环境相关浓度双酚AF暴露对成年雌性弓背青鳉生长和生殖的毒性效应
2023-05-23黄泽胤谢明花陈月碧黎学友郭昱嵩王中铎董忠典
黄泽胤 谢明花 陈月碧 黎学友 郭昱嵩 王中铎 董忠典



摘要 以成年雌性弓背青鳉(Oryzias curvinotus)为研究对象,研究了不同环境相关浓度(0.93、9.33和102.33 μg/L)双酚AF(BPAF)短期暴露对其生存、生长和生殖的影响。结果表明,102.33 μg/L BPAF暴露21 d极显著降低了成年雌性弓背青鳉的存活率和生长状况因子;9.33和102.33 μg/L BPAF暴露导致成年雌性弓背青鳉肝细胞排列松散、脂肪积累减少,102.33 μg/L BPAF暴露还导致肝细胞形态出现明显肿胀;102.33 μg/L BPAF暴露导致其卵母细胞大多停留在初级卵母细胞阶段,抑制了卵母细胞的成熟。另外,102.33 μg/L BPAF暴露显著或极显著上调了vtglike、vtg1、vtg2和chgh在肝脏中的转录水平以及cyp11a、cyp19a1a和gsdf在卵巢中的转录水平。不同环境相关浓度的BPAF暴露对成年弓背青鳉雌鱼有较强的致死效应、生长抑制效应、雌激素效应,并可引起肝损伤和卵母细胞发生障碍。
关键词弓背青鳉;内分泌干扰物;双酚AF;雌激素效应;生殖毒性
中图分类号X174文献标识码A
文章编号0517-6611(2023)08-0079-07
doi:10.3969/j.issn.0517-6611.2023.08.019开放科学(资源服务)标识码(OSID):
Toxic Effects of Bisphenol AF Exposure at Environmental Concentrations on the Growth and Reproduction of Adult Female Oryzias curvinotus
HUANG Ze-yin,XIE Ming-hua,CHEN Yue-bi et al(Key Laboratory of Aquaculture in South China Sea for Aquatic Economic Animal of Guangdong Higher Education Institutes/ Fisheries College of Guangdong Ocean University,Zhanjiang,Guangdong 524088)
AbstractAdult female Oryzias curvinotus were used as research objects to study the effects of short-term exposure with BPAF at different environmental concentrations (0.93,9.33 and 102.33 μg/L) on the survival, growth and reproduction of O.curvinotus.The results showed that the survival rate and condition factor of adult female O.curvinotus were extremely significantly reduced by the exposure with 102.33 μg/L BPAF for 21 days.Exposure with 9.33 and 102.33 μg/L BPAF resulted in loose arrangement of hepatocytes and the reduction of fat accumulation in adult female O.curvinotus.Exposure with 102.33 μg/L BPAF caused significant swelling of liver cell morphology.102.33 μg/L BPAF caused oocytes to stay in the primary oocyte stage and inhibited the maturation of oocytes.102.33 μg/L BPAF significantly or extremely significantly up-regulated the transcript levels of vtglike,vtg1,vtg2 and chgh in the liver,and the transcript levels of cyp11a,cyp19a1a and gsdf in the ovary.Exposure with environmental concentrations of BPAF had strong lethal effects,growth inhibitory effects,and estrogen effects on adult female O.curvinotus,and could cause liver damage and oocyte dysfunction.
Key wordsOryzias curvinotus;Endocrine disruptor;Bisphenol AF;Estrogen effects;Reproductive toxicity
内分泌干扰物(endocrine disrupting chemicals,EDCs)污染已被列为继臭氧层空洞和地球变暖之后迫切需要治理的环境污染问题。双酚A(bisphenol A,BPA)是最常见的內分泌干扰物,具有类雌激素效应、神经毒性、遗传毒性和生殖毒性等[1-4],目前已被多个地区和行业禁用[5-6]。双酚AF(bisphenol AF,BPAF)又名六氟双酚A,是一种新型的含氟双酚类物质,也是BPA的主要替代物之一。它在工业上主要用作氟橡胶硫化促进剂,也可用于电子产品、光纤、食品包装盒加工、医药中间体等[7]。随着BPAF用量的增加,在产品的制造、消费和废弃过程中BPAF不可避免会释放到各类环境介质中[6,8-9]。
水体作为容纳BPAF的主要介质,近年来水体中BPAF含量呈现增加的趋势[9-13]。德国地表水中BPAF的浓度为0.1~180.0 μg/L[13];嘉兴市BPAF工厂附近河水中BPAF浓度高达15.3 μg/L[14]。2006—2007年,美国74个污水处理厂污水污泥样品中BPAF检出率为46%,干重1.79~72.20 μg/kg[15]。目前饮用水中BPAF的相关报道较少,但BPAF在水源和饮用水样本中的检出率仅次于BPA[16-17]。Mandrah等[18]在瓶装碳酸饮料样品中也检测到BPAF。
研究表明,BPAF具有内分泌干扰效应、生殖毒性、生长和发育毒性以及神经毒性[19-26]。尽管现阶段水环境中BPAF含量较低,但BPAF具有半衰期长、易富集、难降解等特性,同时由于其CF3基团具有更高的电负性和活性,即使低剂量的BPAF也可能具有更高的结合激素受体潜力,严重威胁水生生物的健康[27]。污染物的毒性受水体理化因子的影响,同一种污染物在不同盐度、温度、pH等环境条件下可能具有不同的毒性,此外不同的物种对相同污染物的敏感度也可能存在差异[28-29]。目前对BPAF的水生生态毒理研究主要集中在斑马鱼上[24-26,30-31]。若要准确评估BPAF的水生生态风险,有必要开展BPAF暴露对不同种类水生生物的生态毒性效应研究。
弓背青鳉(Oryzias curvinotus)体型小、适盐范围广、易于实验室养殖、世代周期短、产卵量大、胚胎发育透明且遗传性别明确,是理想的海洋环境监测模型[32-34]。研究环境相关浓度BPAF对弓背青鳉生长和生殖的影响,有助于阐明BPAF对鱼类的内分泌干扰效应机制,也为确定水体中BPAF的安全浓度提供支持。笔者以雌性弓背青鳉成鱼为研究对象,使用不同环境浓度的BPAF对其进行短期暴露,从个体、组织、细胞、分子水平研究BPAF暴露对弓背青鳉生存、生长、组织结构及繁殖相关基因转录表达的影响,旨在为全面评估BPAF的生态风险提供科学依据。
1材料与方法
1.1试验材料试验所用弓背青鳉为南海水产经济动物增养殖重点实验室繁育的高桥群体F代成年雌鱼(10月龄),试验水体为经过曝气的自来水。养殖水温为(26 ± 1)℃,光暗周期为14 h∶10 h,pH 7.2~8.4,每天投喂新鲜孵化的丰年虫(Artemia salina)2次。BPAF(CAS 1478-61-1)购自Sigma公司。取适量BPAF粉末溶于二甲基亚砜(dimethyl sulfoxide,DMSO,国产分析纯),配制浓度分别为10、1和100 μg/mL 的储存液,用棕色玻璃瓶储存,并置于-20 ℃冰箱中暗处,备用。
1.2BPAF暴露试验设计选取120条生长规格一致、健康的成年雌性弓背青鳉用于BPAF暴露试验;选取12 L的玻璃缸作为暴露容器,暴露溶液体积为8 L,每缸随机放入10条弓背青鳉。将弓背青鳉暴露于不同环境相关浓度(100、10和1 μg/L)的BPAF(即BPAF暴露处理组),另设置对照组(含0.01‰的DMSO)。所有BPAF暴露处理组均含有0.01‰(V/V)的DMSO。每个处理设置3个平行,试验暴露周期为21 d,暴露水体每48 h全部更新。试验过程中水温、光照周期和投喂方式与常规饲养保持一致。暴露期间,每天用一次性吸管吸取残饵和粪便,挑出并记录死亡个体。分别在暴露第8天和第16天从每个玻璃缸中取相同体积暴露48 h的水体和新更换的水体,混合后送至广东省科学院测试分析研究所(中国广州分析测试中心)测定暴露水体中BPAF的浓度。暴露结束后,将所有鱼置于冰上麻醉后立刻测量鱼的体长和湿重,计算生长状况因子(condition factor,K)。生长状况因子计算公式为K=湿重/(体长)×100。测量结束后解剖,取肝脏和卵巢组织:肝脏和卵巢组织分成2份,1份置于4%的多聚甲醛(PFA)中,用于组织切片制备;另1份置于RNALater中,用于总RNA提取。
1.3组织切片制备和总RNA提取将获得的部分肝脏和卵巢组织于4%的PFA中固定18 h,随后进行乙醇溶液系列脱水、二甲苯透明、石蜡包埋、切片(厚度6~8 μm)、苏木精-伊红(H.E)染色,置于光学显微镜下观察并拍照。取用RNALater保存的肝脏和卵巢组织,参照Trizol 试剂盒(Invitrogen,美国)说明书提取总RNA,通过琼脂糖凝胶电泳检测总RNA的完整性;然后,使用美国NanoDrop 2000(赛默飞,美国)测定总RNA样品的A/A。所有RNA样品18S和28S条带清晰,A/A为1.8~2.0,符合后续试验要求。
1.4cDNA制备和荧光定量分析参照HiScript 1st Strand cDNA Synthesis Kit(诺唯赞,南京)使用说明书,配制反转录体系,使用PCR仪进行反转录,获得cDNA。将获得的cDNA用双蒸水(ddHO)稀释20倍,-20 ℃下保存备用。参考笔者所在实验室弓背青鳉转录组数据,获取部分与生殖相关的基因序列[35-37],并设计荧光定量引物(表1)。qPCR反应在罗氏LC96荧光定量PCR仪上运行,反应体系共15.0 μL,包括2×PerfectStart TM Green qPCR SuperMix(全式金,北京)7.5 μL,上下游引物(10 μmol/L)各0.3 μL,cDNA 2.0 μL,ddHO 4.9 μL。反應程序如下:95 ℃ 预变性 30 s;95 ℃ 5 s,60 ℃ 15 s,72 ℃ 10 s(收集荧光),40个循环;随后进行熔解曲线分析。以eef1b和rps4x为内参基因[37-38],对目的基因进行归一化处理。定量结果采用2-ΔΔCt方法评估目的基因相对于对照组的相对表达量。
1.5数据统计与分析试验数据均以平均值±标准差的形式表示,使用GraphPad Prism 8软件进行数据统计分析并绘图。采用单因素ANOVA检验分析方法中的Tukey多重比较,分析对照组与BPAF暴露处理组各数据的差异显著性。
2结果与分析
2.1BPAF在暴露水体中的实际浓度如表2所示,BPAF在暴露过程中的浓度保持稳定,3个BPAF处理组在试验过程中BPAF实际浓度分别为0.93、9.33和102.33 μg/L,實测浓度与理论浓度接近;在对照组中未检测到BPAF,说明试验水体未被BPAF污染。所有处理组BPAF暴露浓度均以实测浓度表示。
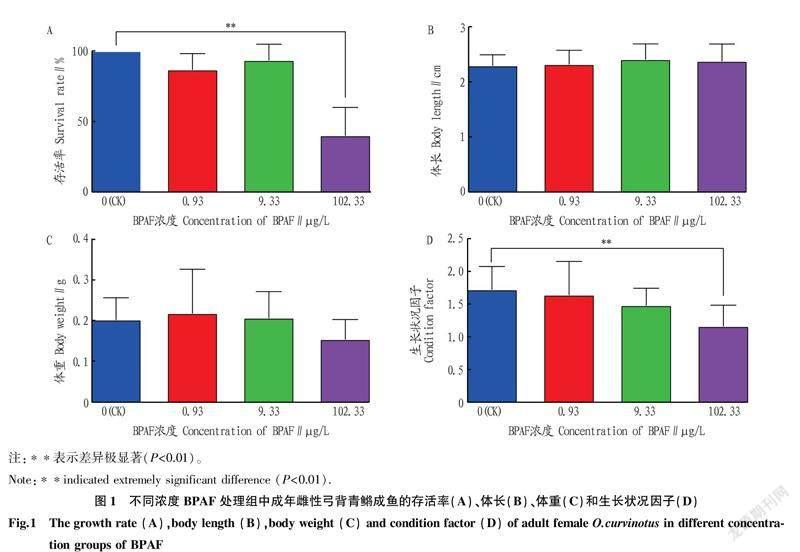
2.2BPAF暴露对雌性弓背青鳉成鱼生存、生长的影响0.93和9.33 μg/L的BPAF暴露处理21 d未对雌性弓背青鳉成鱼的生存造成影响,而102.33 μg/L的BPAF暴露处理极显著降低了弓背青鳉的存活率(图1A)。各浓度BPAF暴露没有对弓背青鳉体长和体重造成显著影响(图1B、C),但102.33 μg/L BPAF暴露极显著降低了弓背青鳉生长状况因子(图1D)。
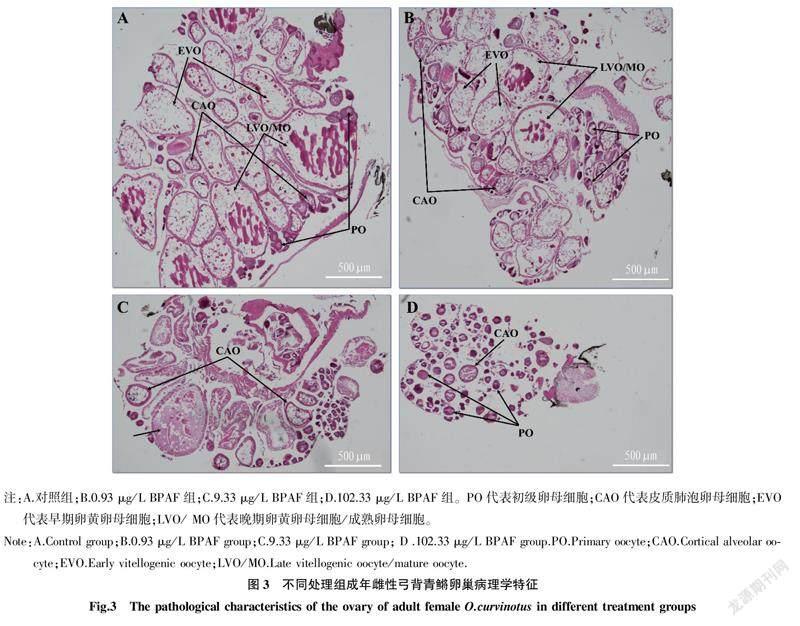
2.3BPAF暴露对雌性弓背青鳉成鱼肝脏和卵巢组织学结构的影响0.93 μg/L BPAF暴露处理21 d后弓背青鳉肝脏组织学结构(图2B)在形态上与对照组(图2A)相比没有明显差异,肝细胞呈多边形且排列紧密,仅脂肪含量与对照组相比略有降低;9.33和102.33 μg/L BPAF暴露处理组肝细胞排列松散,肝脏细胞间间隙增大,脂肪含量降低(图2C、D);102.33 μg/L BPAF暴露还导致肝细胞出现明显的肿胀和形状改变(图2D)。对照组弓背青鳉的卵巢含有各期卵母细胞(图3A),0.93 μg/L BPAF暴露处理未对卵巢结构造成明显的影响(图3B),但9.33和102.33 μg/L的BPAF暴露处理抑制了弓背青鳉卵母细胞的成熟,卵母细胞多停留在初级卵母细胞阶段(primary oocyte,PO)(图3C、D)。
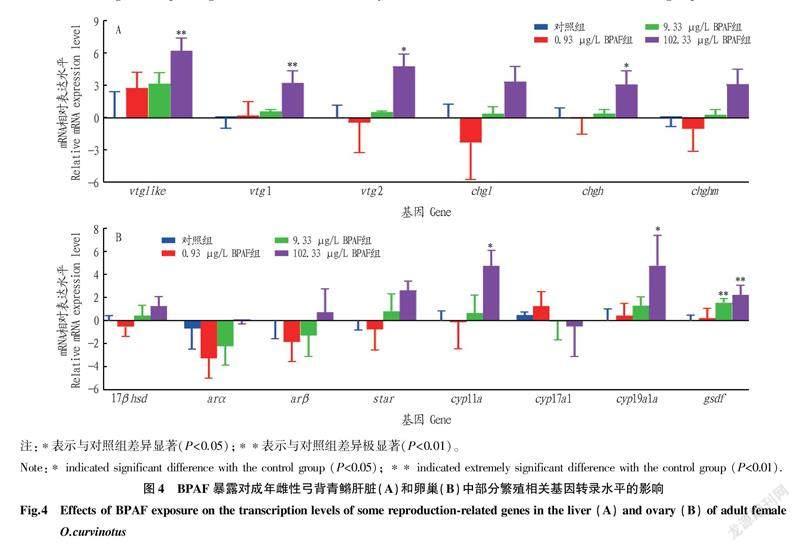
51卷8期黄泽胤等环境相关浓度双酚AF暴露对成年雌性弓背青鳉生长和生殖的毒性效应2.4BPAF暴露对雌性弓背青鳉成鱼基因转录水平的影响在肝脏中,102.33 μg/L的BPAF暴露可以显著上调卵黄蛋白原(vitellogenins,vtgs)和透明带蛋白前体(choriogenins,chgs)基因的转录水平,其中vtg2和chgh的上调显著(P<0.05),vtglike和vtg1的上调极显著(P<0.01),chgl和chghm的上调不显著(P>0.05);0.93和9.33 μg/L的BPAF暴露未对vtgs和chgs的表达造成显著影响(图4A)。在卵巢中,102.33 μg/L的BPAF暴露显著上调了细胞色素P450芳香化酶(cyp19a1a)基因、细胞色素p450介导侧链裂解酶(cholesterolside chain cleavage enzyme,cyp11a)基因,另外9.33和102.33 μg/L的BPAF暴露极显著上调了gsdf的转录水平(图4B)。
3讨论
该研究在雌性弓背青鳉成鱼暴露过程中BPAF的实测浓度与理论浓度基本一致,表明在48 h暴露时间内BPAF的稳定性较高,这与其半衰期较长且难降解的特性[27]相符,提示BPAF对水生生物具有持久性危害。
该研究中102.33 μg/L BPAF暴露导致成年弓背青鳉雌鱼存活率显著下降。目前关于BPAF对水生生物的致死浓度研究较少。Yang等[31]使用1.0 mg/L的BPAF对2月龄斑马鱼进行28 d暴露处理,结果发现试验组雌、雄斑马鱼的存活率与对照组相比没有显著差异;陈亚文[24]研究发现用500 μg/L 的BPAF处理成年斑马鱼30 d并未导致斑马鱼死亡;杨洋等[30]研究发现BPAF暴露24 h对斑马鱼胚胎致畸的半数效应浓度为2.00 mg/L,暴露96 h的半数致死浓度为1.84 mg/L;Mu等[39]研究了BPAF、BPA、BPF和BPS对斑马鱼胚胎的半数致死浓度,分别为1.95、10.43、19.59和>50 mg/L。郭婧颖等[40]通过毒性试验测得BPAF对大型溞(Daphnia magna)的24 h和48 h半数致死浓度分别为9.70和5.02 mg/L。综合以上研究可推测出成年雌性弓背青鳉对水体中BPAF的耐受程度较斑马鱼和大型溞等水生动物更低,水体中BPAF会对弓背青鳉的生存造成威胁。
肝脏在鱼类物质能量代谢、激素合成等生理过程中具有重要作用,是鱼类监测环境污染物毒性效应的主要靶点[41-44],也是动物或人体富集BPAF的重要器官[45]。该研究中短期BPAF暴露导致雌性弓背青鳉成鱼肝脏细胞排列松散、肝细胞体积变大。类似的结果也出现在BPAF和其他含氟类有机物的研究中,250和1 000 μg/L BPAF暴露组雄性斑马鱼中出现肝细胞排列松散,1 000 μg/L BPAF暴露组肝细胞明显肿大、形状不规则,但相同处理的雌性斑马鱼肝脏并未发生明显改变[31];全氟十二烷酸腹腔注射斑马鱼,短时间即导致斑马鱼出现肝损伤,包括肝细胞肿胀、空泡化和核固缩等[46];全氟辛烷磺酸暴露处理雌性大鼠后,导致幼鼠肝细胞肿大[47]。因此,雌性弓背青鳉成鱼肝脏在组织学上可作为监测水体中BPAF等含氟类化合物的靶点,显著的肝脏病理学损伤可能是102.33 μg/L BPAF处理组弓背青鳉成鱼死亡率高的诱因之一。另外,成年雌鱼肝脏是卵黄蛋白原(vitellogenins,vtgs)、透明带蛋白前体(choriogenins,chgs)等基因的主要表达器官。这些基因对环境中雌激素类物质敏感,在无外界因素刺激的情况下仅在雌鱼肝脏中转录,在雄鱼及幼鱼中不发生转录,而当水环境中存在雌激素类物质时则诱导这些基因的大量表达[48-50]。该研究中102.33 μg/L BPAF诱导vtglike、vtg1、vtg2和chgh的转录水平显著上升,chgl和chghm的转录水平也明显上调。该结果符合BPAF具有雌激素效应的特性,类似的结果在250 μg/L BPAF暴露处理的雌性斑马鱼肝脏中也有发现[31];Mu等[39]研究表明BPAF显著提高了斑马鱼胚胎中vtg1的mRNA 水平。综合BPAF对弓背青鳉肝脏组织病理学特征和基因转录水平的影响,推断BPAF对雌性弓背青鳉成鱼既有雌激素效应又有肝脏毒性效应。
9.33 和102.33 μg/L BPAF暴露阻滞了雌性弓背青鳉成鱼卵母细胞的发育,这与以前的研究结果相一致。Yang等[31]研究发现BPAF短期暴露(250和1 000 μg/L暴露28 d)导致雌性斑马鱼卵母细胞发育阻滞;陈亚文[24]研究表明经BPAF处理的斑马鱼卵巢中卵母细胞发育受到抑制,其成熟卵母细胞数量较对照组显著减少;BPAF急性暴露还可能导致性成熟雌性小鼠性腺形态异常和卵巢未分化的导管末端小葉增生[51]。因此,水体中环境相关浓度的BPAF对雌性弓背青鳉成鱼具有较强的生殖毒性。该研究中BPAF暴露改变了雌性弓背青鳉成鱼卵巢中cyp11a、cyp19a1a和gsdf的转录水平。在性激素合成过程中,CYP11A1是已知的唯一催化胆固醇向孕烯醇酮转化的酶,后者是所有类固醇激素的共同前体[52]。cyp19a1a编码的性腺芳香化酶可将雄激素转化为雌激素,从而促进卵巢分化[53-54]。该研究中BPAF暴露上调了雌性弓背青鳉成鱼卵巢中cyp11a和cyp19a1a的转录水平。性腺体细胞衍生因子(gonadal soma derived factor,GSDF)属于TGF-β超家族成员,主要参与鱼类性别分化,对于维持鱼类精原细胞的正常增殖发育具有重要作用[55-56];敲除罗非鱼的gsdf导致遗传雄鱼性逆转为雌鱼,而过表达gsdf致使遗传雌鱼的性腺发育为精巢[57]。该研究中9.33和102.33 μg/L BPAF暴露显著上调了雌性弓背青鳉成鱼卵巢gsdf转录水平,这可能抑制了卵巢的成熟。据此推测BPAF对雌性弓背青鳉成鱼的生殖毒性可能与BPAF改变了繁殖相关基因的转录水平有关。
4结论
该研究结果表明,水体中环境相关浓度的BPAF暴露对雌性弓背青鳉成鱼的生存和生长造成了不利影响。BPAF暴露还导致雌性弓背青鳉成鱼肝损伤,上调了肝脏中vtgs和chgs转录水平,导致了卵巢结构异常,阻碍了卵母细胞的成熟,影响了卵巢中cyp11a、cyp19a1a和gsdf的转录水平。作为一种新型的内分泌干扰化合物,BPAF致毒机理及其对水生生物的危害需要引起更多的关注。
参考文献
[1] SAKAUE M,OHSAKO S,ISHIMURA R,et al.Bisphenol-A affects spermatogenesis in the adult rat even at a low dose[J].Journal of occupational health,2001,43:185-190.
[2] MA A T,YANG X Z,WANG Z X,et al.Adult exposure to diethylstilbestrol induces spermatogenic cell apoptosis in vivo through increased oxidative stress in male hamster[J].Reproductive toxicology,2008,25(3):367-373.
[3] NAKAMURA D,YANAGIBA Y,DUAN Z W,et aNl.Bisphenol A may cause testosterone reduction by adversely affecting both testis and pituitary systems similar to estradiol[J].Toxicology letters,2010,194(1/2):16-25.
[4] VANDENBERG L N,CHAHOUD I,HEINDEL J J,et al.Urinary,circulating,and tissue biomonitoring studies indicate widespread exposure to bisphenol A[J].Ciência & saúde coletiva,2012,17(2):407-434.
[5] YE X B,PIERIK F H,ANGERER J,et al.Levels of metabolites of organophosphate pesticides,phthalates,and bisphenol A in pooled urine specimens from pregnant women participating in the Norwegian Mother and Child Cohort Study (MoBa) [J].International journal of hygiene & environmental health,2009,212(5):481-491.
[6] 宋作栋,仇雁翎,张华,等.水体中双酚类物质的赋存现状及研究进展[J].环境化学,2020,39(6):1496-1503.
[7] AKAHORI Y,NAKAI M,YAMASAKI K,et al.Relationship between the results of in vitro receptor binding assay to human estrogen receptor alpha and in vivo uterotrophic assay:Comparative study with 65 selected chemicals[J].Toxicology in vitro,2008,22(1):225-231.
[8] YANG Y J,YU J L,YIN J,et al.Molecularly imprinted solid-phase extraction for selective extraction of bisphenol analogues in beverages and canned food[J].Journal of agricultural & food chemistry,2014,62(46):11130-11137.
[9] JIN H B,ZHU L Y.Occurrence and partitioning of bisphenol analogues in water and sediment from Liaohe River Basin and Taihu Lake,China[J].Water research,2016,103:343-351.
[10] YAN Z Y,LIU Y H,YAN K,et al.Bisphenol analogues in surface water and sediment from the shallow Chinese freshwater lakes:Occurrence,distribution,source apportionment,and ecological and human health risk[J].Chemosphere,2017,184:318-328.
[11] LIU Y H,ZHANG S H,SONG N H,et al.Occurrence,distribution and sources of bisphenol analogues in a shallow Chinese freshwater lake (Taihu Lake):Implications for ecological and human health risk[J].Science of the total environment,2017,599/600:1090-1098.
[12] SI W,CAI Y F,LIU J C,et al.Investigating the role of colloids on the distribution of bisphenol analogues in surface water from an ecological demonstration area,China[J].Science of the total environment,2019,673:699-707.
[13] FROMME H,KCHLER T,OTTO T,et al.Occurrence of phthalates and bisphenol A and F in the environment[J].Water research,2002,36(6):1429-1438.
[14] SONG S J,RUAN T,WANG T,et al.Distribution and preliminary exposure assessment of bisphenol AF (BPAF) in various environmental matrices around a manufacturing plant in China[J].Environmental science & technology,2012,46(24):13136-13143.
[15] YU X H,XUE J C,YAO H,et al.Occurrence and estrogenic potency of eight bisphenol analogs in sewage sludge from the U.S. EPA targeted national sewage sludge survey[J].Journal of hazardous materials,2015,299:733-739.
[16] SUN X L,PENG J Y,WANG M H,et al.Determination of nine bisphenols in sewage and sludge using dummy molecularly imprinted solid-phase extraction coupled with liquid chromatography tandem mass spectrometry[J].Journal of chromatography A,2018,1552:10-16.
[17] ZHANG H F,ZHANG Y P,LI J B,et al.Occurrence and exposure assessment of bisphenol analogues in source water and drinking water in China[J].Science of the total environment,2019,655:607-613.
[18] MANDRAH K,SATYANARAYANA G N V,ROY S K.A dispersive liquid-liquid microextraction based on solidification of floating organic droplet followed by injector port silylation coupled with gas chromatography-tandem mass spectrometry for the determination of nine bisphenols in bottled carbonated beverages[J].Journal of chromatography A,2017,1528:10-17.
[19] YAMASAKI K,TAKEYOSHI M,SAWAKI M,et al.Immature rat uterotrophic assay of 18 chemicals and Hershberger assay of 30 chemicals[J].Toxicology,2003,183(1/2/3):93-115.
[20] YAMASAKI K,TAKEYOSHI M,YAKABE Y,et al.Comparison of reporter gene assay and immature rat uterotrophic assay of twenty-three chemicals[J].Toxicology,2002,170(1/2):21-30.
[21] MATSUSHIMA A,LIU X H,OKADA H,et al.Bisphenol AF is a full agonist for the estrogen receptor ERα but a highly specific antagonist for ERβ[J].Environmental health perspectives,2010,118(9):1267-1272.
[22] KITAMURA S,SUZUKI T,SANOH S,et al.Comparative study of the endocrine-disrupting activity of bisphenol A and 19 related compounds[J].Toxicological sciences,2005,84(2):249-259.
[23] GU J,WANG H Y,ZHOU L J,et al.Oxidative stress in bisphenol AF-induced cardiotoxicity in zebrafish and the protective role of N-acetyl N-cysteine[J/OL].Science of the total environment,2020,731[2021-11-15].https://doi.org/10.1016/j.scitotenv.2020.139190.
[24] 陈亚文.六氟双酚A暴露对成鱼期斑马鱼的毒性效应[D].海口:海南大学,2016.
[25] 唐天乐.六氟双酚A对斑马鱼(Danio rerio)甲状腺轴及认知能力的干擾效应研究[D].海口:海南大学,2016.
[26] 杨洋.双酚AF暴露对胚胎/幼鱼期斑马鱼的毒性效应及甲状腺内分泌系统的干扰效应[D].海口: 海南大学,2016.
[27] 吴迪.双酚AF对小鼠血睾屏障和精子发生的影响及其机制研究[D].武汉:华中农业大学,2020.
[28] YOU C,JIA C X,PAN G.Effect of salinity and sediment characteristics on the sorption and desorption of perfluorooctane sulfonate at sediment-water interface[J].Environmental pollution,2010,158(5):1343-1347.
[29] 黄乾生,陈亚檞,方超,等.盐度影响全氟辛烷磺酸对海水青鳉(Oryzias melastigma)的毒性[J].科学通报,2013,58(2):151-157.
[30] 杨洋,陈亚文,唐天乐,等.双酚AF暴露对胚胎期和幼鱼期斑马鱼的毒性效应[J].环境科学研究,2015,28(8):1219-1226.
[31] YANG X X,LIU Y C,LI J,et al.Exposure to bisphenol AF disrupts sex hormone levels and vitellogenin expression in zebrafish[J].Environmental toxicology,2016,31(3):285-294.
[32] 董忠典,龙水生,黄承勤,等.一种快速鉴定弓背青鳉遗传性别的方法[J].广东海洋大学学报,2018,38(3):25-29.
[33] 黄顺楷,郭昱嵩,汪淳,等.弓背青鳉3种雌激素受体基因的克隆及其表达分析[J].广东海洋大学学报,2019,39(2):8-19.
[34] 张海瑞,王中铎,黄顺楷,等.弓背青鳉的胚胎发育及自发荧光观察[J].广东海洋大学学报,2019,39(2):38-44.
[35] 董忠典,黎学友,廖健,等.雌、雄弓背青鳉(Oryzias curvinotus)肝脏转录组比较分析[J].海洋与湖沼,2020,51(5):1203-1213.
[36] DONG Z D,LI X Y,YAO Z B,et al.Oryzias curvinotus in Sanya does not contain the male sex-determining gene dmy[J].Animals,2021,11(5):1-13.
[37] DONG Z D,LI X Y,HUANG S K,et al.Vitellogenins and choriogenins are biomarkers for monitoring Oryzias curvinotus juveniles exposed to 17 β-estradiol[J].Comparative biochemistry and physiology part C:Toxicology & pharmacology,2020,236:1-10.
[38] DONG Z D,CHEN P S,ZHANG N,et al.Evaluation of reference genes for quantitative real-time PCR analysis of gene expression in Hainan medaka (Oryzias curvinotus)[J].Gene reports,2019,14:94-99.
[39] MU X Y,HUANG Y,LI X X,et al.Developmental effects and estrogenicity of bisphenol A alternatives in a zebrafish embryo model[J].Environmental science & technology,2018,52(5):3222-3231.
[40] 郭婧穎,刘建超,李帅衡,等.双酚AF对大型溞生殖、生长等生态行为的影响[J].中国环境科学,2019,39(10):4394-4400.
[41] MONDON J A,HOWITT J,TOSIANO M,et al.A simple osmium post-fixation paraffin-embedment technique to identify lipid accumulation in fish liver using medaka (Oryzias latipes) eggs and eleutheroembryos as lipid rich models[J].Marine pollution bulletin,2011,63(5/6/7/8/9/10/11/12):86-90.
[42] ZHANG X Y,WEN H S,WANG H L,et al.RNA-Seq analysis of salinity stress-responsive transcriptome in the liver of spotted sea bass (Lateolabrax maculatus)[J].PLoS One,2017,12(3):1-18.
[43] NI X M,WAN L,LIANG P P,et al.The acute toxic effects of hexavalent chromium on the liver of marine medaka (Oryzias melastigma) [J].Comparative biochemistry and physiology,part C:Toxicology & pharmacology,2020,231:1-11.
[44] HOSHIKAWA Y,FURUKAWA S,IRIE K,et al.Sequential histological changes in the liver of medaka exposed to methylazoxymethaol acetate[J].Journal of toxicologic pathology,2020,33(4):219-226.
[45] YANG Y J,YIN J,YANG Y,et al.Determination of bisphenol AF (BPAF) in tissues,serum,urine and feces of orally dosed rats by ultra-high-pressure liquid chromatography-electrospray tandem mass spectrometry[J].Journal of chromatography B,2012,901:93-97.
[46] LIU Y,WANG J S,WEI Y H,et al.Induction of time-dependent oxidative stress and related transcriptional effects of perfluorododecanoic acid in zebrafish liver[J].Aquatic toxicology,2008,89(4):242-250.
[47] BJORK J A,LAU C,CHANG S C,et al.Perfluorooctane sulfonate-induced changes in fetal rat liver gene expression[J].Toxicology,2008,251(1/2/3):8-20.
[48] CHEN X P,LI V W,YU R M K,et al.Choriogenin mRNA as a sensitive molecular biomarker for estrogenic chemicals in developing brackish medaka (Oryzias melastigma)[J].Ecotoxicology & environmental safety,2008,71(1):200-208.
[49] LEE C,NA J G,LEE K C,et al.Choriogenin mRNA induction in male medaka,Oryzias latipes as a biomarker of endocrine disruption[J].Aquatic toxicology,2002,61(3/4):233-241.
[50] LAI K P,LI J W,WANG S Y,et al.Tissue-specific transcriptome assemblies of the marine medaka Oryzias melastigma and comparative analysis with the freshwater medaka Oryzias latipes[J].BMC genomics,2015,16(1):1-13.
[51] TUCKER D K,BOUKNIGHT S H,BRAR S S,et al.Evaluation of prenatal exposure to bisphenol analogues on development and long-term health of the mammary gland in female mice[J].Environmental health perspectives,2018,126(8):1-17.
[52] HU M C,HSU H J,GUO I C,et al.Function of Cyp11a1 in animal models[J].Molecular and cellular endocrinology,2004,215(1/2):95-100.
[53] SI Y F,DING Y X,HE F,et al.DNA methylation level of cyp19a1a and Foxl2 gene related to their expression patterns and reproduction traits during ovary development stages of Japanese flounder (Paralichthys olivaceus)[J].Gene,2016,575(2):321-330.
[54] KOBAYASHI Y,HORIGUCHI R,MIURA S,et al.Sex-and tissue-specific expression of P450 aromatase (cyp19a1a) in the yellowtail clownfish,Amphiprion clarkii[J].Comparative biochemistry and physiology part A:Molecular & integrative physiology,2010,155(2):237-244.
[55] SAWATARI E,SHIKINA S,TAKEUCHI T,et al.A novel transforming growth factor-beta superfamily member expressed in gonadal somatic cells enhances primordial germ cell and spermatogonial proliferation in rainbow trout (Oncorhynchus mykiss)[J].Developmental biology,2007,301(1):266-275.
[56] SHIBATA Y,PAUL-PRASANTH B,SUZUKI A,et al.Expression of gonadal soma derived factor (GSDF) is spatially and temporally correlated with early testicular differentiation in medaka[J].Gene expression patterns,2010,10(6):283-289.
[57] JIANG D N,YANG H H,LI M H,et al.gsdf is a downstream gene of dmrt1 that functions in the male sex determination pathway of the Nile tilapia[J].Molecular reproduction and development,2016,83(6):497-508.
