Acceptance, availability and feasibility of RTS, S/AS01 malaria vaccine: A review of literature
2023-05-15AbdullahNadeemWajeehaBilal
Abdullah Nadeem, Wajeeha Bilal
Department of Medicine, Dow University of Health Sciences, Karachi, Pakistan
ABSTRACT Malaria remains the most serious infectious disease and is one of the leading causes of death among children in malaria endemic areas.The development of malaria vaccines has been underway since 1960s.Significant progress in the development of vaccine has been made in the last decade.On 6 October 2021, World Health Organization recommended widespread use of the RTS, S/AS01 malaria vaccine.The level of acceptance of RTS, S/AS01 malaria vaccine is relatively low in middle-income countries.This might be because of lack of information regarding vaccine implementation in such countries.The proper and efficient execution of the malaria vaccination program necessitates careful consideration of each community's socio-cultural setting.The most prominent RTS, S/AS01 vaccine trial was conducted from 2009 to 2011 in which eleven sites in seven African countries participated.Results of the trial, published in 2015, provided a promising advance in the development of a malaria vaccine for African children.As of 2019,large-scale pilot studies of the vaccine have been conducted in Ghana, Kenya, and Malawi, involving several hundreds of thousands of infants.The RTS, S/AS01 vaccine shows modest efficacy against malaria and has a feasible mode of administration.Although there is increased risk of meningitis, cerebral malaria, pneumonia, anemia,febrile convulsions and gastroenteritis, the vaccine still has a feasible mode of administration and high cost effectiveness and can be easily implemented in resource-limited settings.
KEYWORDS: RTS, S/AS01; Malaria; Plasmodium; Vaccine
1.Introduction
Malaria is the most important parasitic disease of humans caused by infection with a protozoan parasite of the genus of the order Haemosporida in the phylum Apicomplexa[1].In terms of mortality and morbidity, Plasmodium (P.) falciparum fection remains the most serious of all other malarial species infections.It causes asymptomatic infection in as much as 87% of individuals in endemic areas[2].The systematic signs and symptoms of malaria include fever, chills, headache, diarrhea, nausea, vomiting, muscle and joint pain, increased breathing and heart rate.With almost 5 million cases and 9 000 fatalities from malaria in 2020, the South-East Asia region was the second highest contributor to the disease worldwide, right behind the African region[3].India alone was responsible for 82.5% of malaria cases in the South-East Asian nations, with Indonesia(15.6%) coming in second and Myanmar (1.6%) coming in third[3].There were an estimated 619 000 malaria deaths globally in 2021 according to World Malaria Report 2022.It is also known to be the fourth leading cause of death among children of less than 5 years[4].
With varying degrees of success, attempts have been conducted over the past century to control, decrease, and eventually eradicate the effects of malaria, particularly in tropical and subtropical Africa and some regions of Asia[1].Significant progress has been made in the development of a malaria vaccine over the last two decades.Around 90% of the annually reported malaria cases occur in Africa, where 1 million children under the age of 5 died because of malaria[5].The recent development and phase 3 testing of the most advanced malaria vaccine, RTS, S/AS01, indicates that malaria vaccine is moving into a new phase[6].
2.Acceptance of malaria vaccine
A large-scale phase 3 study was conducted between 2009 and 2014 among children aged 5-17 months, and the results revealed that the vaccination averted 4 in 10 instances of uncomplicated malaria and 3 in 10 cases of severe malaria.It is one of the biggest reasons for monetary burden and stunted GDP growth owing to loss of productivity and absenteeism among school-age children.Malaria prevention and control efficiency are being hampered by rising parasite resistance to anti-malarial medications, poor accessibility and unequal distribution of health care, inadequate healthcare service infrastructure, and an increasing population[7].A vaccine capable of lowering severe and complex malaria as well as malaria-related mortality is an essential public health strategy.Malaria transmissionblocking vaccines can help break the malaria transmission cycle by focusing on community protection rather than individual protection.However, when introducing new therapeutic techniques into a community, acceptance and adoption are as important as efficacy[8].
Individuals' understandings of ailments and the desirable impacts of therapies that aim to prevent diseases, according to the health belief model, are major drivers of people’s attitudes towards health interventions[9].Historically, the failure of an approved vaccine in a region is always due to lack of community participation.In recent years, there has been a growing recognition of the importance of community participation in the implementation of modern clinical research and community-based preventive strategies.However, the effective use of community participation is hampered by several factors, including identifying actual stakeholders of interest and evaluating their level of involvement[10].The recent RTS, S/AS01 malaria vaccine is a remarkable milestone towards the eradication of malaria, but the level of acceptance, especially in low and middleincome countries, may pose an obstacle that must be overcome for effective implementation of the project.Historically, poor vaccination campaigns in different regions, including African and Asian territories, have prompted reservations regarding the successful adoption of malaria vaccines in endemic regions[11].For example,Nigeria formerly had the largest number of polio cases, which was blamed on the country's low vaccination coverage and compliance.Similarly, Pakistan and Afghanistan are also blamed for the failure to eradicate polio due to ineffective vaccination programs[12].
The malaria vaccine has shown a very good response in several African countries, and the majority of parents are willing to administer the recommended dosage to their children.Four recent studies from Ethiopia, Tanzania, Nigeria, and Kenya found acceptance rates of 32%, 97%, 98%, and 88%, respectively[13-16].This demonstrates that while malaria vaccine acceptability in African countries is good, there are still areas where vaccine acceptance is subpar[17].In contrast, the COVID-19 acceptance rates were found to be 51%, 60%, 34%, and 63% in Ethiopia, Tanzania, Nigeria, and Kenya, respectively[17-20].This disparity shows that the acceptance rate of the malaria vaccine is relatively higher than the COVID-19 vaccination.This might be due to the fact that malaria has been a known disease for several decades and has caused millions of deaths,with children accounting for a sizable fraction of those deceased.On the other hand, COVID-19 is a relatively new disease and several myths circulated around the world due to emergency measures taken by governments all over the world and the economic halt[21].
However, there are still several factors causing hindrance to the maximum attainable coverage of the malaria vaccine that can prevent thousands of deaths.Literature has shown that the major factors contributing to hesitancy towards malarial vaccine include inadequate community engagement as the authorities fail to engage with the general public.People do not have sufficient knowledge about vaccines, their efficacy, mechanism of action, and significance,which leads to denial of immunization programs[22].Another important hurdle to vaccination acceptance is the fear that the active elements in the injectable vaccine may harm their children, as well as other beliefs that vaccines contain harmful substances and transmit disease rather than protecting[23].Inadequate delivery of child immunization services, including huge distance to vaccination facilities, lengthy queues, crowding, and limited-service hours,were reported to decrease the intent to vaccinate[14].Besides that,a fragile healthcare system with inadequate facilities, understaffed hospitals, poor communication between healthcare workers and patients, and a lack of faith in the local healthcare system are all contributing factors to the low vaccination rate[19].Traditional cultural practices and beliefs, single family member taking healthcare decisions in households and relationship of the caregiver with the child are considered as minor factors towards vaccine hesitancy[21-23].The main proposed solutions to decrease vaccination hesitancy include using versatile and broad communication models and trusted sources to deliver vaccine-related health information to communities; encouraging community participation at both the national and district levels; and implementing new vaccine services alongside existing health services.The proper and efficient execution of the malaria vaccination program necessitates careful consideration of each community's socio-cultural setting[24].Several studies on vaccine acceptance advocate the use of initiatives that increase positive community understanding and perceptions regarding vaccine efficacy.Immunization success is determined by both clinical efficacy and the community's perception.Lack of community engagement, awareness and misconceptions results in higher rejection rates.In such contexts, aligning stakeholders is an important input, as suggested in the network analysis to examine decision-making[25].
3.Availability of malaria vaccine
The development of malaria vaccines has been underway since 1960s, with substantial progress being made in the last decade.It was on October 6, 2021, that the World Health Organization (WHO)released its recommendation for widespread use of the malaria vaccine RTS, S/AS01 among children living in sub-Saharan Africa and other malaria-stricken regions.There were 11 sites in seven African countries which participated in the trial from 2009 to 2011.The children were aged 6-12 weeks and 5-17 months[26,27].This trial was conducted at one site in western Kenya by Centers for Disease Control and Prevention (CDC) in collaboration with Kenya Medical Research Institute.Number of children aged 6 weeks-17 months enrolled by site in Phase 3 trial of RTS, S/AS01 (2009-2011) is shown in Figure 1.
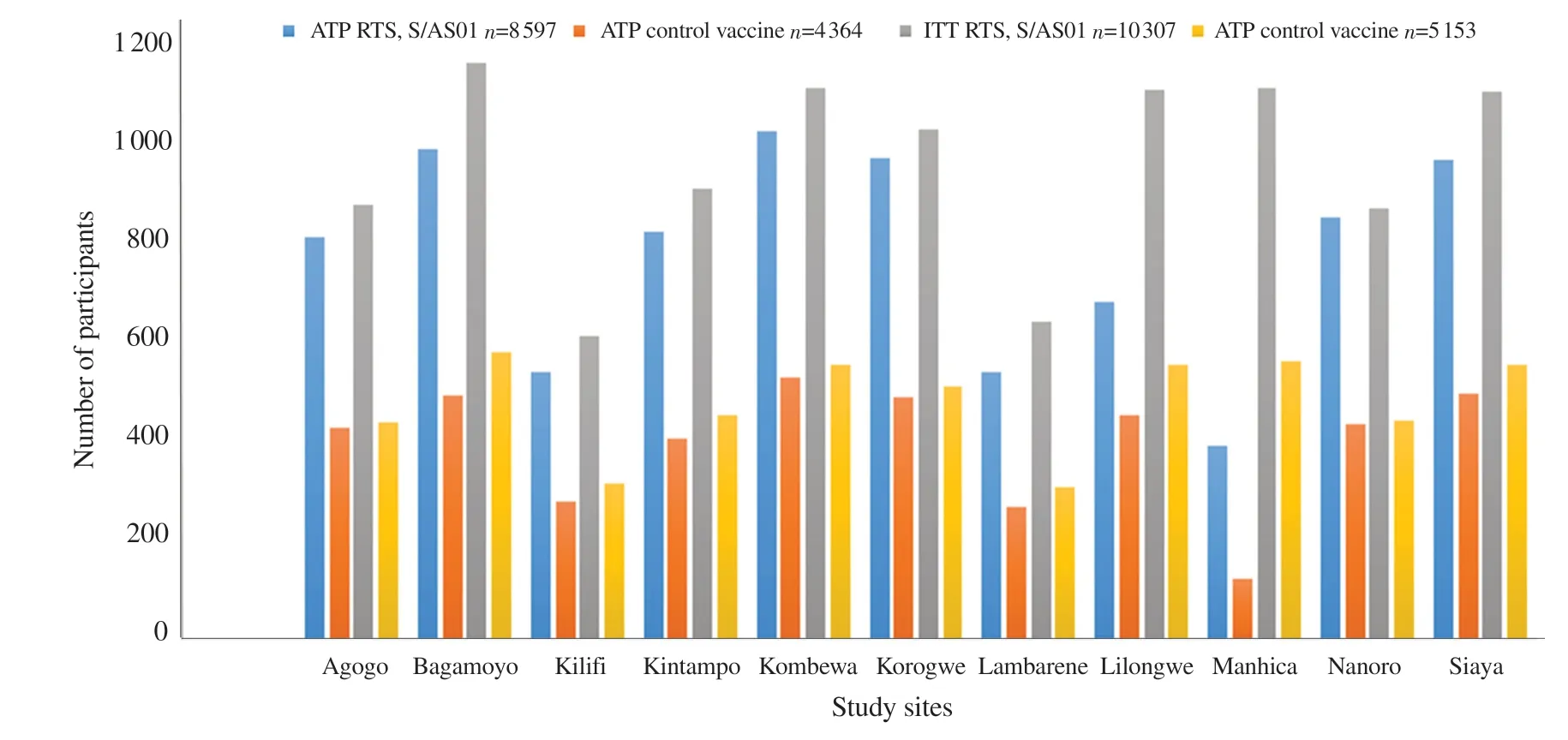
Figure 1.Number of children aged 6 weeks-17 months enrolled by site in Phase 3 trial of RTS, S/AS01 (2009-2011).ATP: active trial participants; ITP:inactive trial participants.
The results of the trial, published in 2015, provided a promising advance in the development of a malaria vaccine for African children.Children who received the three-dose vaccine series plus the booster dose of the RTS, S/AS01 vaccine experienced a decrease of one-third in clinical and severe cases of malaria over a period of four years[27,28].Vaccine effectiveness was lower among young infants.Vaccine safety was generally found to be satisfactory, but a few safety signals warranted further investigation, including febrile convulsions, meningitis, and cerebral malaria.A notable aspect of this vaccine was its ability to provide protection in settings where other effective malaria preventions and treatment interventions were ongoing: bed nets, antimalarial drugs for disease treatment, residual insecticide spraying in the home to prevent man-mosquito contact,and medications preventing malaria's adverse effects from occurring in pregnant women and newborns[27].
Despite the positive regulatory assessment by the European Medicines Agency in July 2015, WHO recommended in October 2015 that a large-scale pilot study be undertaken before recommending RTS, S/AS01 vaccine for children aged 5-17 months.As of 2019, large-scale pilot studies of the vaccine have been conducted in Ghana, Kenya, and Malawi, involving several hundreds of thousands of infants.A large-scale RTS, S/AS01 pilot program is currently being evaluated by the CDC in western Kenya, in collaboration with Kenya Medical Research Institute (KEMRI) and several other organizations.This pilot evaluation aims to determine whether the three-dose vaccine series plus booster can be delivered through routine healthcare systems and to carefully analyze the vaccine's impact on all-cause mortality, as well as its relationship to specific adverse events (febrile seizures, meningitis, cerebral malaria)[27,28].
It has also been proposed that whole sporozoite that is extracted from mosquito salivary glands, may be used as a potential malaria vaccine candidate, either after being rendered non-infectious throughirradiation or following the administration of chemoprophylaxis[27].A recent study has demonstrated the safety and well-tolerance of the irradiated sporozoite PfSPZ vaccine produced by Sanaria®.This vaccine shows promising protection against malaria when administered intravenously.Based on the results of a collaborative CDC/KEMRI study in western Kenya, the PfSPZ vaccine seems to be safe and well tolerated in infants and young children.The vaccine failed to provide significant protection against P.falciparum infections at 6 months, preventing further evaluation for this age group, although other studies are currently testing the efficacy of the PfSPZ vaccine in Mali, Gabon, Tanzania, and Equatorial[27].Nevertheless, this study provided valuable information regarding the immune response to the vaccine, which will aid researchers in developing a vaccine for young children that is more effective.Currently, RTS, S/AS01 and PfSPZ vaccine products are two of the most promising malaria vaccine candidates.In Burkina Faso,an early trial testing a pre-erythrocytic vaccine candidate, R21,demonstrated good efficacy among children 5 to 17 months of age[27].A variety of other malaria vaccine candidates are in the development or clinical trial stage, including transmission-blocking vaccines that target the sexual stage of the parasite's development in the mosquito, as well as malaria mRNA vaccines.To accelerate the development of a highly effective malaria vaccine, the leading global health organizations have developed a Malaria Vaccine Technology Roadmap.As part of the strategic objectives, the following goals will be established for malaria vaccines by 2030: Provide malaria vaccines with protective efficacy of at least 75% against clinical malaria in areas where malaria transmission is ongoing and ensure the elimination of malaria through mass vaccination campaigns in multiple settings using malaria vaccines that reduce transmission and human malaria infection[27].
4.Feasibility of malaria vaccine
The malaria vaccine RTS, S/AS01 has been developed against P.falciparum infections.In numerous clinical settings around the world, it has been discovered that the WHO-recommended vaccine lowers mortality.After successfully completing Phase 3 clinical testing, RTS, S/AS01 from GlaxoSmithKline Biologicals was the first malaria vaccine to be recommended by the WHO for broad use among children in regions with moderate to high malaria transmission on October 6, 2021.
The implementation of a vaccine depends mainly on its feasible mode of administration.The lyophilized injection used to administer the RTS, S/AS01 vaccination is given intramuscularly.Hence it makes it more feasible for the vaccine to be delivered easily, even in low-income countries.In African settings with moderate-tohigh malaria transmission, the WHO advised pilot deployment of RTS, S/AS01 as a four-dose regimen[28].The first dose should be administered at the age of 5 months.The third dose should be finished by the age of 9 months after the first two are given monthly.The fourth dose needs to be given between 15 and 18 months[29].The pneumococcal conjugate vaccination plus rotavirus vaccine are all safe to co-administer with RTS, S/AS01, according to prior research.Furthermore, the immunological response to vaccinations in a person is unaffected by the co-administration of RTS, S/AS01 with vitamin B6 from 6 to 7.5 months of age and with the yellow fever and measles, rubella rabies vaccines at 9 months of age[30].
Vaccination is a financially viable public health approach[31].Various articles have reported high cost-effectiveness of malarial vaccines[32,33].Cost-effectiveness of interventions affects decisions to introduce and invest in their sustainable use.In low and middle income countries like Bangladesh, the introduction of new health technology such as new vaccines is expected to implement with delays.The estimated cost of Bangladesh’s malaria vaccine is expected to be around US $0.16[32].The vaccine cost will vary from US$ 0.1 up to US$ 5.0[33] (not including the vaccine delivery costs, travel costs and other interventional costs).However, the implementation of RTS, S/AS01 in low- and middle-income countries of South Asia like Pakistan, India, Nepal and Bangladesh is required through an Expanded Program on Immunization (EPI)programme to facilitate rapid vaccination[34].
The RTS, S/AS01 malaria vaccine is offered in a two-vial liquidsolid format.One vial includes the lyophilized antigen (RTS, S),which must be reassembled using the liquid Adjuvant System AS01 included in the second vial[34].Due to the susceptibility of liquid AS01 to higher temperatures, this vaccine must now be kept between 2 and 8 ℃[35].PfSPZ vaccination challenges include the need for liquid nitrogen storage and intravenous injection.
Clinical studies on neonates aged 6 to 14 weeks revealed that the vaccine's efficacy was considerably lower than that of children aged between 5 to 7 years[36].Low efficiency of RTS, S/AS01 vaccine in infants was reportedly due to the immature immune system and the intervention of maternal antibodies in neonates[36].Despite the RTS, S/AS01 vaccine's limited efficiency, there are still several benefits for people's general health.A total of 1 744 cases of malarial infection were prevented for every 1 000 children who received four vaccinations.Furthermore, it was discovered that four doses of the vaccine would prevent 484 fatalities per 100 000 immunized children and 116 480 cases of malarial infection, according to data from phase 3 clinical studies carried out under the supervision of the WHO[37].Infants have relatively little protection from the RTS, S/AS01 vaccine, and the higher rate of effectiveness seen in older children quickly declines[38,39].Feasibility of malaria vaccine is shown in Table 1.
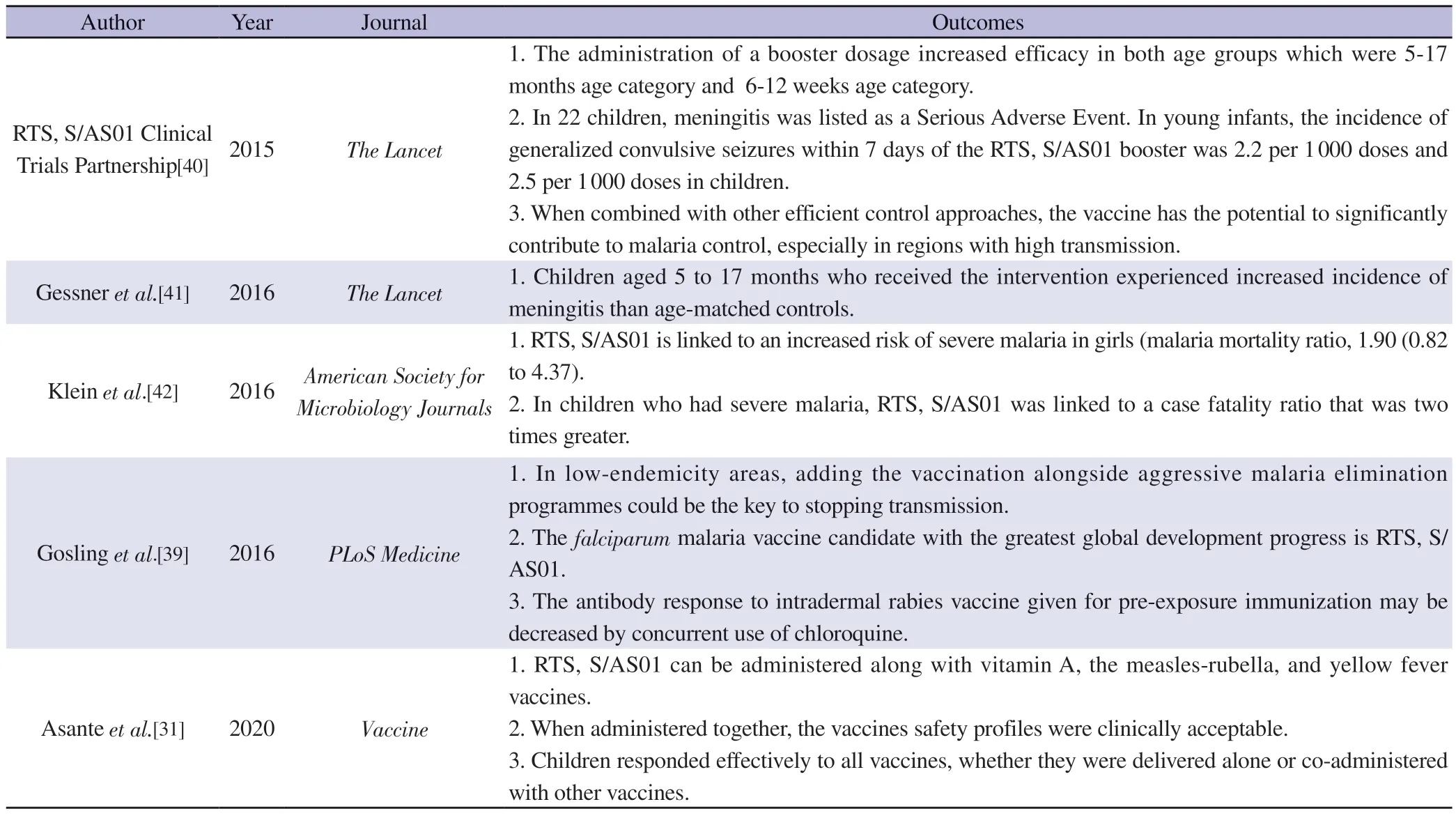
Table 1.Feasibility of malarial vaccine.
Numerous studies have reported various adverse effects of RTS, S/AS01 vaccine including meningitis and cerebral malaria cases[40,41,43].Increased female mortality has also been observed in malaria vaccination groups[42].Increased incidence of pneumonia,anemia, febrile convulsions and gastroenteritis has also been observed in all age groups including infants[30].
5.Conclusions
The RTS, S/AS01 vaccine has achieved an important milestone in the development of malaria vaccine as it is the first malaria vaccine recommended by European Medicines Agency and the WHO.Although the vaccine has moderate efficacy against malaria, it can still be employed in malaria endemic areas, especially those of South Asia and Africa.The vaccine has few adverse effects like meningitis,cerebral malaria, anemia, convulsions etc, it still has a feasible mode of administration and high-cost effectiveness and can be easily implemented in resource-limited settings.At the national, regional,and local levels, concerns about vaccine storage and delivery as well as training for medical staff would need to be addressed.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Funding
The authors received no extramural funding for the study.
Authors’ contributions
A.N.developed the theoretical formalism, performed the analytic calculations and performed the numerical simulations.Both A.N.and W.B.contributed to the final version of the manuscript.A.N.supervised the project.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Medicinal and biological potential of Thuja occidentalis: A comprehensive review
- Early efficacy of individual regimens containing bedaquiline in patients with drug resistant tuberculosis
- COVID-19 vaccine uptake and its determinants among teenagers and their parents in Zhejiang, China: An online cross-sectional study
- Myositis and rhabdomyolysis in scrub typhus infection: A case report
- Dengue positivity among blood donors in hyper-endemic region of southern India
