Banking of perinatal mesenchymal stem/stromal cells for stem cellbased personalized medicine over lifetime: Matters arising
2023-05-25ChengHaiLiJingZhaoHongYanZhangBinWang
Cheng-Hai Li, Jing Zhao, Hong-Yan Zhang, Bin Wang
Cheng-Hai Li, Stem Cell Program of Clinical Research Center, People’s Hospital of Zhengzhou University and Henan Provincial People’s Hospital, Zhengzhou 450003, Henan Province,China
Jing Zhao, Department of Clinical Laboratory, People’s Hospital of Zhengzhou University and Henan Provincial People’s Hospital, Zhengzhou 450003, Henan Province, China
Hong-Yan Zhang, Department of Pharmacy, Fuwai Central China Cardiovascular Hospital,Zhengzhou 450000, Henan Province, China
Bin Wang, Department of Neurosurgery, People’s Hospital of Zhengzhou University and Henan Provincial People’s Hospital, Zhengzhou 450003, Henan Province, China
Abstract Mesenchymal stromal/stem cells (MSCs) are currently applied in regenerative medicine and tissue engineering. Numerous clinical studies have indicated that MSCs from different tissue sources can provide therapeutic benefits for patients.MSCs derived from either human adult or perinatal tissues have their own unique advantages in their medical practices. Usually, clinical studies are conducted by using of cultured MSCs after thawing or short-term cryopreserved-then-thawed MSCs prior to administration for the treatment of a wide range of diseases and medical disorders. Currently, cryogenically banking perinatal MSCs for potential personalized medicine for later use in lifetime has raised growing interest in China as well as in many other countries. Meanwhile, this has led to questions regarding the availability, stability, consistency, multipotency, and therapeutic efficiency of the potential perinatal MSC-derived therapeutic products after longterm cryostorage. This opinion review does not minimize any therapeutic benefit of perinatal MSCs in many diseases after short-term cryopreservation. This article mainly describes what is known about banking perinatal MSCs in China and,importantly, it is to recognize the limitation and uncertainty of the perinatal MSCs stored in cryobanks for stem cell medical treatments in whole life. This article also provides several recommendations for banking of perinatal MSCs for potentially future personalized medicine, albeit it is impossible to anticipate whether the donor will benefit from banked MSCs during her/his lifetime.
Key Words: Mesenchymal stromal/stem cells; Adult mesenchymal stromal/stem cells; Perinatal mesenchymal stromal/stem cells; Perinatal tissue; Stem cell bank; Personalized medicine
INTRODUCTION
Mesenchymal stromal/stem cells (MSCs) possess their unique properties that have attracted great attention in regenerative medicine and tissue engineering. Understanding of the biological properties of MSCs has been continued over a long period of time. Approximately 50 years ago, Fridenshteĭnet al[1]found a minor subpopulation of transplanted bone marrow (BM) cells cultured in the diffusion chambers that can act as osteogenic stem cells to show the differentiation of these cells towards osteogenesis. MSCs were initially identified in animal BM in a series of studies as fibroblast-like cells that were plastic-adherent and formed discrete fibroblast colonies[1-3]. Such fibroblast colony-forming cells were also shown to display high proliferative capacity and osteogenic potential[3]. Caplan first coined the name “mesenchymal stem cells” in 1991 on the basis of theirin vitrocapacity to give rise to bone, cartilage,etc.[4], but, to date, the stem cell properties of these cells remain controversial due to the multipotent uncertainty of MSCsin vivo. The essential features of MSC population have been identified as the minimal criteria by the International Society for Cell & Gene Therapy (ISCT) based on the specific phenotypic markers, adherence to plastic, and the potential of tri-lineage differentiation (adipogenesis,chondrogenesis, and osteogenesis)[5]. Given the unknown self-renewal and differentiation propertiesin vivo, later, Caplan[6] proposed to change the name of MSCs to more accurately reflect their main immunomodulatory and trophic potential not for multipotency of MSCs[7]. In 2019, to further consolidate and clarify the nomenclature of MSCs unless rigorous evidence for stemness exists, the ISCT MSC committee offers a position on the functional definition of mesenchymal stemvsstromal cells[8].
MSCs are well known to be isolated from various adult tissues including BM, adipose (AD) tissue,skeletal muscle, dental pulp, and blood[9,10], as well as vascularized tissues[11,12]. Given the immunomodulatory functions, paracrine capacity, and tropic aspects of MSCs, as well as the lacking of human leukocyte antigen (HLA)-DR, a major histocompatibility complex class II molecule, the potential therapeutic properties of MSCs in clinical trials are being explored for MSC-based regenerative medicine. Typically, MSCs are expandedin vitro, tested, cryopreserved, and banked for later use in preclinical and clinical studies[13-15]. Biobanking of MSCs from perinatal/neonatal tissues such as umbilical cord tissue, placental tissue, as well as placenta-associated amniotic fluid (AF) and amniotic membrane (AM) for potentially personalized medicine in the future has become more popular over the last few years in China. These newborn tissues would be routinely thrown away after birth. Importantly, these neonatal tissues are an abundant and easily available source of MSCs at birth. In this rapidly growing field, usually, stem cell banking companies involved in the stem cell industry in close collaboration with hospitals in China take over neonatal tissue collection, and preparation and cryobanking of the perinatal MSCs proposed for personal or family use for future stem cell therapies when a person develops a disease. Banking of perinatal MSCs has attracted renewed attention not only in China and probably in many other countries as well. However, serious concerns have been also raised pertaining to the maintenance of functionality and stability of stem cells along with therapeutic potential of MSCs at the time of release years. It is hard to predict the true likelihood of perinatal MSC transplantation later in whole lifetime and, noticeably, there is great uncertainty regarding whether or not these MSCs can be used to treat certain diseases after decades. As such, this opinion review article highlights several key observations in regard to the limitation of perinatal MSCs stored in stem cell cryobanks for later personal or family use in the future, which should be widely considered in the settings of cryostorage to minimize the possible side effects of these MSCs for future stem cell therapies.Importantly, this review provides several practical recommendations for banking of perinatal MSCs to better serve patients who might be desperately in need for potential personalized medicine for future stem cell-based treatments.
PERINATAL MSCS VS ADULT MSCS IN THEIR BIOLOGICAL AND THERAPEUTIC PROPERTIES
MSCs from adult and perinatal sources exhibit differentiated biological and therapeutic properties. The preclinical and clinical data reported in the literature are varied to this end. With a focus on whether the perinatal MSCs are worth biobanking, the pros and cons of perinatalvsadult MSCs need to be addressed in this section.
Compared to adult MSCs, the newborn stem cells obtained from perinatal tissues rarely carry any infectious diseases and reduce risk of exposure to environmental toxins. Perinatal MSCs have their own advantages such as easy availability, lacking stem cell variability, and comparably low risk of donor environmental effects, as well as immune privileged property. The diversity of MSC differentiation potential is also observed among MSCs from different source tissues. For example, Houet al[16]conducted an analysis of single-cell RNA-seq using MSCs derived from various tissues. By comparison,umbilical cord-derived MSCs (UC-MSCs), one of the most explored perinatal MSC types, and BMderived MSCs (BM-MSCs) exhibited the highest osteogenic potential, while AD-derived MSCs (ADMSCs) and BM-MSCs had the highest potential of adipogenesis and chondrogenesis, respectively[16].Meanwhile, UC-MSCs showed the highest immunosuppression as well as the highest stemness among all MSC samples in this study by Houet al[16]. Although human UC-MSCs and BM-MSCs exhibited similar immunosuppressive properties, the differences in immunomodulatory effects of UC-MSCs and BM-MSCs were also suggested in a previous study[17]. In contrast, BM-MSCs expressed more indolamine 2,3-dioxygenase (IDO) inin vitroinflammatory environment, while UC-MSCs expressed more prostaglandin E2, interleukine-6, and programmed death-ligands 1 and 2. In addition, there were more T helper 17 cells inhibited and more regulatory T cells induced by UC-MSCs compared with BMMSCs in co-culture[17]. In an experimental model of sepsis, BM-MSCs but not UC-MSCs were proposed to improve survival rate in septic mice due to the enhanced immunoregulatory properties through a variety of mechanisms[18]. In anin vitrostudy, UC-MSCs showed a higher angiogenic capacity in comparison with BM-MSCs and AT-MSCs[19]. Parket al[20] conducted anin vivostudy to test the angiogenic potential of perinatal chorion-derived MSCs (C-MSCs), which revealed that C-MSCs significantly increased the capillary formation in the C-MSCs injected myocardium compared to UCMSCs and BM-MSCs. Transplantation of UC-MSCs and BM-MSCs also showed similar therapeutic effects for type I diabetes in a mouse model as well as in treated patients with type I diabetes,respectively[21]. Compared to the mice with BM-MSCs treatment, the UC-MSCs treated mice had seemingly higher B-cell mass post-transplantation, although no significant difference was observed between the two treatment groups[21]. There was no difference in the therapeutic efficacy of BM-MSCs and UC-MSCs at one-year follow-up, albeit both types of MSCs decreased the levels of haemoglobin A1c and fasting and postprandial C-peptide[21].
Overall, the relevant preclinical and clinical studies to date by the use of adult and perinatal MSCs have yielded comparable results, which may contribute to a fuller understanding of their therapeutic potential in laboratory and clinical settings. The biological and therapeutic properties of both perinatal and adult MSCs are summarized in Table 1.
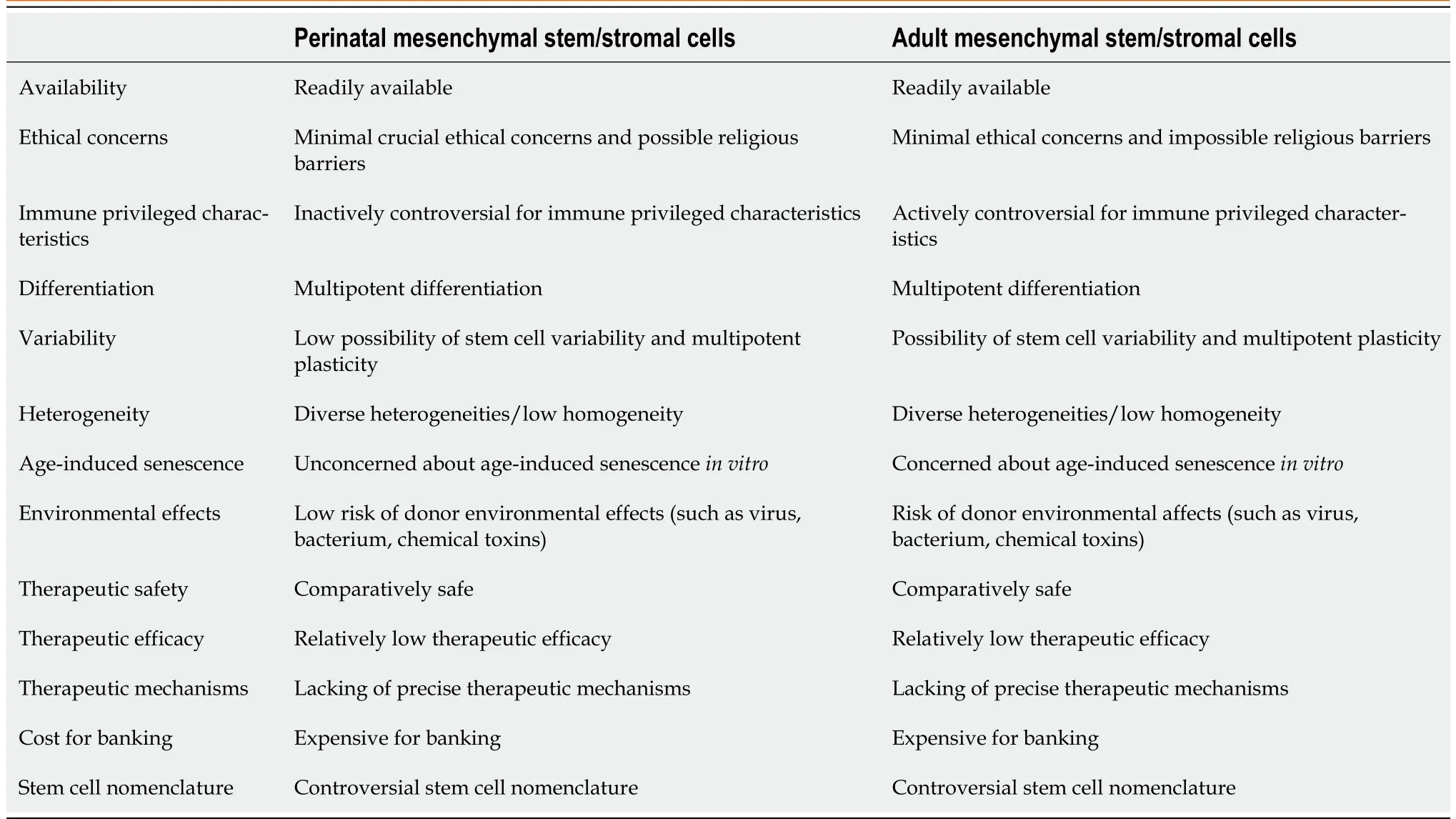
Table 1 Perinatal vs adult mesenchymal stem/stromal cells in their biological and therapeutic properties
PERINATAL MSC BANKING FOR PERSONALIZED MEDICINE OVER LIFETIME:CONCERNS AND UNCERTAINTIES
Over the past 20 years of MSC translational research, clinical experiences have shown that MSCs are seemingly unmet medical needs[22-24]. Translation of stem cell potential into medical practices still confronts many challenges. Clearly, these challenges include the long- and short-term therapeutic safety and efficacy of transplanted MSCs, the sufficient capability of homing and engraftment, the long-term cultivation associated alteration of MSC therapeutic properties, loss of stem cell potency with culture time, heterogeneous functions, and consistency and stability of MSCs or MSC-based therapeutic products. While clinical studies have shown the therapeutic benefits of using MSCs in various human diseases, including cardiomyopathy, autoimmune diseases, diabetes and diabetic complications, bone and cartilage repair, and fibrosis[9], rigorous clinical evidence for MSC therapeutics is still actually lacking. The positive, negative, or mixed clinical results have also been frequently observed in MSC clinical studies. Currently, MSCs based medicine still remains unproven and experimental. Therefore, it is possible to consider the limitation and uncertainty of perinatal MSC biobanking for personalized medicine in the context of the future therapeutic potential. Several important issues illustrated by the use of adult MSCs but not well discussed in perinatal MSCs are exemplified in this section to extend thediscussion regarding these relevant aspects of perinatal MSCs. The key issues have been identified to date, including the following.
Quality assurance before cryobanking for likely future personalized medicine
It is well known that functional properties and intrinsic multipotency of MSCs can be negatively affected by donor factors such as increased donor age, genetics, and health status. Banking of MSCs at their most potent state from perinatal tissues, the “best” cell source over one’s lifetime, has been supposed for future use in need of regenerative therapies. Certainly, cryobanking of perinatal MSCs is a prerequisite in personalized medicine strategy; however, the great uncertainty remains concerning the final function of perinatal MSCs, the accessibility of MSCs (e.g., a change in business circumstance), and therapeutic potential for the intractable diseases (e.g., cancer) decades later. Due to no expiration date of the perinatal MSCs cryopreserved in biobanks, exploiting and developing new approaches to testing cellular variability and stability, functionality, and heterogeneity during the processing of preparation and banking of the end MSC products should be considered carefully for cell quality assessed over longer time periods. The quality assurance programs should be performed to ensure the quality of stem cell products during the whole banking process including the perinatal tissue collection, processing,testing, preparation, and storage, as well as additional analysis (Figure 1). It should be also considered that there should be minimal levels of differentiation of perinatal MSCs in the course of the overall culture period to preserve stem cells at their most multipotent state for future use. New quality control to meet the product standards is required to be developed during the process of banking of perinatal MSCs. Except for routine multiple experiments for cell quality assessment (e.g., cell viability, proliferation, and differentiation potential), it is important to determine a panel of predicativein vitrotests for a system of quality assurance and these may be applied including but not limited to stem cell potency assay, spectral karyotyping, and genetic etiologies (Figure 1). Perinatal MSC potency assessment needs to be further validated for their therapeutic safety and effectiveness in the future. Specially, genetic etiologies associated with multi-factorial or monogenetic diseases may potentially influence stem cell safety. The wide analysis in identifying genetic/epigenetic etiologies is necessitated for therapeutic safety. For example, considering the importance of disease-associated individual single-nuleotide polymorphisms (SNPs), it is enabling to analyze and investigate whether stem cells carry diseaseassociated SNPs. Therefore, there is a critical need to further consider about whether it is worth biobanking of perinatal MSCs and whether it is to be stopped early if there is a family history of genetic conditions. Careful measuring and monitoring are extremely important before biobanking.
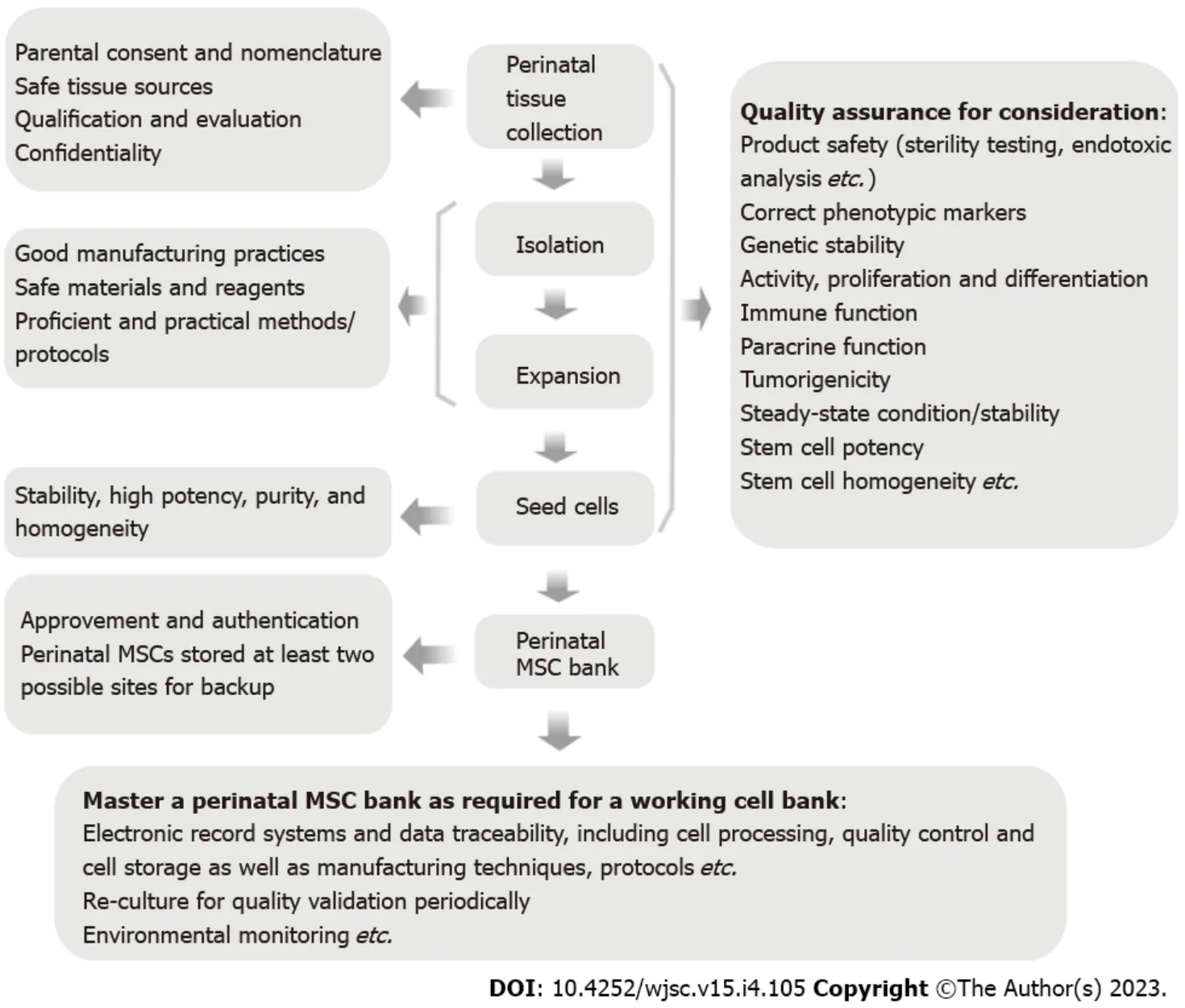
Figure 1 Operating model of banking perinatal mesenchymal stromal/stem cells.
Reconsidering of pretreatment of perinatal MSCs before cryobanking
There are more and morein vitroandin vivostudies on pretreatment of MSCs,e.g., pretreatment with cytokines or growth factors and hypoxia-priming, to improve their biological properties and therapeutic effectiveness. Acknowledging and understanding a beneficial role of pretreatment, the details given in this subsection describe the feature of pretreatment among MSCs from different source tissues including adult and perinatal sources, specifically, with the BM appearing the most common. This would be conducive to a better understanding of the stem cell therapeutic advantages and matters needing attention before banking of perinatal MSCs.
Biological properties could be altered by pre-conditioning of MSCs duringin vitroexpansion. Cell culture variables should be documented and, conventionally, growth medium containing fetal bovine serum and supplemented with basic fibroblast growth factor (bFGF) is considered to be the “gold standard” medium for primary human MSC expansion[25]. To remove animal components, serum-free medium throughout the process of cell preparation is being developed for clinical applications and MSC cryobanking. Usually, serum-free medium contains various cytokines/growth factors. Medium supplementation with bFGF is shown to increase the proliferation capacity of BM-MSCs over multiple passages[26]. Expansion is further improved in UC-MSCs by addition of bFGF in growth medium and the highest cell yield is detected in UC-MSCs among MSC lines pre-treated with bFGF including BMMSCs and AD-MSCs during the whole culture period[27]. Additionally, despite the increased expression of HLA-DR induced by bFGF in AD-MSCs, the addition of bFGF in culture media seems not to affect the expression levels of HLA-DR in UC-MSCs and BM-MSCs in the course of passages[27].Furthermore, the percentage of HLA-DR positive cells increases after inflammatory priming of all these three types of MSCs expanded in the bFGF-supplemented media[27]. As regards MSC differentiation,on the one hand, pretreatment with bFGF enhances BM-MSC chondrogenesis during chondrogenic pellet culture, resulting in upregulation of collagen type X and matrix metalloproteinase 13, the hypertrophic markers, in pellet cultures after 5 wk of endochondral ossification[26]. On the other hand,the supplementation with bFGF in medium has been also shown to have a negative effect on chondrogenesis of BM-MSCs[28,29] as well as their adipogenesis and osteogenesis[29,30]. Studies in laboratories demonstrate a time-dependent contribution of bFGF signaling to the reduced osteogenic/adipogenic differentiation of BM-MSCs throughout the culture[30,31]. For instance, the activity of alkaline phosphatase, a marker of osteogenesis, is significantly reduced during osteogenic differentiation in the case of addition of bFGF after passage 4 compared to the control at the same passage of MSCs and,similarly, adipogenesis potential is also significantly decreased by time during differentiation[30].
Pretreatment of MSCs has been observed to enhance the biological properties of the frozen and thawed MSCs. For example, one study reports that there are more apoptotic cells in the post-thawed cryopreserved BM-MSCs than the live BM-MSCs in active culture[32]. These frozen-thawed BM-MSCs are susceptible to complement-mediated lysis[33] and T-cell mediated apoptosis[34]. Interestingly,frozen-thawed MSCs primed by interferon-gamma (IFN-γ) in the culture medium for 48 h prior to cryopreservation may partly avoid the lysis by activated T cells[34]. The mechanism of MSC-mediated immunosuppression has been previously proposed as IDO expression in BM-MSCs[35] as well as UCMSCs[36] induced by IFN-γ. However, while pre-licensing MSCs with IFN-γ have been seen to enhance their IDO expressionin vitro[37,38], the pre-licensed MSCs by IFN-γ, compared to the unstimulated MSCs, have let to the loss of their effectiveness in rescuing retinal ganglion cells in a retinal ischemia/reperfusion injury mouse model[37]. Together, the results of pretreatment with cytokines including but not limited to bFGF and IFN-γ indicate the different effects on the biological properties of MSCs depending on their existing microenvironment (e.g., inflammation) or the specific pathological contexts.
Apart from pretreatment with cytokines, other approaches can also be proposed to boost the therapeutic potential of MSCs, for example, microenvironmental hypoxia-primed MSCs[39]. One previous study shows that, compared to standard 21% O2tension, 3% O2can increase clonogenic potential,in vitromigration, and stemness of MSCs from the Wharton’s jelly (WJ) of human umbilical cord (WJ-MSCs)[40]. Another previous study indicates that umbilical cord blood (UCB)-derived MSCs(UCB-MSCs) primed with hypoxia and calcium ions exhibit improved self-renewal, migratory, antiinflammatory and immunomodulatory capacities, resulting in further improving therapeutic potential for graftvshost disease in an animal model[41].
As mentioned above, in theory, pretreatment of MSCs from adult or perinatal sources with cytokines can improve their biological and functional properties. However, previousin vitroandin vivostudies could not prove the therapeutic efficacy of the freshly cultured or post-thawed MSCs with cytokine pretreatment. Microenvironment conditions (e.g., hypoxia) have become a promising strategy prior to cryopreservation, which is possible for the enhancement of MSC-based therapeutic potential.In vitroandin vivostudies indicate that pretreatment strategy may benefit patients in a disease specific context.However, without knowing the context of a specific pathology, it is not possible to assess and predict the function of perinatal MSCs pretreated with cytokines for stem cell medical treatments after many years. Therefore, whether pretreatment of perinatal MSCs should become the standardization for biobanking for their future applications as stem cell-based treatments remains to be further investigated.
Pre-selection for biobanking to decrease the heterogeneity of perinatal MSCs
As known, there are diverse heterogeneities of MSCs including inter-donor/individual, inter-tissue/source, inter-cellular, and inter-colony heterogeneities involved in the manufacturing and biobanking of MSCs. MSC heterogeneity reflects the diversity of MSC-associated environments or niches. The present paper extends the relevant discussion regarding the pre-selection required prior to banking with the aim to control product consistency for a stem cell bank for maximizing the “homogeneity” of perinatal MSCs. As well known, MSCs including adult and perinatal MSCs derived different tissues differ in their functional characteristics. Wegmeyeret al[42] conducted one previous study to evaluate the source- and donor-dependent differential stem cell properties and found UC-MSCs, AM-derived MSCs (AM-MSCs),and BM-MSCs exhibited comparable differences between each other. Interestingly, UC-MSCs and AMMSCs exhibited different growth characteristics and morphologies as well as high inter-donor variability of AM-MSCs but not UC-MSCs. In contrast, another previous report indicated that,compared to UCB-MSCs, placental tissue MSCs, and WJ-MSCs, umbilical cord lining-derived MSCs showed the highest proliferation and migration rates and prolonged survival in immunodeficient mice[43]. Concerning immunosuppressive properties, in contrast to placenta MSCs, both UC- and BM-MSCs significantly reduced the proliferation of activated CD4+ and CD8+ T cells[44]. Additionally, the heterogeneous proangiogenic properties of perinatal and adult MSCs were observed in one previous study,which showed that both BM-MSCs and placental chorionic villi MSCs had significant proangiogenic activities on endothelial cells in matrigelin vitrocompared to AD-MSCs and UC-MSCs[45]. Importantly,compared to dental pulp MSCs and AD-MSCs, WJ-MSCs showed the strongest therapeutic efficacy in reducing fasting glucose levels in type II diabetic mice in one previousin vivostudy[46].
As mentioned above, due to inconsistency of MSCs, different perinatal MSCs populations may have heterogeneous functional properties, consequently leading to different therapeutic efficiency. Therefore,a pre-selection may be required before stem cell banking to identify relatively homogeneous perinatal MSCs for potential later use in life. As known, pretreatment with cytokines before banking may be a suitable approach to support therapeutic potential of perinatal MSCs in the future as exemplified by IFN-γ pre-licensing. Peltzeret al[47] conducted a previous study to create anin vitro“tolerogenic” niche priming mimicking placental environment, which showed that primed perinatal MSCs (UC-, UCB-,AM-, and chorionic-MSCs) with IFN-γ could enhance their immunomodulatory potential in a dose- and donor-dependant manner. This study by Peltzeret al[47] may suggest that an approach to screen the large number of perinatal MSCs with cytokine priming will be beneficial for the consistency of banking MSCs. Sorting of perinatal MSCs may be another effective approach for pre-selection, for example, preselection of MSC subpopulation using surface markers to increase the purity of the expanded MSCs. A series of markers need to be considered in sorting of perinatal MSCs, including cell surface markers related to MSC clonogenicity, potency, differentiation, and immunomodulatory properties[48].Practically, a few conventional approaches to increasing the consistency of perinatal MSCs may be also considered, such as the use of the single colony of MSCs and specific biomaterials (see below), and the handing of MSCs by the standardized protocols for manufacturing consistency.
Biomaterial scaffold approach to maintain the maximal biological properties of perinatal MSCs
MSCs within the body have their own physical microenvironments or niches to maintain their unique biological properties. In order to mimic a specific stem cell niche, various biomaterials such as alginate,chitosan, collagen hyaluronic acid, silica gel, silk fibroin, poly(lactic acid), and graphene-base materials are being explored for use as scaffolds/surfaces supporting the growth and differentiation of MSCsin vitroandin vivo. By comparing the conventional techniques with monolayer culture of MSCs,biomaterial scaffold approach such as cell surface modification and encapsulation technology for tissue engineering exhibits improved MSC survival and proliferation, increased differentiation potential, and maintenance of stem cell stemness[49,50]. Further studies indicate that biomaterials can enhance the paracrine function of MSCs[51]. While technical challenges for development of biomaterials remain the degradability, viscoelasticity/elasticity, architecture property, and compatibility, the non-toxic 3D porous biomaterials are already widely used for delivery of encapsulated UC-MSCs or BM-MSCs in preclinical studies[52,53] as well as in clinical trials[54,55]. Interestingly, cryopreservation of microencapsulated stem cells in alginate hydrogel has been reported to suppress ice formation that contributes to the effect of cryoprotection[56]. Importantly, there are no significant differences in cell viability and multi-lineage differentiation potential between the MSCs post cryopreservation either encapsulated or without encapsulation[56]. Similar studies have been also conducted with MSCs cryopreserved in degradable hyaluronic-acid based hydrogel[57]. In addition, intravenous injection of freeze-thawed mouse MSC encapsulates with microgel into recipient mice shows similar levels of cell survival as fresh non-cryopreserved MSC encapsulates[58]. As mentioned above, previous and current studies are instructive to note that using special biomaterial scaffold may be considered as a suitable new strategy for banking of MSCs from different source tissues including but not limited to perinatal tissues.
Dimethyl sulfoxide and dimethyl sulfoxide-free cryoprotectants
Dimethyl sulfoxide (DMSO), as a key cryopreservation agent, is most often used in the cryostorage to protect cells from mechanical and osmotic stress due to the formation of ice crystals[59]. DMSO is also observed to have significant influence on the viability, phenotype, and proliferation of MSCs, as well as cellular epigenome, and to induce changes in cellular processes[60,61]. As such, low concentrations of DMSO and DMSO with combinations of non-cytotoxic biocompatible agents/substances are being explored for MSC culture and cryopreservation in preclinical and clinical studies. For example, one previous study demonstrated that human BM-MSCs modified to express tumor necrosis factor-related apoptosis inducing ligand were cryopreserved in a low concentration (5%) of DMSO (accepted as nontoxic concentrations of below 10%) with 95% human serum albumin without affecting their biological properties[62]. However, a question may be raised as to whether DMSO at a very low concentration may not be sufficient to prevent freezing damage to MSCs.
Currently, there are different types of effective and non-toxic cryoprotective agents/compounds used as suitable replacements for DMSO, such as glycerol, hydroxyethyl starch, trehalose, and dextran[59,61]. One study reports that DMSO-free cryopreservation solutions composed of sugars, sugar alcohols,and small-molecule additives have been showed to retain MSC post-thaw viability, cell surface markers,and proliferation and differentiation potential[60]. The osmolyte-based freeze solutions also exhibit a more normal alignment of the actin cytoskeleton of MSCs compared to DMSO frozen cells[60]. A nontoxic cryoprotective agent, a combination of trehalose and glycerol, has been tested in another study for cryopreservation of AD-derived stem cells, and the cells cryopreserved with this cryoprotective agent presented high cell viability and proliferation and migration capacity after thawing[63]. Overall, while various studies are being devoted to possible improvement of DMSO as a cryoprotectant, some alternatives to DMSO are being evaluated as cryopretectants for cryopreservation of MSCs from different tissue sources including perinatal tissues. DMSO together with alternatives to DMSO have not been tested enough to advocate their use for biobanking of MSCs stored over many years. Therefore, the use of DMSO alone as a cryopreservation agent may not insure the final functional properties of MSCs for stem cell-based treatments after many years and the new freezing solutions need to be intensively investigated for long-term therapeutic stem cell cryopreservation.
Ethical and regulatory concerns
Banking stem cells using neonatal birth-associated tissues or other related sources began with the establishment of banking cord blood for hematopoietic cell transplantation in the early 1990s[64]. Given that perinatal MSCs have the positive characteristics of both embryonic stem cells and adult stem cells,the ethical issue involvement may be related to the use of embryonic stem cells. In particular, there are further ethical and regulatory issues that will challenge banking of perinatal MSCs. In China, currently,there are so many exaggerate advertisements directly to consumers on banking of life-saving perinatal stem cells to insure infants or family members against serious illnesses in the future. Parents are encouraged to make decision for the preservation of their children’ stem cells in a private stem cell bank for the future health of their children. Indeed, the current applications of MSCs as stem cell medical treatments are sometimes also exaggerated by the social media. Parents or requestors need to pay thousands of dollars for the commercial banks of perinatal MSCs as well as an annual fee for the maintenance of stem cells. Therefore, physicians and clinical investigators in hospitals who have financial conflicts of interest associated with stem cell banking should disclose any financial relationship. Currently, there is a lack of clarity in country-level regulations in China for the management of stem cell banking and the guidance document for quality control on the banking of MSCs. Accordingly, technical and ethical guidelines are imperative for the appropriate governance and restriction of banking perinatal MSCs to insure standardization for MSCs with good quality for future stem cell therapies. Technical and ethical guidelines should also be in line with the international standardized regimens such as guidelines from the International Society for Stem Cell Research and the International Stem Cell Banking Initiative. Lastly, the most established private banks of perinatal MSCs in China, previously engaged in cord blood storage, are paid by the parents and these banks store the source of stem cells for future use by the donor and, probably, the donor’s relatives.
Correspondingly, an additional challenge is whether the source of MSCs with potential applications in the future can be shared by the donor-unrelated recipients. In this regard, many public banks that store the perinatal MSCs derived from the donated newborn tissues should be established free charge of anything for banking. In accordance, these banks are open for research and they store stem cells for all recipients in future personalized medicine including the donors, the donor’s families, and those unrelated with donors when they might be desperately in need due to life-threatening diseases. Due to no data available for the use of public banks of perinatal MSCs in China, clinical applications of cord blood stem cells from the banks are illustrated for the perspective in the future. One representative example is that the public cord blood banks have released at least 30 times for the usage rate of previously cryopreserved cord blood stem cells as compared to the private banks worldwide[65].Almost over the past 30 years, cord blood banks have been successfully achieved for safe storage and rapid availability for cord blood stem cell-based treatments[66]. Another example is that, in a cord blood bank (MEDIPOST Co., Ltd., Seongnam-si, Gyeonggi-do, Korea), cell populations were evaluated and,among 557 UCB units, 128 units cryopreserved for more than 10 years were used for transplantation[67]. Therefore, the useful value of public or private stem cell banks to society or the donors is important for the stem cell medicine potential in the future.
Reculturing post-thawed cryopreserved perinatal MSCs from stem cell banks at the release time
Usually, the freshly cultured perinatal MSCs are seemingly to be more potent than freshly thawed cells in therapeutic properties of MSCs. Some studies suggest that perinatal MSCs may lose their functionality with cryopreservation, which could in turn affect the efficacy. Whether the characteristics of thawed perinatal MSCsvscultured MSCs would be changed needs to be further discussed here. One previous study showed that freshly cultured UC-MSCs were superior to cryopreserved and thawed UCMSCs in regard to cell viability[68]. Another previous study demonstrated that osteogenic and chondrogenic capacities were slightly reduced in cryopreserved human UC-MSCs for one year,compared to non-cryopreserved UC-MSCs from the same donor[69]. In contrast, one previous study showed that short-term cryopreservation and subsequent thawing of UC-MSCs could not alter the specific MSC surface markers and the proliferation capacity[70]. There was no obvious difference in fibroblast-like morphology and cellular viabilities between freshly frozen thawed and culture-rescued UC-MSCs[71,72]. Frozen thawed and culture-rescued UC-MSCs also displayed similar osteogenesis,chondrogenesis, and adipogenesis[71]. Similarly, one previous study by Narakornsaket al[73] to compare the post-thawed human AF MSCs (AF-MSCs) after one-month cryopreservation to the nonfrozen AF-MSCs showed no statistical differences between these cells in MSC surface markers, cell proliferation, chondrogenic differentiation, and immune privileged properties. Still, one previous study to analyze immunomodulatory activityin vitroindicated no significant differences in the suppression of activated T cells in the freshly thawed UC-MSCsvsthe freshly cultured UC-MSCs[70]. Comparatively,there were different observations reported by assessing the potential in immunosuppression between freshly thawed and cultured UC-MSCs[71], as showed the freshly culture-rescued UC-MSCs to be more potent in immunosuppression than the frozen-thawed UC-MSCs.
In animal experiments, immunomodulatory activity in an adjuvant-induced arthritis rat model and angiogenic potential using a mouse model for hindlimb ischemia were observed andin vivostudies showed no significant difference in immunomodulatory and angiogenic potential of freshly thawed and freshly cultured UC-MSCs[70]. Treatment with the freshly thawed UC-MSCs improved the regeneration of rotator cuff tendon in an animal model after injection at 2 and 4 wk, respectively, which was comparable to the therapeutic potential for tendon regeneration by the use of the culture-rescued UCMSCs[72]. In a ventilator-induced lung injury animal model, the fresh UC-MSCs as well as the fresh BM-MSCs were found to enhance injury resolution and repair, while the cryopreserved UC-MSCs were comparably effective[74]. As mentioned above, these experimental and preclinical data were provided to support short-term cryopreserved perinatal MSCs (e.g., UC-MSCs) for stem cell-based applications in translational medicine. However, long-term cryopreserved perinatal MSCs need to be further explored to achieve preclinical and clinical safety and efficiency after intervention. Accordingly, it is advisable to continuously culture post-thawed perinatal MSCs for at least an additional passage at the release time for restoration of stem cell biological properties and, meanwhile, it is also required to conduct extra assessment of safety and therapeutic effectiveness.
Short-term but not long-term cryopreservation of perinatal tissues for MSC preparation
Last but not least, a key issue has also been identified to date: Instead of banking perinatal MSCs, shotterm cryopreservation of perinatal tissues (e.g., umbilical cord tissue) potentially for perinatal MSC storage for future stem cell-based treatments. Freezing of umbilical cords that yield post-thaw MSCs with high scalability is still controversial. One previous study reported that the frozen umbilical cord tissues for at least 2 wk were thawed and minced to generate a large number of MSCs byin vitroexpansion[75]. UC-MSCs from cryopreserved and thawed umbilical cord tissues exhibited characteristics such as cellular phenotypes, immunosuppressive properties, and differentiation potentials similar to those of UC-MSCs from fresh tissues[75]. Another previous study reported that the fresh WJ cryopreserved for 30 d in liquid nitrogen can yield large numbers of post-thaw MSCs[76]. However, one previous study reported that although the cryopreserved umbilical cord tissue segments were thawed 5 years later to release MSCs after enzymatic digestion, those displayed poor recoveries and produced few viable UC-MSCs compared with fresh cord tissue samples[77]. Still, one previous study to assess the optimal isolation culture and cryopreservation methods to facilitate cord WJ-MSCs banking failed as no MSC cultures were obtained from the thawed cord WJ samples stored for 1 wk, 1 mo, or 6 mo in liquid nitrogen, respectively[78]. In addition, one study showed that cryopreservation of umbilical cord tissues for at least 1 mo in liquid nitrogen did not prevent the later collection of UC-MSCs by the explant method[79]. Currently, transplantation of UC-MSCs from fresh and short-term cryopreserved-thenthawed cord tissues is an issue of increasing interest. Similar to cord blood banks, perinatal umbilical cord tissue cryobanks are present in many other countries including United States, United Kingdom,Australia, and South Africa[80]. As a whole, given that cryopreservation cannot prevent the high cord quality, long-term restoration of perinatal umbilical cord tissue in banks may be unadvisable for potentially later use for post-thaw MSC preparation.
Collectively, when considering subsequent events, there are many limitations and uncertainties for stem cell medical treatments using perinatal MSCs banked over many years. Although most (if not all)aforementioned key issues, as summarized in Figure 2, are not specific to perinatal MSCs, such as cell quality insurance, heterogeneity, genetic etiologies, supplementation of scaffold, and use of cryoprotectants, these should be considered for the application of biobanking of perinatal MSCs. Research and development of different types of stem cell-based medication must be viewed with a forward-looking perspective. Importantly, when contemplating these issues including biological and technical concerns,this is a question as to whether any specific types of perinatal MSCs are worth biobanking. Without knowing the specific clinical applications, it is impossible to develop (e.g., potency assay), and without a potency assay, it is not possible to assess the quality of perinatal MSCs stored by cryopreservation over many years. The question will always be raised as to what functions perinatal MSCs banked over many years can still actually play for the possible applications in their clinical practices. Realizing that shortterm banking and restoration of perinatal MSCs is being routinely applied particularly in regenerative medicine and tissue engineering, long-term banking of perinatal MSCs as well as perinatal tissues for stem cell medical treatments within the lifetime should not be encouraged.
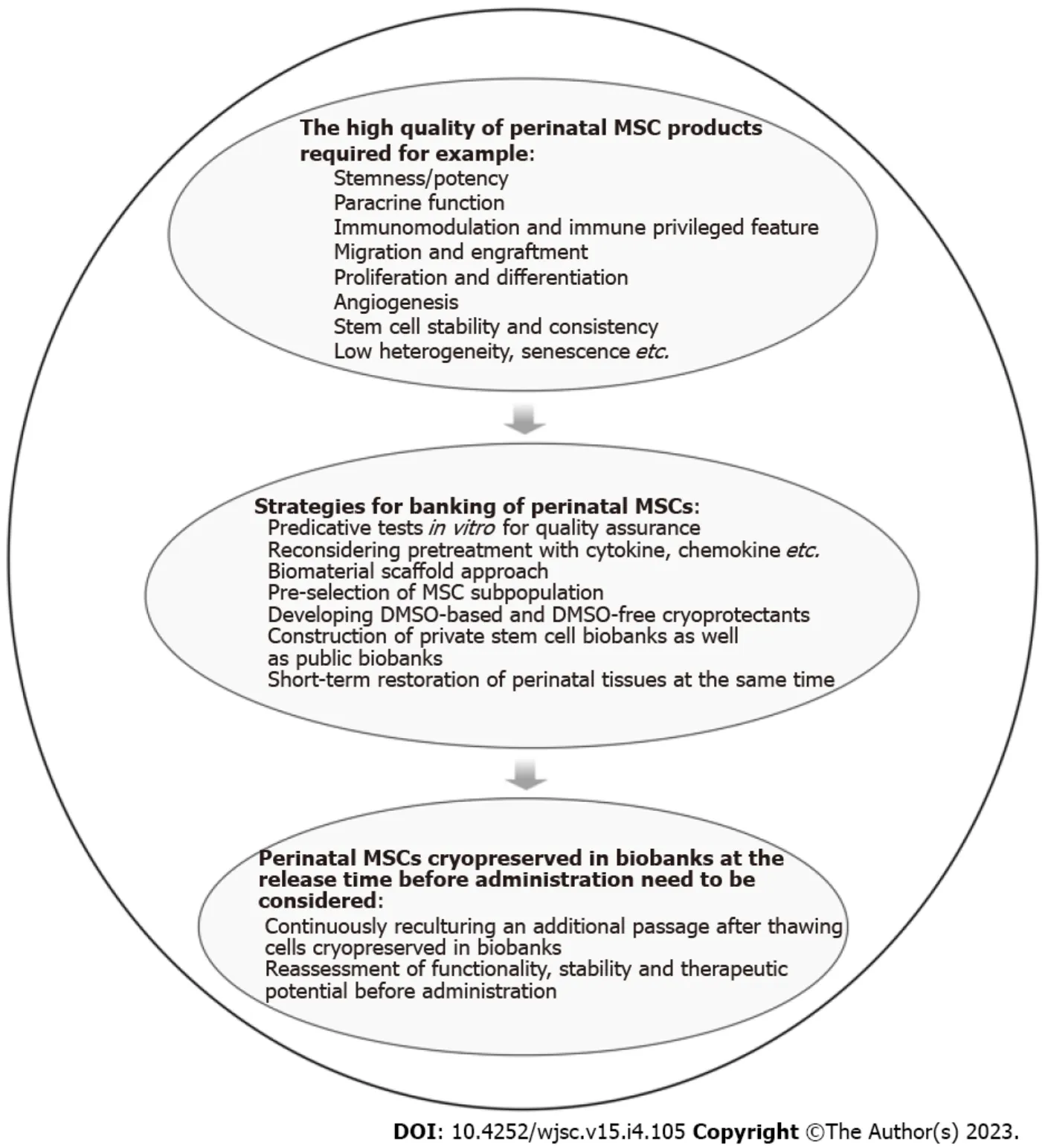
Figure 2 Strategies and administrative requirements for high-quality stem cell banks.
DISCUSSION
This opinion review focuses on several important points of view that are currently attracting people’s attention. The review article does not diminish the clinical translational perspective of MSCs from either adult or neonatal tissues. Given the current situation of banking perinatal MSCs, the aim of this article is to contribute a balanced, comprehensive, and critical view in the settings of cryobanking of perinatal MSCs to probably maximize their potentially therapeutic activity in the future. As described above,there is no expiration date of cryostorage of the perinatal MSCs and it is not known about future timeline of stored perinatal MSCs for potential therapeutic applications. Therefore, the key issues have been identified in this article and, on the one hand, it is possible that the stored perinatal MSCs would be used for potential personalized medicine for a child when he/she develops a disease later on. On the other hand, it is too remote to assess the therapeutic benefit in the next decades. For example, by the time when a baby reaches the age of 50, no one knows whether these cells could be accessible and useful for personalized medicine at that time because there is a potential change for therapeutic benefit of perinatal MSCs. Although the short-term cryopreserved-then-thawed perinatal MSCs or the recultured MSCs are being widely used in pre-clinical and clinical studies, there are no data provided to support applications of long-term low-temperature storage of perinatal MSCs in banks,e.g.,cryopreservation of MSCs over a period of more than 10 years. Furthermore, it is not clear about how much the advanced techniques in science would be developed, for example, genome editing and precision medicine technologies to provide the best tools, probably, as opposed to MSC-based therapies looking forward 50 years.Science still drives the development of the advanced techniques and, eventually, the emerging advanced technologies are likely to influence the direction of therapeutic strategies in MSC-based translational medicine.
To date, banking perinatal stem cells and stem cell-derived newborn tissues have raised growing interest potentially for future stem cell applications. Due to less information available in scientifically understanding stem cell biologyin vivofrom the transplanted patients, such as stem cell proliferation,differentiation, immunomodulation, homing, and fate of MSCs, the true function and the precise mechanisms of the therapeutic benefit remain largely unclear. More importantly, given the unknown context of a specific pathology in the future, it should be noted as to whether perinatal MSCs are worth biobanking for a long period of time to achieve clinical efficiency. People believe that banking of perinatal MSCs may serve patients well one day in the foreseeable future, ideally for several years,when they may really need for the personalized medicine. But instead, the great uncertainties remain for potential use of the cryopreserved perinatal MSCs for stem cell-based treatments in the unforeseeable future. Acknowledging the limitations and uncertainties of banking perinatal MSCs for future potential personalized medicine, the following key recommendations should be addressed and the most of the recommendations proposed do not apply to the perinatal MSCs only.
For the sake of therapeutic safety and effectiveness in the future, a series of predicative testsin vitrofor quality assurance as well as the additional analysis of genetic/epigenetic etiologies should be considered before cryobanking of perinatal MSCs. The novel strategic approaches, for example,biomaterial scaffold techniques, should be encouraged to use for maintaining the maximal biological and functional properties of perinatal MSCs in the routine banking practices.
Emerging practicable technologies would also be applied to yield a number of the desired stem cell types,i.e.,homogeneous and consistent perinatal MSCs, and, consequently, it is essential to develop novel biological technologies with a high yield of stem cells for cryobanking. The appropriate governance is required and banking of the perinatal MSC-based therapeutic products should comply with accreditation standards and the international standardized guidelines.
The public banks are to be built as a priority to better serve all recipients who might be desperately in need in the future and, therefore, the roles played by the public banks should not be underestimated.Lastly, instead of banking perinatal MSCs, short-term but not for long-term restoration of perinatal tissues, for instance umbilical cord tissue, may be suggestive of a possible approach of cell preparation for stem cell-based medicine.
CONCLUSION
MSCs can provide therapeutic benefit given their unique biological characteristics, but, to date, there is still much to learn about stem cell science and medicine. MSCs derived from diverse tissues have their different functional properties and perinatal MSCs have their own advantages. Therapeutic properties of perinatal MSCs have been shown by multiple preclinical and clinical studies; however, banking of perinatal MSCs for personalized medicine in whole life remains to be unforeseeable. Therefore, it is extremely important for parents, physicians, and clinical investigators to be aware that there are limitations and uncertainties of banking perinatal MSCs for the future personalized medicine. Based on the above consideration, this opinion review article is conducted to address the concerns raised and provides several practical recommendations for banking perinatal MSCs for future potential personalized medicine. Accordingly, different strategic approaches can be employed in this rapidly growing field to improve this process, making perinatal MSCs available for future stem cell based therapies when needed to avoid banking poor-quality MSCs discarded as novel medical waste products in the future.
FOOTNOTES
Author contributions:Li CH, Zhao J, and Zhang HY contributed equally to this work; and all authors contributed to conception, design, data analysis and interpretation, drafting, critical revision, and approval of the final version of the article.Supported bythe Henan Province Science and Technique Bureau R&D Project, No. 222102310228.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORCID number:Cheng-Hai Li 0000-0002-1922-2784; Jing Zhao 0000-0002-7962-1656; Hong-Yan Zhang 0000-0001-5424-050X; Bin Wang 0000-0002-6816-2212.
S-Editor:Wang JJ
L-Editor:Wang TQ
P-Editor:Wang JJ
杂志排行
World Journal of Stem Cells的其它文章
- Obesity and cancer stem cells: Roles in cancer initiation,progression and therapy resistance
- Clinical application prospects and transformation value of dental follicle stem cells in oral and neurological diseases
- Current status and prospects of basic research and clinical application of mesenchymal stem cells in acute respiratory distress syndrome
- Extracellular vesicles: Emerged as a promising strategy for regenerative medicine
- Human pluripotent stem cell-derived B cells: Truly immature islet B cells for type 1 diabetes therapy?
- Mechanisms of analgesic effect of mesenchymal stem cells in osteoarthritis pain
