Cysteine dioxygenase and taurine are essential for embryo implantation by involving in E2-ERα and P4-PR signaling in mouse
2023-06-14DiZhangZhijuanWangXuanLuoHongzhouGuoGuobinQiuYunengGongHongxuGaoandShengCui
Di Zhang, Zhijuan Wang, Xuan Luo, Hongzhou Guo, Guobin Qiu, Yuneng Gong, Hongxu Gao and Sheng Cui,4*
Abstract Background Taurine performs multiple physiological functions, and the maintenance of taurine level for most mammals relies on active uptake from diet and endogenous taurine synthesis through its synthesis enzymes, including cysteine dioxygenase (CDO). In addition, uterus tissue and uterus fluid are rich in taurine, and taurine synthesis is regulated by estrogen (E2) and progesterone (P4), the key hormones priming embryo-uterine crosstalk during embryo implantation, but the functional interactions and mechanisms among which are largely unknown. The present study was thus proposed to identify the effects of CDO and taurine on embryo implantation and related mechanisms by using Cdo knockout (KO) and ovariectomy (OVX) mouse models.Results The uterine CDO expression was assayed from the first day of plugging (d 1) to d 8 and the results showed that CDO expression level increased from d 1 to d 4, followed by a significant decline on d 5 and persisted to d 8,which was highly correlated with serum and uterine taurine levels, and serum P4 concentration. Next, Cdo KO mouse was established by CRISPER/Cas9. It was showed that Cdo deletion sharply decreased the taurine levels both in serum and uterus tissue, causing implantation defects and severe subfertility. However, the implantation defects in Cdo KO mice were partly rescued by the taurine supplementation. In addition, Cdo deletion led to a sharp decrease in the expressions of P4 receptor (PR) and its responsive genes Ihh, Hoxa10 and Hand2. Although the expression of uterine estrogen receptor (ERα) had no significant change, the levels of ERα induced genes (Muc1, Ltf) during the implantation window were upregulated after Cdo deletion. These accompanied by the suppression of stroma cell proliferation.Meanwhile, E2 inhibited CDO expression through ERα and P4 upregulated CDO expression through PR.Conclusion The present study firstly demonstrates that taurine and CDO play prominent roles in uterine receptivity and embryo implantation by involving in E2-ERα and P4-PR signaling. These are crucial for our understanding the mechanism of embryo implantation, and infer that taurine is a potential agent for improving reproductive efficiency of livestock industry and reproductive medicine.
Keywords CDO, E2, Embryo implantation, P4, Taurine
Background
High rate of embryo loss in early pregnancy is a major constraint both in livestock industry and human reproduction, whereas much pregnancy wastage is caused by the failure of embryo implantation [1–3]. The implantation of the blastocyst into the maternal uterus is a crucial step in mammalian reproduction [4, 5]. It is generally accepted that embryo implantation depends on blastocyst quality, endometrial receptivity, and the synchronization of their development [6]. In mice,embryo implantation consists of apposition between the trophectoderm layer of blastocyst and the luminal epithelium (LE), attachment and final invasion into the LE [7]. Upon embryo invasion, the uterine stromal cells are rapidly remodeled in the process of decidualization,which is characterized by morphological and functional changes in stromal cells in the form of proliferation and differentiation into large epithelioid decidual cells [8]. However, the cell proliferation and differentiation of specific uterine cell types in early pregnancy are dependent on the coordinated actions of ovarian steroid hormones, including progesterone (P4) and estrogen (E2). During mouse pregnancy, an E2surge on d 1 stimulates uterine epithelial cell proliferation, and the decline of E2level on d 2 leads to apoptosis of a large number of epithelial cells. P4, from the newly formed corpora lutea on d 3 [8], initiates uterine stromal cell proliferation. In conjunction with P4, an acute E2spike on d 4 further stimulates uterine stromal cell proliferation and renders the uterus receptivity for the blastocyst to implant [8–10]. However, it is still largely unknown about the molecular mechanisms of coordinate proliferative events induced by E2and differentiative processes directed by P4.
Although the receptivity of the uterus during implantation is primed by E2and P4, their actions on cell proliferation and differentiation are complicated, and the relative molecular mechanisms have been extensively studied.The related molecular and genetic studies indicate that E2and P4act respectively via estrogen receptor 1 (Esr1,ERα) [11] and progesterone receptor (Pgr, PR) [12] to govern the embryo-uterine crosstalk during peri-implantation stage by targeting local transcriptional factors, signals or paracrine molecules [13], some of which include Muc1 [14], Ltf [15], Hox10a [16, 17], Hand2 [17] and IHH[18, 19]. In addition, uterine epithelium and its secretions are essential for uterine receptivity and embryo implantation. Uterine epithelium includes LE and glandular epithelium (GE), which directly synthesize, secrete or selectively transport a wide variety of substances from serum and transudate, collectively termed histotroph[20], into uterus lumen. Whereas histotroph is complex and comprised of many different substances, such as leukemia inhibitory factor (LIF), ions, sugars, lipids,proteins and amino acids [20–24], among which taurine is included [25]. It is much interested that taurine concentration in the uterine luminal fluid (UFL) of mouse is much higher during the implantation [25]. But as we know, there is no direct evidence about the functional relations of taurine with uterine receptivity and embryo implantation, although it is reported thatCdoknockout(KO) mice exhibit impaired reproductive capacity [26].
Taurine is one of the most abundant non-essential amino acids in most mammals [27], and performs numerous physiological functions, including bile salts synthesis and hepatoprotection [28], energy metabolism[29], maintenance of Ca2+homeostasis [30], anti-oxidative, osmoregulation, anti-inflammatory and anti-apoptotic [31–33]. While the maintenance of the body taurine level mainly relays active uptake from diet and the endogenous taurine synthesis through the sequential actions of its synthesis enzymes, including cysteine dioxygenase(CDO) [34].
CDO is a critical enzyme for taurine synthesis and CDO expression has been detected in liver, adipose tissue, pancreas, kidneys, lungs and reproductive system[35].CdoKO results in a higher incidence of postnatal mortality, retards postnatal growth and damages male fertility [26, 36]. In addition, CDO is highly expressed in mouse ovary and uterus [37], but the functions of CDO in female reproduction remains unclear. Interestingly,uterus tissue and ULF are rich in taurine, and ULF taurine concentration is increased during embryo implantation [25]. Furthermore, CDO expression in uterus is up-regulated by P4, whereas E2decreases CDO expression [37]. In addition, our recent study shows that E2regulates taurine synthesis through E2-ERα-CSD signaling[38]. These make us to hypothesize that CDO may play important roles in E2and P4primed embryo implantation, possibly through the physiological actions of taurine. The present study was thus proposed to identify the effects of CDO and taurine on embryo implantation and illustrate the related mechanisms by usingCdoKO and ovariectomy (OVX) mouse models.
Materials and methods
Animals and treatments
Eight weeks old ICR mice were used in fertility test and other animal experiments.CdoKO mice were generated by using 129 mice (Additional file 1). Mice were raised in controlled temperature (25 ± 1 °C) and humidity(60%–70%) with a 12 h light, 12 h dark cycle. The animal experiments were approved by the Chinese Association for Laboratory Animal Sciences. Virgin female mice were mated with sexually matured males to induce pregnancy(day 1 is the day of vaginal plug checked, d 1). Embryos were collected from oviducts on d 1. The implantation sites (IS) were visualized by intravenous injection of 0.1 mL 1% Chicago blue dye (Sangon Biotech, Shanghai,China) in saline on d 5 [39]. To investigate the ovarian hormonal influence on uterine CDO expression, wildtype (WT) mice were ovariectomized. Oil, 100 ng/mouse 17β-estradiol (E2; MedChemExpress, NJ, USA) or 2 mg/mouse progesterone (P4; MedChemExpress, NJ, USA)was injected 7 d later [40]. The treated mice were then sacrificed at indicated times for further experiments.
Real-time quantitative PCR (RT-qPCR) and common PCR
Total RNA of the uterus tissues was isolated using the TRIzol reagent (Takara, Dalian, China), purified by DNase I and quantified by spectrophotometry. 1 μg purified total RNA was used as a template for cDNA synthesis using HiScript Reverse Transcriptase (Vazyme, Nanjing,China) according to the manufacturer’s instructions.RT-qPCR was performed using SYBR Green master mix (Vazyme, Nanjing, China) in the StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA,USA) and reactions were done in triplicate. RT-qPCR conditions were as follows: 95 °C for 2 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. Relative gene expressions were normalized to endogenous controlGapdh. All Primers listed in Table S3 were designed using NCBI.
The genotype identification of theCdoKO mice was performed by common PCR using primers as described in Table S4. Amplifications were carried out on PCR instrument (Bio-Rad, Hercules, CA, USA) using the following protocol: 94 °C for 5 min (one time); 94 °C for 50 s, 65 °C for 30 s, 72 °C for 30 s (35 times); 72 °C for 10 min; and holding at 4 °C.
Western blotting (WB)
The uterus tissues were lysed with RIPA buffer (Beyotime,Shanghai, China) containing 1 mmol/L phenylmethanesulfonyl fluoride (PMSF, Sangon Biotech, Shanghai,China). The protein concentration of each group was determined by using the BCA assay reagent (CoWin Biosciences, Jiangsu, China) according to the manufacturer’s recommendations. Equal amounts of 50 μg proteins were electrophoresed on 12% sodium dodecyl sulfate–polyacrylamide gel (SDS-PAGE), and the bands were transferred to 0.45 μm polyvinylidene difluoride (PVDF)membrane (Millipore, MA, USA). The membrane was blocked with 5% (w/v) nonfat dry milk in 0.05 mol/L pH 7.4 Tris buffered saline (TBS) for 3 h and incubated with CDO antibody (ab53436, abcam, Cambridge, UK;1:2000), internal control GAPDH antibody (AM4300,Ambion, TX, USA; 1:10,000), PR antibody (ab2765,abcam, Cambridge, UK; 1:2000) or ERα antibody(ab32063, abcam, Cambridge, UK; 1:2000) overnight at 4 °C. The PVDF membrane was then washed 3 times for 30 min in TBST (0.1% Tween-20 in TBS) and incubated for 2 h with horseradish peroxidase-conjugated goat antirabbit IgG or horseradish peroxidase-conjugated goat anti-mouse IgG (Zhongshan, Beijing, China). After washing for 30 min with 3 changes of TBST, the membrane was treated with the ECL kit (Vazyme, Nanjing, China)and visualized by Tannon gel imager (Tanon, Shanghai,China). The intensity values pertaining to each group were normalized against the optical density of GAPDH corresponding to the same group.
Immunohistochemistry (IHC) and immunofluorescence (IF)
Tissues were fixed in 4% paraformaldehyde, dehydrated via graded ethanol solutions, and then embedded in paraffin to obtain 5 μm thick sections. IHC was performed as previously described [41]. The sections were incubated with CDO antibody (ab53436, abcam, Cambridge, UK;1:200), PR antibody (ab2765, abcam, Cambridge, UK;1:200), ERα antibody (ab32063, abcam, Cambridge, UK;1:200) or Ki67 antibody (D385, CST, MA, USA; 1:200)diluted in PBS overnight at 4 °C. After washing with PBS for 30 min, the sections were incubated with biotinylated goat anti-rabbit/mouse IgG (31820/31802, Thermofisher,Waltham, MA, USA; 1:200) for 3 h at RT. After washing with PBS for 30 min, the sections were incubated with streptavidin peroxidase complex (SA10001, Thermofisher, Waltham, MA, USA; 1:200) for 30 min at room temperature. Finally, the signals were visualized by incubating the sections with 0.05 mol/L Tris–HCL (pH 6.5)containing 0.06% (w/v) diaminobenzidine (DAB, ZSGBBio, Beijing, China) and 0.03% (v/v) H2O2. For IF staining, Hand2 (ab200040, abcam, Cambridge, UK; 1:200),Muc1 (ab15481, abcam, Cambridge, UK; 1:200) primary antibodies, and their respective secondary antibodies (Jackson Immuno Research, Philadelphia, PA, USA;1:200) were used. Nuclear staining was performed using 4’,6-diamidino-2-phenylindole (DAPI) dye (0.1 μg/mL,Beyotime, Shanghai, China). The signals were captured using a microscope (Olympus, Tokyo, Japan).
In vivo production of embryo
Non-superovulated virgin female mice (8–9 weeks)were mated with adult males. The males and females(1:2) were housed overnight and the presence of a vaginal plug in the following morning was regarded as successful mating (d 1 is the day of vaginal plug checked).Mice were killed by cervical dislocation and oviducts were immediately dissected and placed in M2 solution (CaCl2·2H2O 1.71 mmol/L, Glucose 5.56 mmol/L,HEPES 20.85 mmol/L, KCl 4.78 mmol/L, MgSO4·7H2O 1.19 mmol/L, NaCl 94.66 mmol/L, NaHCO34.15 mmol/L, Sodium lactate 23.28 mmol/L, Sodium pyruvate 0.33 mmol/L) at 11:00 on d 2. Embryos punched out from the ampulla were digested with hyaluronidase(HA) followed by three times washing with M2 and cultured in KSOM at 37 °C until the time of embryo transfer.
Embryo transfer
The uterine horn was exposed by flank laparotomy and six expanding embryos were transferred with a minimal amount of medium into the uterine cavity of pseudopregnant (d 3) recipients. The uterine horn was then placed back into the abdominal cavity and the incision site was closed. The procedure was repeated in the opposite flank where another six expanding embryos were transferred. The recipients were then placed individually in clean cages to recover from anesthesia in a warm room(28–30 °C).
Fertility test
CdoKO females and their nesting WT females were caged with adult wild-type males (8–9 weeks) at the rate of male:female = 1:2. Vaginal plug was examined the next morning. Female mice with vaginal plug withdrew from the experiment, while the none plugged females were backed to the test after one day’s rest. The plugged females were caged alone to observe their pregnancy and parturition.
Measurement of taurine
Uterus, liver, and serum taurine contents were measured by HPLC–UV (HPLC). Firstly, samples were weighed,homogenized and deproteinized using 0.2 mol/L sulfosalicylic acid. After being centrifuged at 14,000 ×gfor 20 min, the supernatants were added into a dual-bed column containing cation exchange resins to remove other amino acids and metabolic precursors of taurine. Secondly, samples were added with 100 μmol/L glutamine as an internal standard. All samples were then filtrated through a 0.22-μm PVDF membrane and saved in -80 °C refrigerator until use. The samples and the standard samples of taurine which were 100, 50, 25,10, 5, 2 and 1 μmol/L were derivated with OPA (Sigma-Aldrich, St. Louis, MO, USA) solution (20 mg OPA,2 mL methanol, 80 μL 2-hydroxy-1-etanethiol, 18 mL 0.1 mol/L borate buffer (pH 9.6)) for 3 min. Then 20 μL sample was automatically injected into a six-port valve to analysis with Waters Symmetry C18 Column(4.6 μm, 150 mm × 5 mm) (Waters, Milford, MA, USA)on a Shimadzu HPLC system (Shimadzu, Kyoto, Japan).The HPLC conditions were: flow A: 100% methanol,flow B: sodium phosphate buffer pH 4.7 containing 50%methanol. Flow rate was 1.2 mL/min, and the detection wavelength was 340 nm. The duration times were 2.3 min and 4.95 min for the internal standard and taurine.
Radioimmunoassay (RIA)
They belonged to her daughter; and surely no one who had such adaughter could be silly. The mother was like a fountain ofquestions; and the daughter, who listened but never spoke8, mighthave passed for the beautiful maid of the fountain. How charming shewas! She was a study for the sculptor to contemplate9, but not toconverse with; for she did not speak, or, at least, very seldom. Has the pope a great family? inquired the lady.
The only problem with being in this class was that I was surrounded by at-risk students. These were kids who did not do well in school and didn t want to be there most of the time. Their home lives were obviously much different from mine, and they were constantly in trouble with the school and the law.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 6.0. Data from at least three independent samples were expressed as mean ± SEM. Two group comparison studies were performed using Student’st-test and one-way analysis of variance (ANOVA) for data comprising three or more groups.P< 0.05 was considered to be statistically significant.
Results
Uterine CDO expression and taurine levels during early pregnancy
Now, an eagle with a King s heart in his breast is apt to be bold, and accordingly he instantly made up his mind to carry off the lovely damsel, feeling sure that having once seen her he could not live without her

Fig. 1 Uterine CDO mRNA and protein expressions and their relations to taurine levels in uterus from d 1 to d 8 of pregnancy mice. A, Cdo mRNA expression in mouse uteri detected by RT-qPCR (n = 3). B, CDO protein expression in uteri of d 1 to d 8 pregnancy mice assayed by WB. C, Serum and uterine taurine concentrations assayed by HPLC (n = 3). D, Serum P4 concentrations assayed by RIA (n = 5). E, CDO IHC staining of uterine cross-sections. Data shown as Mean ± SEM. Different letters represent significant differences (P < 0.05). d, days post coitum; E2, estrogen; GE,glandular epithelium; LE, luminal epithelium; P4, progesterone; St, stromal cells. Bars: 50 μm
Establishment of Cdo KO mouse
OVX Ovariectomy
The eyes are not the same. They no longer reflect the soul of the person because that person no longer exists. The eyes now are deep and cold and empty -- pools of color that have been filled with something reaching far beyond the happiness that once was there. Like the yellow brick road it stretches endlessly, maddeningly, winding9 through valleys and woodlands, obscuring her vision until she has somehow lost sight of the Emerald City.

Fig. 2 Generation of Cdo KO mouse. A, Cas9n target scheme in the first and the third exons of Cdo gene. The blue lines indicate sgRNAs’ spacer and the red lines indicate PAM (protospacer adjacent motif). B, Sanger sequencing of the target region in F0 mice. The Cdo KO mice get a miss of 7489 nucleotides. C, Genotype identification results using PCR. The 325 bp strand indicates wild type and the 245 bp strand indicates mutant type. D, IHC staining of CDO in WT and Cdo KO mouse uterus. Bars, 50 μm. E, RT-qPCR analysis of Cdo mRNA levels in WT and Cdo KO mouse uteri.F, Representative patterns of WB analysis of CDO expressions. G-I, Taurine levels in WT and Cdo KO mice liver, serum and uterus detected by HPLC.Data are the Mean ± SEM. (n ≥ 3). Different letters above columns indicate significant differences (P < 0.05). GE: glandular epithelium. LE: luminal epithelium. Sm: smooth muscle cells. St: stromal cells. Scale bars: 50 μm
Cdo KO causes impairment of embryo implantation and severe subfertility
In order to identify the effects ofCdodeletion on the embryo implantation, the serum and uterine taurine levels of theCdoKO females from d 1 to d 8 were assayed by HPLC. The results showed thatCdoKO sharply decreased the taurine levels in the serum and uterus tissue compared with that of WT mice, and there were no significant differences from d 1 to d 8 (Fig. 3A,B). However, there was a rising spike on d 4 in WT mice(Fig. 3A, B).

Fig. 3 CDO deletion causes impairment of embryo implantation and severe subfertility. A and B, Serum (A) and uterus (B) taurine concentrations of pregnancy Cdo KO and WT females from d 1 to d 8, assayed by HPLC (n = 3). C, Pregnancy rates of WT and KO females. The number within brackets indicate females with pups over total number of plug-positive females. D, Average litter sizes of WT and Cdo KO females (n = 7). E, Representative images of d 5 pregnant uteri from WT and Cdo KO females. Bars, 1 cm. F, Blastocysts flushed from Cdo KO uterus. Bar, 50 μm. G, Average numbers of implanted embryos represented by implantation sites (n = 5). H, Representative patterns of d 5 WT receptive mice uteri transferred with WT and Cdo KO embryos. I, d 5 KO receptive mice uteri transferred with WT embryos. Arrowheads indicate the location of blastocysts. Bars, 1 cm. K, IS numbers of different treated mice uteri (n = 3). Data are presented as Mean ± SEM. Different letters above columns indicate significant differences(P < 0.05)
Further, in order to dissect the cause of the subfertility inCdoKO females, the effect ofCdodeletion on the embryo implantation was examined. The plug positive mice were euthanized on d 5 and the numbers of embryos implanted to the uterine epithelium were identified by employing the Chicago blue dye assay [39].The implantation sites (IS) were clearly observed in WT females, but only a few or no IS were detected inCdoKO females (Fig. 3 E, G). However,CdoKO females had blastocysts which could be flushed out from uterus(Fig. 3 F).
My mind was subdued10 as my heart overflowed11 with the magic of gratitude12 and wonder. I slipped my ring onto my trembling hand, and a smile filled my soul as I whispered, Thanks Mom.
In order to answer whether the subfertility ofCdoKO mice was resulted from the abnormalities in the ovaries or embryos, the embryos were flushed out from oviducts of d 2CdoKO females, which naturally mated with WT males. The flushed 2-cell embryo numbers did not have significant difference between WT andCdoKO mice(Fig. S2 B), and morphological defect was not observed either (Fig. S2 A). In addition, the histological abnormality was not observed in 10 weeksCdoKO mouse ovaries(Fig. S2 C). Serum P4and E2levels did not exhibit significant differences between theCdoKO and WT females on d 4 (Fig. S2 D and E).
Further, the effects ofCdodeletion on the implantation and fertility were examined. Adult WT andCdoKO females were respectively mated with ICR males, the parturition and pups of each litter were then recorded. The results showed that the plug-positive WT females exhibited normal fecundity, but only 38.8% (7/18) of the plugpositiveCdoKO females produced litters (Fig. 3 C), and the litter size (5.429 ± 0.65 pups/litter,n= 7) was much smaller than that of the WT females (11.29 ± 0.68 pups/litter,n= 7) (Fig. 3 D).
In order to confirm whether the subfertility ofCdoKO females was resulted from the defects of uterus receptive status,CdoKO and WT females were separately mated withCdoKO and WT males to obtainCdoKO and WT embryos. The harvestedCdoKO and WT embryos were respectively transferred to WT female uteri. On d 5, IS numbers were assayed and there was no significant difference between the WT females to which the WT andCdoKO embryos were respectively transferred (Fig. 3 H, K). However, the IS number inCdoKO receptive females was much less than in WT receptive females after both of which accepted WT embryos (Fig. 3 I, K). Meanwhile, blastocysts could be flushed out fromCdoKO uterus (Fig. 3 J). These infer that the failure of embryo implantation and subfertility inCdoKO females are resulted from the defects of uterine receptivity.
Cdo KO results in the defects of uterine receptivity by dysregulating PR and ER signaling in mouse
Our results showed thatCdoKO had no significant effects on ovary morphology and functions, including E2and P4secretions (Fig. S2C–E). As the window of uterine receptivity coincides with the P4-mediated down-regulation of ERα activity in uterine LE, we thus assayed the effects ofCdodeletion on uterus PR expression on d 4 by RT-qPCR and WB. The results showed that PR mRNA and protein levels inCdoKO uteri respectively decreased by 28.74%and 26.63% compared with that in WT uteri (Fig. 4A–C).IHC results showed that PR staining intensity on LE, GE and stromal cells were much weaker inCdoKO mice than in WT mice (Fig. 4 G). In addition, the uterine mRNA expression levels of the known PR responsive genesIhh,Hoxa10andHand2decreased by 56.33%, 35.49% and 45.38% in KO mice than that of the WT mice (Fig. 4 H).Further, Hand2 IF staining was performed and it was observed that Hand2 was located only in the stroma cells,but its staining intensity was much weaker inCdoKO mice than that in WT mice (Fig. 4 J), which was consistent with PR IHC staining result (Fig. 4 G).

Fig. 4 Effects of Cdo deletion on PR and ER signaling transduction and the cell proliferation in the uteri of d 4 pregnancy mice. A, RT-qPCR analysis of relative Pgr mRNA expression levels. B and C, WB detection and analysis of PR protein expression. D, RT-qPCR analysis of relative Esr1 mRNA levels.E and F, WB detection and analysis of ERα protein expression levels. G, IHC staining of PR and ERα. Scale bars: 50 μm. H, Relative mRNA expressions of Ihh, Areg and Hand2. I, Relative mRNA levels of Ltf and Muc1. J, IF staining of Hand2 and Muc1. Scale bars: 50 μm. K, Ki67 (proliferative cell marker)IHC staining. Scale bars: 50 μm. GE: glandular epithelium. LE: luminal epithelium. St: stroma. Data are presented as Mean ± SEM, n ≥ 4. Different letters represent significant differences (P < 0.05)
In addition,Cdodeletion had no significant effects on ERα mRNA and protein levels (Fig. 4D–G). Whereas the expression levels of E2-responsive genesMuc1andLtfelevated over 2.5 and 8 times inCdoKO uterus than that of the WT uterus on d 4 (Fig. 4 I). The further IF staining confirmed the Muc1 expression in LE and GE cells ofCdoKO mouse uteri, but which was not detected in WT mice (Fig. 4 J).
Another important character of uterus receptivity to embryo is a cessation of epithelial cell proliferation and robust proliferation of stroma prior to implantation. We thus detected the cell proliferation in the uterine tissues ofCdoKO and WT mice on d 4 by Ki67 IHC staining.The results showed that Ki67 was not detected in LE and GE cells of WT andCdoKO mice uterus, but Ki67 staining intensity in the stroma of WT uteri was much stronger than that ofCdoKO uteri. Moreover, Ki67 positive cells were concentrated around the LE in WT uteri(Fig. 4 K), but Ki67 positive cells inCdoKO uteri were irregularly scattered (Fig. 4 K). These indicate thatCdoKO results in the defects of uterine receptivity by dysregulating PR and ER signaling in mouse.
P4 and E2 are involved in regulating uterine CDO expression
As the dynamic patterns of uterine CDO expression and taurine level were parallel to serum P4concentration and uterine PR expression during early stage of pregnancy(Fig. 1A–D and Fig. 3A, B), we further identified the effects of P4and E2on uterine CDO expression by using OVX mouse model. RT-qPCR results showed that uterineCdomRNA levels were decreased by 54.65%, 90% and 93% after 2, 6 and 12 h after 100 ng E2treatments in OVX mice (Fig. 5 A). These were further confirmed by the CDO IHC staining results that the CDO staining intensity got much weaker after 12 h E2treatment (Fig. 5 C).It was opposite that 2 mg P4injection sharply increased uterine CDO mRNA and protein levels, which were several folds higher than that of the controls after 12 h P4treatment (Fig. 5 B and C). Further, in order to identify whether the regulating effects of P4and E2on uterine CDO expressions were through P4-PR and E2-ERα signaling, OVX mice were respectively pretreated with PR inhibitor RU486 and ERα inhibitor ICI182780, followed by P4or E2treatment. The uterine CDO expressions were then assayed and the results showed that RU486 blocked the enhancing effect of P4on CDO expression (Fig. 5G–I), and ICI182780 restrained the inhibiting effect of E2on CDO expression (Fig. 5D–F).
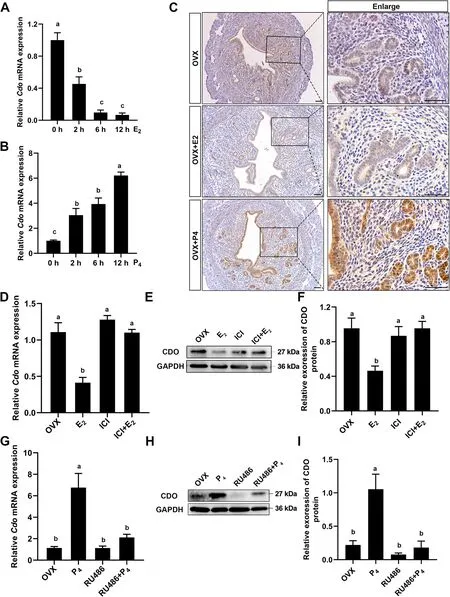
Fig. 5 The relations of P4-PR and E2-ERα signaling to uterine CDO expression. A and B, Relative Cdo mRNA levels in OVX mouse uteri after 0(control), 2, 6 and 12 h treatments with 100 ng E2 (A) and 2 mg P4 (B). C, CDO IHC staining in OVX mouse uteri after 0 (control) and 12 h 100 ng E2 or 2 mg P4 treatments. Scale bars: 50 μm. D, E and F, Relative Cdo mRNA (D) and protein levels (E and F) in OVX mouse uteri treated with E2 and E2 inhibitor ICI182780 (ICI). G, H and I, Relative Cdo mRNA (G) and protein levels (H and I) in OVX mouse uteri treated with 2 mg P4 and PR inhibitor RU486. Data are presented as Mean ± SEM. Different letters represent significant differences (P < 0.05, n = 3)
Taurine supplement partly recues the defects of embryo implantation and subfertility caused by Cdo KO
As the maintenance of the global taurine level relays on the endogenous taurine synthesis through the action of CDO and the active uptake from the diet, we thus supplied extra taurine in the regular diet ofCdoKO mice to confirm whether the defects of uterine receptivity and subfertility caused byCdoKO could be rescued. Adult femaleCdoKO mice were supplemented with drinking water containing 0.2% (w/v) taurine. The uterine and serum taurine levels were assayed. The results showed that taurine supplement markedly elevated taurine levels in uteri and serum, but all of which were significantly lower than that in wild mice with regular diet (Fig. 6A,B). In addition, the embryo implantation was detected on d 5. The results showed that taurine supplement significantly increased the IS number ofCdoKO females,although it was still lower than that of the WT females(Fig. 6C–E). These results demonstrate that taurine supplementation can partly rescue the subfertility ofCdoKO females and suggest that CDO and taurine are essential factors for embryo implantation.
Discussion
The present study, for the first time as we know, demonstrates that CDO and taurine play important roles for embryo implantation in mouse. This is supported by our results here that uterine CDO expression and serum taurine level during early stage of mouse pregnancy are parallel to the uterine taurine level, which sharply increases and reaches a peak just on d 4, the window of implantation [42], followed by a steadily decline under physiological condition. In addition, serum P4, a key factor to regulate the embryo implantation [7, 43], behaves a similar dynamic pattern with the uterine CDO expression and taurine concentration in the duration examined. Whereas global Cdo KO markedly decreases uterine taurine level and omits the dynamic pattern of uterine taurine, accompanied by the decline of serum taurine level, although Cdo KO does not affect the ovary functions to synthesize P4and E2. These demonstrate that uterus has function to synthesize taurine, whereas Cdo deletion causes a severe subfertility in female mice. CDO and endogenous taurine synthesis are thus essential for embryo implantation and the maintenance of animal reproduction. These are also supported by our results here that taurine supplement to the Cdo KO mice can partly rescue the reproductive capacity.
He found them both sitting hand in hand on the step in front of the altar, and immediately knew his daughter again, and took her in his arms, thanking God and her deliverer
Another important finding of this study is that Cdo KO causes defects in uterine epithelium receptivity and decline of pregnancy rate. It has been well documented that successful embryo implantation depends on the intimate connection between the blastocyst and maternal endometrial surface [43, 44]. Healthy embryos at the blastocyst stage and receptive maternal endometrium are necessary for the implantation [45]. The results of this study show that Cdo deleted embryos do not have morphological defects, and behave normal implantation as that of the WT embryos in WT recipients. However, the implantation and birth rates are dramatically decreased after the WT embryos are transferred to the Cdo deleted recipients. These imply that Cdo deleted embryos have normal capacity to get implantation. Another important character of uterine receptivity is the cessation in epithelial cell proliferation and robust proliferation in stroma cells just prior to implantation [46, 47]. The results of present study are in agreement with reports [44, 46]that the cell proliferation, marked by Ki67 IHC staining,is undetectable in the uterine epithelium, but the number of Ki67 positive stroma cells significantly decreases in Cdo KO mice compared with that of WT mice. These collective data suggest that Cdo deletion leads to the abnormalities of uterine receptivity and embryo implantation, and the subfertility of Cdo KO mice is primarily of uterine origin.
However, it has been reported that Cdo KO females are fertile and carry their pregnancies to term [26], whereas our results here demonstrate that 38.8% of plug-positive Cdo KO females are fertile, and the IS number and litter size of Cdo KO females are much less than that of WT females. These discordant statements might be resulted from the limited assessment, and no description about the statistics analysis of the IS numbers and birth rates by Ueki et al. [26]. Another possible explanation is that different mouse strains have been used in our study and the previous report [26].
In addition, the results presented here demonstrate that Cdo deletion impairs embryo implantation by dysregulating P4and E2signaling in mouse uterus. The related molecular and genetic studies indicate that both E2and P4play functions through their corresponding receptor ERα and PR [48, 49] together with local transcriptional and paracrine factors to govern the complicated embryo-uterine crosstalk [11, 12, 43, 50]. It is much interested that our results here show that CDO involves in regulating embryo implantation by affecting P4and E2signaling. In support, the uterus PR mRNA and protein levels in Cdo deleted mice are significantly decreased,accompanied by decline of Ihh, Hoxa10 and Hand2 expressions, which are known PR targeting molecules[18, 43, 51]. These are also confirmed by PR and Hand2 IHC results of this study. However, Cdo deletion does not affect ERα mRNA and protein levels, but the mRNA levels of E2-ERα responsive genes Lf and Muc1 [14, 15]are increased over 5 and 1.5 time in Cdo KO mouse uteri,especially the abnormal expression of Muc1 in Cdo KO LE and GE cells. In addition, CDO or/and taurine might participate in E2-ERα signaling by affecting the ERα activity through ERα Ser 118 site phosphorylation [43, 52],although we did not detect it in this study. Collectively,the results presented here demonstrate that CDO is a crucial factor to affect uterine receptivity during embryo implantation by involving in P4-PR and E2-ERα signaling in mouse uterus, although the relating signaling pathways and mechanisms need to be clarified in future study.
Furthermore, the regulating effects of P4and E2on uterine CDO are confirmed by using OVX mouse model. The in vivo results show that uterus CDO expression level is decreased over 90% after 12 h E2treatment in OVX mouse, which is in agreement with the reports that E2inhibits taurine synthesis through estrogen-ERs-CDO/CSAD signaling in liver and uterus [37, 38]. It is inversed that uterine CDO expression level is sharply elevated after P4injection in OVX mouse. In addition,it is well known that E2and P4are key drivers of uterine plasticity throughout the sexual cycle and early stage of pregnancy, we thus propose that both E2and P4may play roles through their respective receptors to regulate uterine CDO expression and taurine synthesis, which subsequently affect the uterine receptivity and embryo implantation. However, it remains to be elucidated about the interactions or cross-talks among P4-PR signaling,E2-ERα signaling, CDO and taurine in the duration of embryo implantation.
Sm Smooth muscle cells
I could not believe my eyes. I read the note two or three times, my eyes filling with tears. Had I only known earlier, I would have said, “Use them now. Don’t wait until you pay for them.”
In conclusion, the present study demonstrates that taurine and CDO play prominent roles for uterine receptivity and embryo implantation by involving in E2-ERα and P4-PR signaling pathways. These are crucial for our understanding the mechanism of embryo implantation, and infer that taurine is a potential agent for improving reproductive efficiency of livestock industry and/or for reproductive medicine. But it still remains to be elucidated about the interactions among P4-PR signaling, E2-ERα signaling, CDO and taurine in the duration of embryo implantation.
Serum P4and E2were analyzed using RIA reagents provided by the Beijing North Institute Biological Technology (Beijing, China). The minimum detectable concentrations were 2 pg/mL for E2and 0.2 ng/mL for P4.For each RIA the intra and inter assay coefficients of variation were respectively less than 15% and 10%.
Conclusions
The present study demonstrates that taurine and CDO play prominent roles for the uterine receptivity and embryo implantation by involving in E2-ERα and P4-PR signaling in early pregnancy stage. These elucidate a new mechanism for taurine regulating embryo implantation. On the other side, abuse of steroid hormones in stockbreeding industry usually gives rise to premature ovary failure and declines animal fertility. The balancing effect of CDO and taurine between P4-PR signaling and E2-ERα signaling shows that taurine is a potential agent for improving reproductive efficiency of livestock industry and reproductive medicine.
Some modern interpretations95 of the tale, such as Shannon Hale s excellent novel, explain that the Goose Girl doesn t reveal her true identity because she fears no one will believe her. She awaits the best opportunity to reveal her identity with the least amount of blood shed available.
Abbreviations
CDO Cysteine dioxygenase
E2Estrogen
In order to evaluate the physiological significance of CDO and taurine in adult female mouse, we firstly examined the CDO expression profiles in different organs by RT-qPCR and WB. The results showed that CDO mRNA and protein were highly expressed in mouse uterus(Fig. S1). Next, the uterine CDO mRNA and protein expressions, their relations to the changes of uterine taurine concentrations and serum steroid hormones were analyzed in the duration of early pregnancy, from d 1 to d 8. RT-qPCR results showed thatCdomRNA increased from d 1 to d 4 and reached the maximum on d 4, which followed by a sharp decline on d 5 and persisted on d 6 and d 8 (Fig. 1 A). WB results showed that CDO protein levels were highly correlated withCdomRNA levels in the duration examined (Fig. 1 B). In addition, IHC staining revealed that CDO was located in uterine epithelial cells, including LE and GE, and some stromal cells (Fig. 1 E). The taurine concentrations in uterus tissue and serum were assayed by HPLC and the results indicated that the uterine taurine levels exhibited an increasing tendency from d 1 to d 4, and reached the maximum on d 4, followed by a steady decline and returned to the similar level of d 1 on d 8 (Fig. 1 C). The serum taurine level also increased from d 1 to d 4, which significantly declined from d 5 to d 8 (Fig. 1 C). In addition, the dynamic patterns of the uterine CDO expression and taurine concentration corresponded to the change of serum P4from d 1 to d 6 (Fig. 1 D). These suggest that CDO might play important roles in regulating embryo implantation.
Esr1(ERα) Estrogen receptor 1
GE Glandular epithelium
HA Hyaluronidase
The woman returned home, but the time passed slowly for her until the full moon came. Finally the shining disk appeared in the heaven, and she went out to the millpond, sat down, and combed her long black hair with the golden comb. When she was finished she set it down at the water s edge. Before long there came a motion from beneath the water. A wave arose, rolled onto the bank, and carried the comb away with it. In not more time than it took for the comb to sink to the bottom, the surface of the water parted, and the huntsman s head emerged. He said nothing, only looking at his wife with sorrowful glances. That same instant a second wave rushed up and covered her husband s head. Then everything vanished. The millpond lay as peaceful as before, with only the face of the full moon shining on it.
IF Immunofluorescence
IHC Immunohistochemistry
Gold represents virtue, intelligence, superiority, heaven, worldly wealth, idolatry, revealed truth, marriage, and fruitfulness (Olderr 1986).Return to place in story.
LE Luminal epithelium
To detect the functions of CDO in uterus,CdoKO mice were generated by CRISPR/Cas9 technology. Two pairs of single guide RNAs (sgRNAs) (Table S1) targeting exon1 and exon3 ofCdogene respectively (Fig. 2 A), and SpCas9n(D10A) mRNA were co-injected into d 1 embryos (Additional file 1). ACdoKO mutant line with 7489 nucleotide deletion from the first to the third exon was used in the following experiments (Fig. 2 B). The genotypes were identified by PCR that the 245-base pair (bp) strand represent mutant type and the 325 bp strand represent wild type (Fig. 2 C). The knockout efficiency was identified by RT-qPCR, IHC and WB, and the results showed that CDO was totally deleted, at least in mouse uterus (Fig. 2D–F). As CDO is a key enzyme for taurine synthesis, we detected the changes of taurine levels afterCdoknocked out using HPLC. The results showed that the taurine concentrations in the liver, serum and uterus ofCdoKO mice decreased by 76.24%, 51.20% and 70.33%respectively than that of WT mice (Fig. 2G–I). These results demonstrate that CDO is successfully deleted from mouse genome and the lack of CDO leads to taurine deficiency.
P4Progesterone
Pgr(PR) Progesterone receptor
RIA Radioimmunoassay
Finally, the maintenance of taurine levels in uterus and serum relays on the endogenous taurine synthesis through the action of CSAD, CDO, and the active uptake from the diet [34, 35], whileCdodeletion impairs embryo implantation and causes severe subfertility as it is showed in this study. Whereas taurine supplementation significantly increases the litter size and parturition rate ofCdoKO females (Table S2). These provide the in vivo evidence that CDO and taurine are crucial factors for the maintenance and improvement of animal production,and suggest that taurine may be potential agent for animal production.
The needle had barely put in its last stitch when the girl, glancing at the window, spied the white plumed19 hat of the King s son who was being led back by the spindle with the golden thread
St Stromal cells
WB Western blotting
Supplementary Information
The online version contains supplementary material available at https:// doi.org/ 10. 1186/ s40104- 022- 00804-1.
Acknowledgements
This work is supported by the Natural Science Foundation of China (32130098)and the Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Authors’ contributions
Because, because the fish are too lazy! Maybe you could buy some such as Indian blue garra() 4 for 10 quit, they’ll 24 hours on duty and will little bit help you. I didn’t know the proper answer.
DZ, ZW and SC conceived and designed the research; DZ, ZW, GQ, YG and HG (Gao) performed experiments; DZ, ZW, XL and HZ (Guo) analyzed the results and processed data; DZ and SC interpreted results of experiments and drafted the manuscript. The author(s) read and approved the final manuscript.
Availability of data and materials
The datasets supporting the conclusions of this article are available from the corresponding author upon reasonable request.
Declarations
10. Husband: The father/husband s role in the tale is an interesting one. While the father is usually the birth father of the children, he has different levels of responsibility for the abandonment across versions of the tale. In some versions he willingly leaves the children in the forest. In other versions, he ineffectively protests their abandonment. The textual hint that the wife s wishes will win over the children s safety comes from the word choice of husband over father to describe the man s primary role.Return to place in story.
Ethics approval and consent to participate
Institutional Animal Care and Use Committee (IACUC) at the Yangzhou University approved the experimental protocol of this study.Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Received: 19 July 2022 Accepted: 20 November 2022

杂志排行
Journal of Animal Science and Biotechnology的其它文章
- Maintenance of gut microbiome stability for optimum intestinal health in pigs – a review
- Biological function of resveratrol and its application in animal production: a review
- Selective footprints and genes relevant to cold adaptation and other phenotypic traits are unscrambled in the genomes of divergently selected chicken breeds
- The impact of genotyping strategies and statistical models on accuracy of genomic prediction for survival in pigs
- RNA-seq analysis reveals the critical role of the novel lncRNA BIANCR in intramuscular adipogenesis through the ERK1/2 signaling pathway
- Origins, timing and introgression of domestic geese revealed by whole genome data
