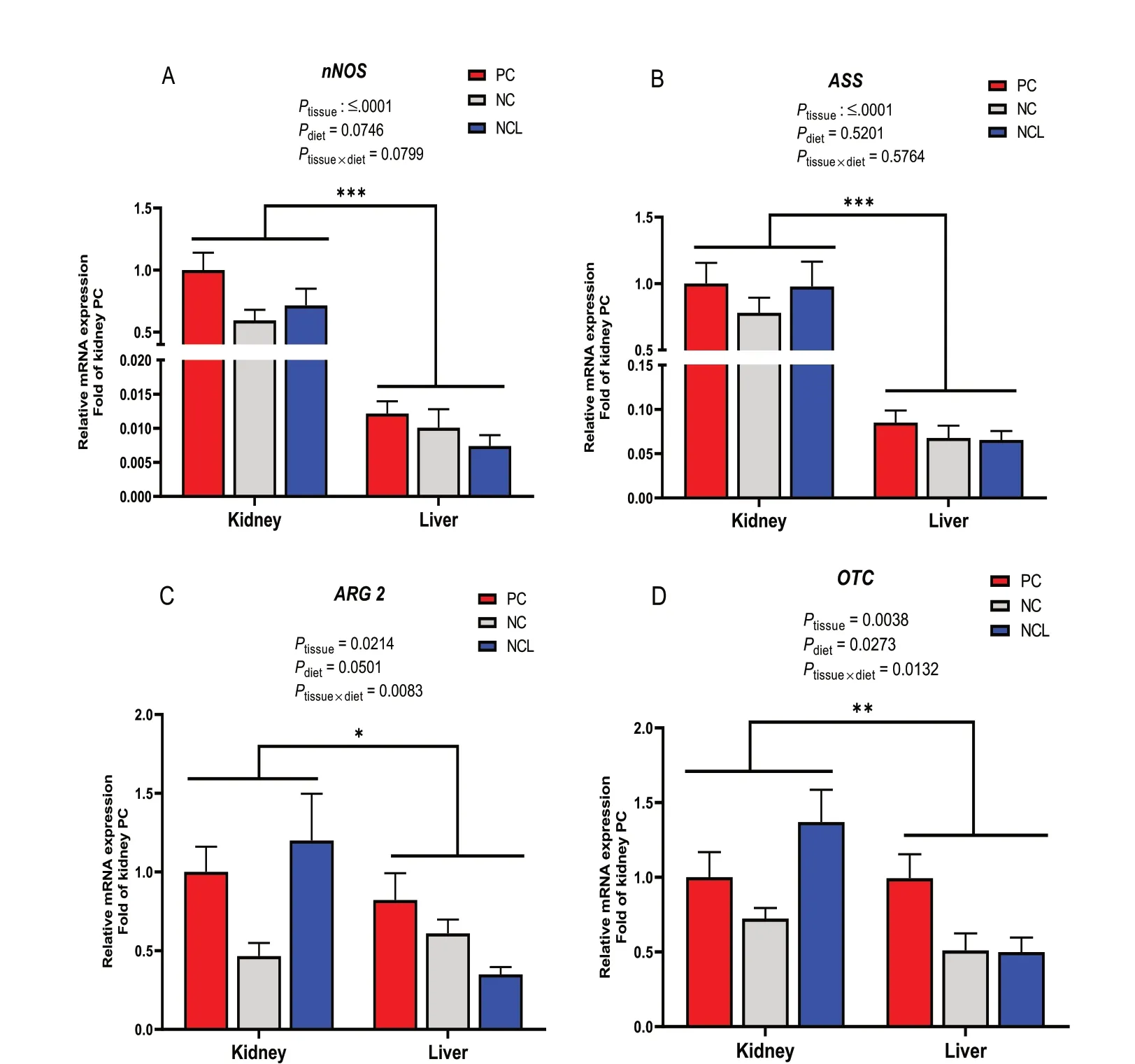
Fig. 6 Comparison of the relative gene expression of arginine metabolizing enzymes in the kidney and liver tissues of broilers fed arginine deficient diets supplemented with arginine or L-citrulline. (A) nNOS (B) ASS (C) ARG2 (D) OTC. PC: Positive control; NC: Negative control; NCL: Negative control + L-citrulline. Data are presented as mean ± SEM (n = 8). Data are significantly different at *P < 0.05; **P < 0.01; ***P < 0.001
Gene expression of nutrient transporters in the intestine of broilers
The ileal expression ofb0,+ATtransporter did not differ between the dietary treatments (Fig. 7A;P> 0.05),whereas, theEAAT3andPepT1transporters were similarly influenced (P< 0.05). Feeding with NCL diet upregulated the mRNA expression ofEAAT3andPepT1in the ileum of broilers compared to the PC and NC diets(P< 0.05).
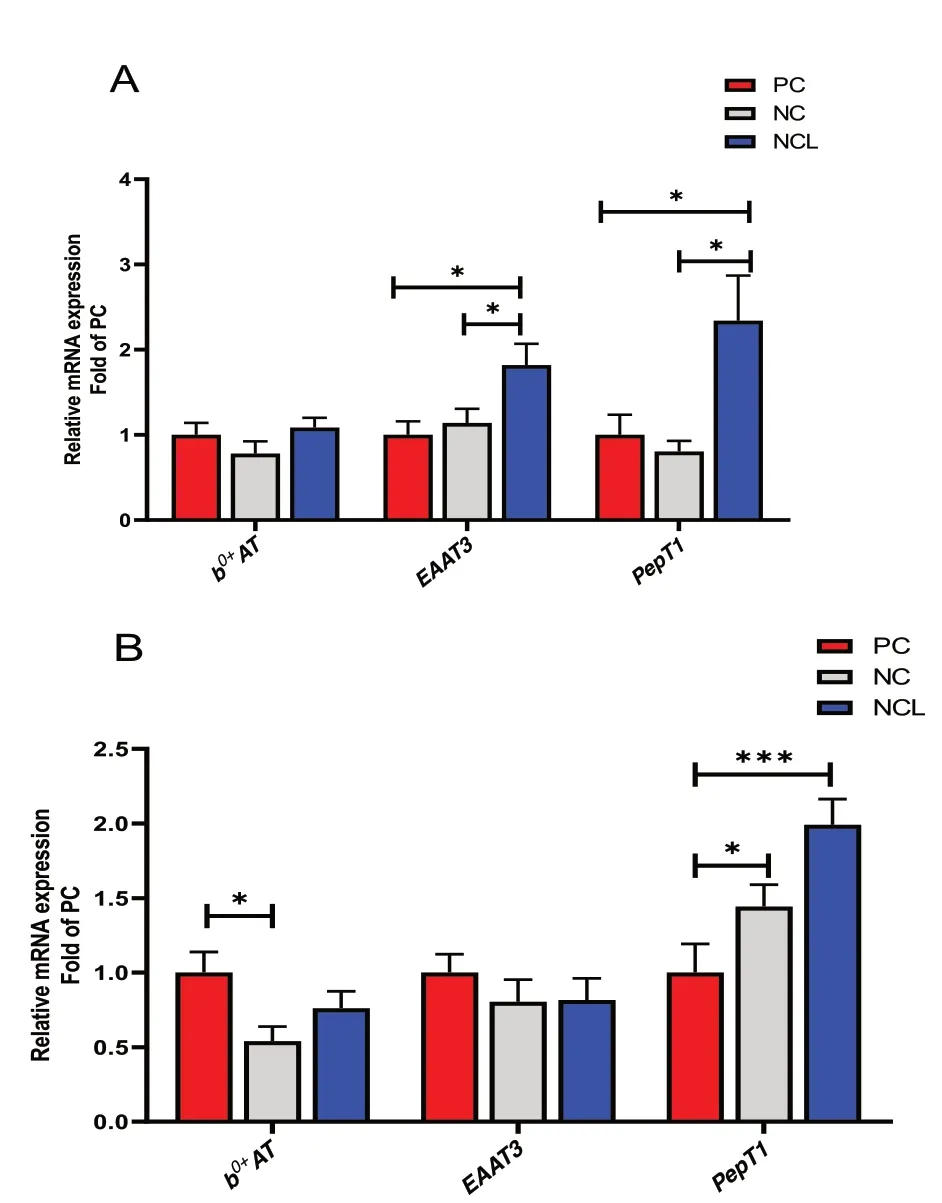
Fig. 7 Gene expression of nutrient transporters in the intestine of broilers fed arginine deficient diets supplemented with arginine or L-citrulline. (A) Ileum tissue (B) Jejunum tissue. PC: Positive control;NC: Negative control; NCL: Negative control + L-citrulline. Data are presented as mean ± SEM (n = 8). Data are significantly different at*P < 0.05; ***P < 0.001
In the jejunum, the mRNA expression ofEAAT3was unchanged (P> 0.05), whereas, PC diet upregulatedb0,+ATexpression higher than the NC diet (Fig. 7B). In addition, feeding with NC and NCL diets significantly upregulatedPepT1mRNA expression in the jejunum compared to the PC diet (Fig. 7B;P< 0.05).
Gene expression of tight junction proteins in the intestine of broilers
The relative mRNA expression of tight junction proteins and immune markers in the ileal mucosa revealed that NCL diet significantly upregulated (P< 0.05) the expression of Claudin 1, Occludin,MUC2,andpIgRhigher than the NC diet (Fig. 8A). Also, the Claudin 1 and Occludin expression were increased (P< 0.05) by NCL diet higher than the PC diet, whereas, NC diet upregulatedpIgRexpression higher than PC diet (Fig. 8A). TheZO-1expression was not affected by dietary treatments(P> 0.05).
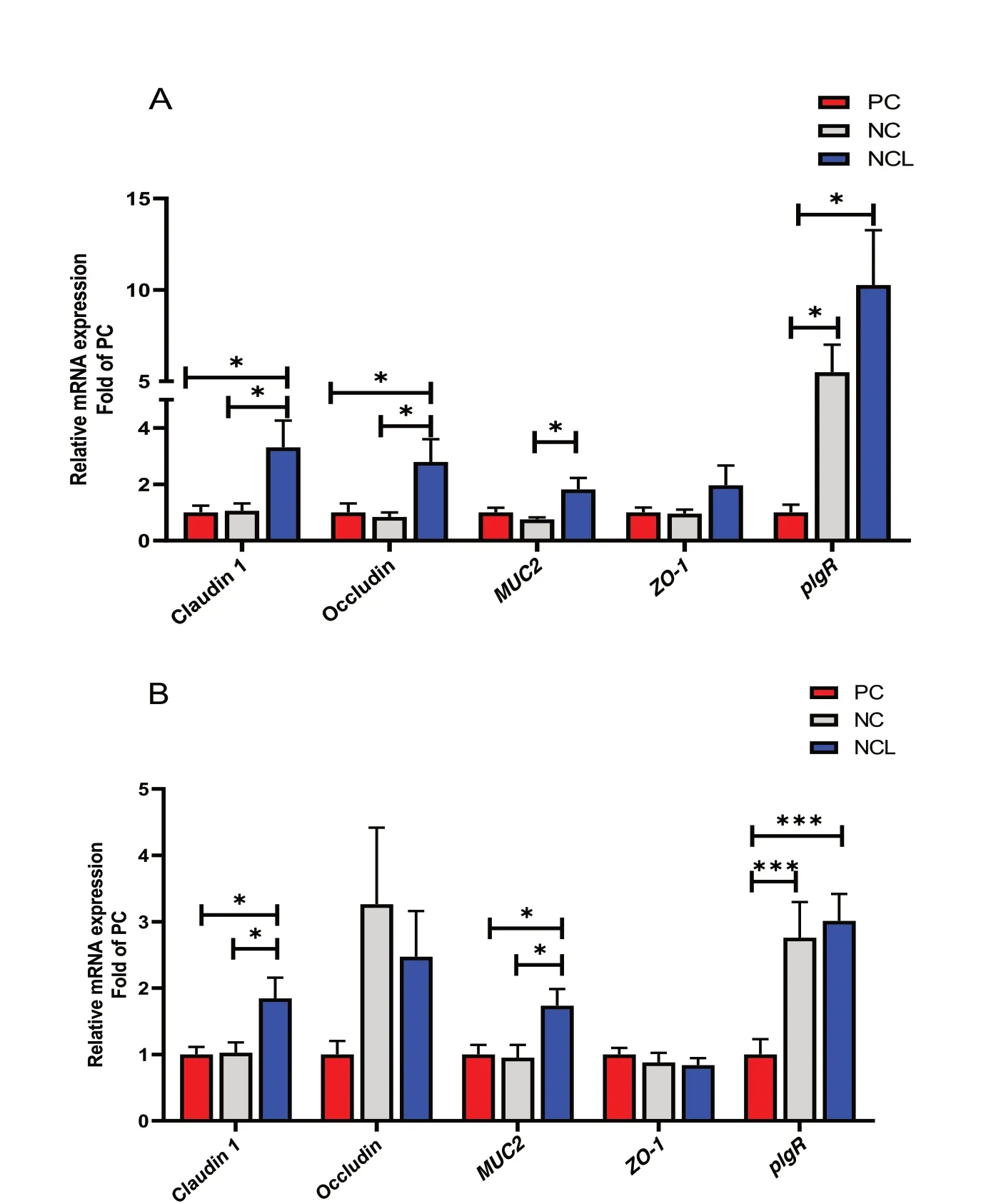
Fig. 8 Gene expression of tight junction proteins in the intestine of broilers fed arginine deficient diets supplemented with arginine or L-citrulline.(A) Ileal mucosa (B) Jejunum tissue. PC: Positive control; NC: Negative control; NCL: Negative control + L-citrulline. Data are presented as mean ± SEM (n = 8). Data are significantly different at *P < 0.05; ***P < 0.001
In the jejunum, feeding with NCL diet upregulated the expression of Claudin 1, andMUC2higher than both the PC and NC diets (Fig. 8B). In addition, both NCL and NC diets significantly upregulatedpIgRexpression higher than the PC diet (P< 0.05). Similar to the ileal expression,ZO-1did not differ with dietary treatments (P> 0.05).
Histomorphometric analysis of the intestine
Table 7 shows that the intestinal morphometry of broilers was significantly affected by dietary treatments, except in the duodenum, where the VH, CD, and VH/CD ratio were not changed (P> 0.05). In the jejunum, the VH was lowered by NC diet, whereas, it was increased with PC and NCL diets (P< 0.05). The jejunal CD was increased in the PC and NC groups but lowered by NCL diet(P< 0.05). Also, the VH/CD ratio of the jejunum was lowered by NC diet relative to the PC and NC diets (P< 0.05).The VH of the ileum was not changed by dietary treatment (P> 0.05), however, NC diet significantly increased the ileal CD compared to the PC and NCL diets, which were similar. In contrast, the VH/CD ratio was lowered by NC diet but increased in both the PC and NCL fed groups (P< 0.05).

Table 7 Intestinal morphometry of broilers fed arginine deficient diets supplemented with arginine or L-citrulline
Discussion
The present study examined the effects of arginine orL-Cit supplementation to broilers under arginine-deficient conditions. It was found that although feeding with the arginine-deficient-NC diet did not elicit significant effects on the growth performance of broilers,it changed the free plasma AA profile, arginine metabolism, intestinal nutrient transport, and intestinal integrity of broilers. Corresponding with our study, Dao et al.[22] demonstrated that in laying hens fed with arginine deficient diet and supplemented with 0.35% arginineor 0.35% citrulline, the birds did not differ in their feed intake and egg production performance. According to the standard guidelines for arbor acre broiler management [23], under optimal management, environmental and nutritional conditions, a body weight of 978 g, along with cumulative feed intake of 1190 g is expected of broilers at 21 days of age. Relative to these standards, our study showed that the experimental birds had depreciated performance, such that at 21 days of age, broilers fed PC diet exhibited about 48% and 42% decline in BW and FI respectively, compared to the standard guidelines. This impaired performance of broilers may likely occur due to nutrient imbalance in the basal diet used in the study.The basal diets were formulated as arginine-deficient,and from the findings of the present study, it is evident that the NC diet could not support the optimal growth performance of broilers regardless ofL-arginine orL-Cit supplementation. In line with these, several studies have also reported that feeding chickens with an arginine deficient diet impeded their growth responses and production performance [12, 24, 25]. Interestingly, both PC and NCL diets increased the relative weight of the thigh muscle, without obvious changes to the final BW of broilers.In line with this, it was reported that the absolute and relative weight of thigh muscles were linearly increased in broilers with increasing levels of digestible arginine/lysine ratio, probably due to the actions of arginine on muscle creatine synthesis [26]. Thus, the potential for increased muscle mass observed with arginine andL-Cit supplementation may be further investigated over longer periods since both AA are involved in protein turnover and protein synthesis [27, 28].
The ALT and AST enzymes are released into the bloodstream when the hepatic parenchymal cells are damaged, along with the associative effects of inflammation and oxidative stress [29]. In this study, PC diet increased the plasma ALT higher than NCL diet and further increased the AST levels higher than both NC and NCL diets. These may be suggestive of liver injury since these enzymes are key markers of liver dysfunction. Although arginine has been shown to exert beneficial effects on liver functioning [30, 31], it was also reported that arginine supplementation potentiated liver injury in mercury chloride-treated rats, which was characterized by elevated ALT and AST levels[32]. Coincidently, this may account for the increased ALT and AST levels with PC diet, such that the added arginine in PC fed broilers would have exerted burden on liver metabolism ultimately affecting its functions.Importantly, the NCL diet afforded protective effects by lowering both plasma ALT and AST compared to the PC diet. This corroborates the report thatL-citrulline treatment decreased ALT, AST, and the ratio of ALT/AST in high-fat and cholesterol-fed rats [33].In addition, feeding broilers with NC diet increased the plasma urate content higher than both the PC and NCL diets. This may be indicative of increased protein catabolism, exposure to stressors, and impaired hepato-renal functions [34, 35]. However, both arginine andL-Cit supplementation were able to reverse these effects by diminishing urate accumulation in the bloodstream.
An examination of the plasma AA profile showed that feeding broilers with NC diet increased the circulation of several essential and non-essential AA than the arginine fortified-PC diet. It is understood that inadequate arginine supply from the NC diet could have activated other metabolic routes for protein synthesis, leading to the increased availability of certain AA. This alteration in protein synthesis was also evidenced by the accumulation of urate, as well as the decrease in thigh muscle weight of NC-fed broilers. Under certain states such as stress and catabolic conditions, some non-essential AA may become essential, especially where the capacity of endogenously synthesized AA is exceeded [36]. In this study, it was found that broilers fed NC diet had increased circulating levels of several AA, except for arginine. We had hypothesized thatL-Cit supplementation would promote arginine availability to meet the body’s metabolic needs since arginine is widely involved in several precursory roles including NO production[37]. Interestingly, NCL diet was able to augment the levels of arginine, citrulline, and ornithine in circulation, whereas, the PC diet could not support circulating citrulline levels. An important finding of this study was that the free plasma citrulline was decreased during arginine supplementation relative to arginine deficient condition. This supports the understanding that citrulline supply is increasingly effective during conditions of arginine deficiency. This also corroborates with previous reports where citrulline proved more effective in providing arginine than direct arginine supplementation [38–40]. Furthermore, it is necessary to report that the experimental birds were examined during the fed state, since the nutrient concentrations in body fluids are closely related to the food composition and anabolic responses of the body during the fed (postprandial) conditions [41, 42].
Since arginine is responsible for NO synthesis, we proposed that the increase in arginine availability would result in increased NO production, similar to previous reports [15, 37]. However, the results revealed that the plasma NO was unaffected by dietary treatments,whereas,L-Cit supplementation to NC diet promotedNO concentration higher than arginine supplementation in both the kidney and liver tissues. Thus, in line with our findings that NCL diet increased both the plasma arginine and citrulline content, it was evident that L-Cit could serve as a potential substrate to restore NO production where the arginine availability was limited. To corroborate this, dietary L-citrulline was reported to promote the circulating arginine and NO levels in a dosedependent manner in laying hens [15]. In addition, it is established that citrulline is directly converted to argininosuccinate via the actions of ASS and further metabolized by ASL to produce arginine, which in turn can be converted to citrulline via NOS activity or ornithine via arginase actions [43–45]. Therefore, an investigation into these enzymes would provide succinct information on the metabolic transformations that occur during citrulline to arginine conversion in poultry. In the present study, PC diet stimulated higher ASS, and ASL activities than NCL diet, suggesting that supplemental arginine promoted citrulline conversion to arginine than directL-Cit supplementation. Importantly, arginine serves as a substrate for three major NOS isoforms, including the constitutive forms of nNOS, which is found in the central nervous system, eNOS which is expressed in the vascular endothelium, and the inducible isoform, iNOS which is stimulated by inflammatory mediators, cytokines and stress signals [46, 47]. In this study, NCL diet induced plasma eNOS activity higher than the PC and NC diets,and also elevated the liver eNOS activity compared to the PC diet. It further upregulated the kidneyeNOSexpression higher than the NC diet. This may account for the increased liver NO concentration by NCL diet, that is,via eNOS catalysis of arginine to release NO. Contradictorily, PC diet was observed to induce kidney eNOS activity, the kidney expression ofnNOSandeNOS, as well asiNOSexpression in the liver. However, it exerted no stimulatory effect on NO production. These contrasting findings cannot be fully explained and may suggest differential responses between the kidney and liver during arginine metabolism in chickens.
Furthermore, earlier studies had demonstrated the absence of CPS 1 and OTC activity in chickens [48,49]. In the present study, changes in the relative mRNA expression ofOTCin the liver, as well asOTCandCPS1in the kidney were observed. This coincides with studies that had identified a functionalCPS1gene in the brain, muscle, and immune tissues of chicken, although its role in the urea cycle was yet to be fully ascertained[50, 51]. In line with this, Li et al. [52] also reported an upregulatedCPS1andOTCgene expression in the liver and breast muscle of chicken embryos following dietaryL-arginine supplementation to broiler breeder hen’s diet.Noteworthy, the gene expression ofOTCin the liver,as well asOTCandCPS1in the kidney were relatively higher thannNOSandeNOS,which were minimally expressed in the liver and kidney respectively. Hence,these findings suggest that both the kidney and liver are active sites for arginine metabolism in chickens, although with distinct peculiarities. Comparatively, it is reasonable that arginine to citrulline metabolism is more efficient in the kidney considering the relatively higher expression ofnNOS, ASS, ARG2,andOTCin the kidney than in the liver. Concurrently, the kidney had been reported as the greatest site for citrulline to arginine conversion [53].
Arginase is an important enzyme that hydrolyzes arginine to produce ornithine and urea [54]. In chicken,the kidney arginase is important in regulating arginine metabolism, since arginine degradation is dependent on both the kidney arginase activity and the plasma arginine level [55]. Earlier works had reported on the molecular characterization of arginase enzyme in the kidney and liver tissues of chickens [56, 57]. Robbins and Baker [58] showed that arginine deficiency slightly increased the kidney arginase activity, however, increasing the arginine concentration in diets that had either 50% or 100% nitrogen requirement markedly increased the kidney arginase by twofold. Chu and Nesheim [55]also showed that arginine supplementation to laying hens induced higher arginase activity in the kidney than the low protein diet. Corresponding with these reports,the arginine-supplemented-PC diet promoted the plasma, kidney, and liver arginase 1 activity higher thanL-Cit supplemented-NCL diets. Interestingly, citrulline can exert a non-competitive inhibition on arginase catalysis of arginine to attenuate arginase actions [59].In this study, NCL diet persistently diminished the arginase 1 activity in the plasma, kidney, and liver. This was also corroborated by the downregulatedARG2gene expression in the liver, but not in the kidney. Moreover,L-Cit inhibition of kidney and liver arginase 1 activity corresponded with its induction of NO concentration in these tissues. Furthermore, arginase directly competes with NOS for the substrate, arginine [60]. Thus,it was also found thatL-Cit inhibition of arginase may have led to its induction of eNOS activity probably to augment NO production.
Studies have shown that arginine is crucial to intestinal health since it maintains intestinal integrity,improves gut immunity, intestinal absorption, gut barrier functions and protects the gut microbial composition [9, 10]. Importantly, different AA transporter systems in the brush border membranes are responsible for the transport of specific free AA into the enterocytes [61]. The b0+AT is a light chain homolog of the heteromeric AA transporter which functions to maintain homeostasis of AA pools in various tissues[62]. EAAT3 is an important anionic AA transporter with an affinity for glutamate [63]. Also, PepT1 is an AA transport system that facilitates the absorption of dietary AA as dipeptides and tripeptides into the intestinal epithelial cells [64]. The present study showed that NCL diet upregulatedPepT1expression in the ileum and jejunum tissues, compared to the PC and NC diet or PC diet, respectively. It was reported that the expression of chickenPepT1gene was regulated in response to changes in the dietary crude protein and AA levels [65, 66]. In another study,PepT1expression was modulated in malnourished rats despite atrophic changes in the intestinal mucosa [67]. This may explain the increasedPepT1expression in the jejunum of NC-fed broilers. Furthermore, citrulline can be transported efficiently across the intestinal lumen by Na+-dependent (system B0,+) and Na+-independent(systems L and b0,+) transporters [16]. Similarly, the cellular transport of arginine involves transit via the system y+and Na-dependent transporters (e.g., b0,+,B0,+, and y+L) in a cell-specific manner [68]. Thus, the upregulation ofPepT1andEAAT3systems with NCL diet suggests thatL-Cit supplementation elicited a positive influence on intestinal AA transport with profound effects on AA uptake in broilers.
Tight junction proteins such as Claudin, Occludin, and ZO-1 play important roles in the intestinal epithelial barrier, and as such, they are crucial for nutrient absorption and intestinal immunity [11, 69]. In addition, pIgR is used to establish the first lines of intestinal defense since it transports the polymeric Immunoglobulin A to the intestinal lumen and across epithelial cells [70]. In this study,NCL diet enhanced Claudin 1 and Occludin expression in the ileal mucosa greater than the PC and NC diets.Alongside this, the jejunal Claudin 1 andMUC2expression were upregulated by NCL diet compared to the PC and NC diets, while thepIgRexpression was also increased by both NCL and NC diets relative to PC diet.Previous studies have shown that arginine is necessary for the maintenance of intestinal epithelial integrity [71],while citrulline is an important biomarker of gut health and intestinal functions [44, 72, 73]. Therefore, these findings suggest thatL-Cit supplementation enhanced the expression of intestinal tight junction proteins, and promoted intestinal mucosal defenses better than arginine supplementation during arginine deficiency. Thus,citrulline may serve as a functional AA during conditions of low arginine availability to support arginine -mediated roles such as nutrient absorption and intestinal immunity.
Proper maintenance of the intestinal morphology is beneficial for nutrient digestion, absorption, and optimal broiler performance. In this study, the VH/CD ratio was lowered in the ileum, while the VH and VH/CD ratios were decreased in the jejunum of NC-fed broilers compared to the PC and NCL groups. This suggests that the nutrient absorption area and efficiency of absorption were compromised in NC-fed broilers, however, supplementation with either arginine orL-Cit would alleviate the gut barrier failure. To corroborate this, studies have shown thatL-arginine supplementation improved VH and preserved the jejunal morphology inClostridium perfringensinfected broilers [10], as well as the VH and VH/CD ratio in coccidiosis-challenged chickens [74]. The beneficial effects of arginine on intestinal microstructure and morphometry have been attributed to its role in the synthesis of polyamines and NO, since these metabolites are vital for intestinal development and nutrient absorption [75]. In addition, citrulline concentration is significantly correlated with intestinal markers of crypt depth,and VH/CD ratio [76], such that plasma citrulline concentration is decreased during conditions of intestinal villus atrophy [77]. Therefore, this study demonstrates that arginine andL-Cit were beneficial in promoting intestinal nutrient absorption, barrier integrity, and gut health during arginine -deficient conditions.
Conclusion
Taken together,L-Cit supplementation effectively replaced arginine in broiler’s diet owing to the ability ofL-Cit to augment circulating arginine and subsequently tissue NO production (Fig. 9). Therefore,L-Cit provided as a crystalline AA to broilers would present an immediate metabolic precursor for arginine supply, tissue NO generation, facilitate nutrient transport and support intestinal integrity under conditions of arginine deficiency. Further studies expounding on the argininesparing effects ofL-Cit would prove useful in optimizing dietaryL-Cit in poultry feeding programs.
Abbreviations
AA Amino acids
ALT Alanine aminotransferase
ASS Argininosuccinate synthetase
ASL Argininosuccinate lyase
ARG2 Arginase 2
AST Aspartate aminotransferase
b0,+AT B0,+AA transporter
BW Bodyweight
BWG Bodyweight gain
CD Crypt depth
CK Creatine kinase
CPS1 Carbamoyl phosphate synthetase 1
EAAT3 Excitatory amino acid transporter 3
eNOS Endothelial nitric oxide synthase
FI Feed intake
FCR Feed conversion ratio
GLU Glucose
iNOS Inducible nitric oxide synthase
L-CitL-Citrulline
LDH Lactate dehydrogenase
MUC2 Mucin 2
NO Nitric oxide
nNOS Neuronal nitric oxide synthase
OTC Ornithine transcarbamylase
PepT1 Peptide transporter 1
pIgR Polymeric Ig receptor
TG Triglyceride
TCHO Total cholesterol
tNOS Total nitric oxide synthase
VH Villus height
VH/CD Villus height to Crypt depth ratio
ZO-1 Zona occludens 1
Acknowledgements
The authors appreciate Zhao Mei, Ding Baofang, Wang Minghui, Li Xin, Gao Ying, Wang Dan and Wang Zhi for their assistance.
Authors’ contributions
VAU and HL conceptualized the research. VAU performed the experiment,data collection and interpretation. LS, YL and MZ assisted with the experimentation. JZ, XW, HJ, OMO, and HL were responsible for supervision and project administration. VAU and HL interpreted the results, edited and reviewed the original draft. All authors have read and approved the final manuscript.
Funding
This work was funded by the Key Technologies Research and Development Program of China (2021YFD1300405), Key Technology Research and Development Program of Shandong province (2019JZZY020602), the Earmarked Fund for China Agriculture Research System (CARS-40-K09), and National Natural Science Foundation of China (31772619).
Availability of data and materials
All relevant data are provided within the paper and the dataset used in this study is fully available from the corresponding author.
Declarations
Ethics approval and consent to participate
All experimental procedures were conducted in compliance with the “Guidelines for Experimental Animals” of the Ministry of Science and Technology(Beijing, P. R. China), and the study was approved by the Ethics Committee of Shandong Agricultural University, China.
Consent for publication
All of the authors have approved the final version of the manuscript for publication.
Competing interests
The authors declare that they have no competing interests.
Received: 7 July 2022 Accepted: 4 December 2022

杂志排行
Journal of Animal Science and Biotechnology的其它文章
- Maintenance of gut microbiome stability for optimum intestinal health in pigs – a review
- Biological function of resveratrol and its application in animal production: a review
- Selective footprints and genes relevant to cold adaptation and other phenotypic traits are unscrambled in the genomes of divergently selected chicken breeds
- The impact of genotyping strategies and statistical models on accuracy of genomic prediction for survival in pigs
- RNA-seq analysis reveals the critical role of the novel lncRNA BIANCR in intramuscular adipogenesis through the ERK1/2 signaling pathway
- Origins, timing and introgression of domestic geese revealed by whole genome data
