Pathogenesis and surgical outcomes of different types of myopic traction maculopathy
2023-05-12,
,
Abstract
•KEYWORDS:myopic traction maculopathy; pathogenesis; surgical outcomes
INTRODUCTION
Myopic traction maculopathy (MTM) describes a wide spectrum of clinical pictures that may affect up to 30% of eyes with pathologic myopia[1-2]. MTM exhibits multiple morphological forms including retinal thickening, foveal detachment (FD), lamellar macular hole (LMH) and foveal retinoschisis-like structures[1]. Traditional fundus examinations hardly distinguish between different types of MTM. The clinical application of optical coherence tomography (OCT) has gradually improved the understanding of MTM in patients with pathologic myopia[3]. A recent study proposed that MTM consists of retinal detachment/FD, LMH, foveoschisis (FS) and full-thickness macular holes with or without retinal detachment[4]. However, these criteria are not fully validated in clinical practice.
Previous studies reported that various factors influence the pathogenesis of MTM, for example, scleral deformation or posterior staphyloma inducing a longitudinal traction of the retina was described as a significant risk factor for MTM[5]. To quantify the extent of scleral deformation, the posterior staphyloma height (PSH) measured by OCT has been proposed as an indicator reflecting the scleral curvature in the macular region[6]. Other vitreoretinal interface abnormalities such as epiretinal membrane (ERM) and internal limiting membrane (ILM) detachment were considered as independent risk factors for MTM[7]. However, the precise pathogenesis of MTM remains controversial.
The treatments for MTM include vitrectomy combined with ILM peeling[8], posterior scleral contraction and reinforcement[9-10]or a combination of them[11]. Among these, vitrectomy combined with ILM peeling represents the proper treatment to release the traction generated by the attached vitreous cortex[12]. Many factors can affect the surgical outcomes of MTM, including the degree of chorioretinal atrophy, baseline visual acuity and disease duration[1,13-15]. Some studies reported that the presence of FD was associated with worse functional and anatomical outcomes[16-19], whereas other studies found that the presence of FD correlated with a better postoperative visual acuity improvements[20-22]. The refore, further studies are needed to explore the relationship between FD and surgical outcomes in MTM patients.
In this study,we aimed at defining several baseline parameters using OCT to explore the pathogenesis and surgical outcomes in different types of MTM.
MATERIALSANDMETHODS
EthicalApprovalThe available information of MTM patients attending the Affiliated Eye Hospital of Wenzhou Medical University from January 2016 to December 2020 was retrospectively reviewed. Due to the retrospective nature of the study, the collection of the informed consent was not required.This study followed the principles of the Declaration of Helsinki and was approved by the Medical Ethics Committee of the Affiliated Eye Hospital of Wenzhou Medical University (No.H2022-011-K-11-01).
CriteriaofPatientSelectionWe included 193 patients (210 eyes) with MTM. The diagnosis of MTM was based on comprehensive clinical examinations including best-corrected visual acuity (BCVA), diopters of ametropia, axial length, fundus photography, and OCT. A total of 74 out of 210 eyes (35.2%) underwent vitrectomy and were further analyzed. Inclusion criteria were as follows: adults older than 18 years old; diagnosis of MTM; availability of OCT scans and BCVA measurements at baseline (M0) and 6mo after surgery (M6). Exclusion criteria were as follows:a history of vitreoretinal surgery or penetrating trauma; eyes with full-thickness macular hole and/or macular retinal detachment; other diseases affecting vision, such as severe cataract, diabetic retinopathy, corneal opacity,or glaucoma; a follow-up duration shorter than 6mo.
SurgicalProcedureA total of 74 eyes underwent a 23-gauge pars plana vitrectomy with ILM peeling. Before surgery, patients experienced a decreased vision even after full correction and complained of metamorphopsia with corresponding OCT changes. All traction forces were removed during vitrectomy, including ERM, ILM, and vitreomacular traction. The ILM was peeled without sparing the fovea. The procedure in patients older than 55 years old with mild lens opacity was combined with phacoemulsification.
MyopicTractionMaculopathyClassificationandOpticalCoherenceTomographyParametersBased on the Spectral-Domain OCT findings (Heidelberg Engineering, Heidelberg, Germany), we categorized the eyes in three groups: FD, FS, and LMH. The FD group was characterized by a physical separation of the retinal neurosensory layer from the retinal pigment epithelium (RPE). We further divided the FD group into the limited FD subgroup with a central foveal thickness (CFT) thinner than 700μm (Figure 1C) and the extensive FD subgroup with a CFT of 700μm or thicker (Figure 1D). Then two assessors independently analyzed the OCT images and reached a consensus.The FS group was characterized by foveal retinoschisis-like structures without a physical separation of the retinal neurosensory layer from RPE or foveal splits in the inner retina (Figure 1A). The LMH group reported foveal splits in the inner retina (Figure 1B).
The CFT was defined as the vertical distance from the ILM’s inner edge to the RPE’s outer edge. According to a previously published study, the PSH was measured vertical distance between the subfoveal RPE and the peripheral RPE 2 mm from the fovea on both temporal (H1) and nasal (H2) sides (Figure 1A)[23]. The relative height was expressed as a positive number if the edge was located anteriorly and negative number if the edge was located posteriorly. Two technicians independently performed the measurements and then calculated the average values.
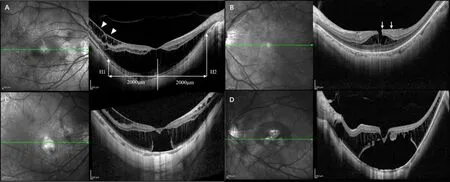
Figure 1 Representative optical coherence tomography images of different types of myopic traction maculopathy. A: Foveoschisis, the arrow heads show the internal limiting membrane detachment. The posterior staphyloma height was measured vertical distance between the subfoveal retinal pigment epithelium (RPE) and the peripheral RPE 2 mm from the fovea on both temporal (H1) and nasal (H2) sides; B: Lamellar macular hole, the arrows show the epiretinal membrane; C: Limited foveal detachment; D: Extensive foveal detachment.
StatisticalAnalysisStatistical analysis was performed using SPSS 26.0 software (SPSS for Windows, Chicago, United States). Before analysis, BCVA was determined using the Snellen chart and converted to the logarithm of the minimum angle of resolution (LogMAR). Data were subjected to a normality test using the Shapiro-Wilk test. Descriptive statistics included mean, standard deviation (SD) and percentages. The Kruskal-Wallis H test was used to compare continuous variables while the Chi-squared test was used to compare categorical variables.Pairwise comparisons were corrected by using the Bonferroni post-hoc test. The Wilcoxon signed-rank test was used to compare variables before and after surgery. Correlations were performed by using Spearman’s correlation analysis or linear regression.P<0.05 was considered statistically significant.
RESULTS


Table 1 Baseline characteristics of patients with myopic traction maculopathy (n=210)
We defined three types of MTM (FD, FS and LMH) with 70 eyes each, and no significant difference in gender (P=0.480), age (P=0.075) and axis length (P=0.057) among groups. There was a significant difference in the incidence of ERM among groups (14.3%, 15.7%, and 40.0% in the FD, FS, and LMH groups, respectively) (P<0.001). The LMH group had a higher incidence of ERM than the FD (P=0.001) and FS (P=0.001) groups, whereas no significant difference was found between FD and FS groups (P=0.813). There was a significant difference in the incidence of ILM detachment among groups (21.4%, 41.4% and 8.6% in the FD, FS and LMH groups;P<0.001). The LMH group had the lowest incidence of ILM detachment when compared to the FD (P=0.033) and FS (P<0.001) groups. The FD group was characterized by the thickest CFT, followed by the FS, and LMH groups (583.47±252.97μmvs.400.54±135.13μmvs.209.20±94.45μm, respectively;P<0.001). Among the three groups, no significant differences were found regarding nasal PSH (P=0.348) and temporal PSH (P=0.496).
CorrelationsbetweenBaselineParametersandtypesofMyopicTractionMaculopathyWe further analyzed potential correlations between the presence of LMH and baseline parameters (Figure 2A). We found that eyes with LMH were positively associated with the presence of ERM (rs=0.28,P<0.001)and were negatively associated with CFT (rs=-0.69,P<0.001). Eyes with FD or FS were associated with the presence of ILM detachment (rs=-0.25,P<0.001). No significant correlation was found between the presence of LMH and nasal PSH (rs=-0.08,P=0.249) or temporal PSH (rs=-0.08,P=0.242).

Figure 2 Correlations between baseline parameters and type of myopic traction maculopathy. A: Correlations between baseline parameters and the presence of LMH; B: Correlations between baseline parameters and the type of FD (extensive or limited). The color, size, and transparency of the circles in the top half reflect the correlation’s trend, degree, and significance. Only significant correlations are presented. LMH: Lamellar macular hole; FD: Foveal detachment; ERM: Epiretinal membrane; ILM: Internal limiting membrane; PSH: Posterior staphyloma height; CFT: Central foveal thickness.
SurgicalOutcomesWe analyzed the anatomical and functional outcomes of 74 eyes that underwent vitrectomy before and after surgery (Table 2). There were 37 eyes in the FD group, 16 eyes in the FS group and 21 eyes in the LMH group. Regarding anatomical outcomes, the CFT of all eyes significantly decreased from 438.77±259.37 to 144.95±68.89μm (P<0.001). There were significant differences in CFT at M0 (P<0.001) and M6 (P<0.001) among the three groups. At M0, the CFT of the LMH group (201.10±115.10μm) was thinner than that of FD (562.59±262.60μm,P<0.001) and FS (464.38±163.14μm,P<0.001) groups. After surgery, the CFT significantly decreased in eyes with FD (P<0.001) and FS (P<0.001) but not in the LMH group (P=0.255). At M6, the CFT of the FD group (110.38±40.53μm) was thinner than that of FS (176.88±90.76μm,P=0.028) and LMH (181.52±61.29μm,P=0.005) groups. Considering functional outcomes, the BCVA of all eyes significantly improved from 0.96±0.49 to 0.62±0.44LogMAR (P<0.001). There were significant differences in BCVA at M0 (P=0.008) and M6 (P<0.001) among groups. At M0, the BCVA of the FD group (1.14±0.50LogMAR) was worse than that of FS (0.80±0.35LogMAR,P=0.028) and LMH (0.78±0.46LogMAR,P=0.005) groups. After surgery, the BCVA was significantly improved in all groups (FD:P<0.001; FS:P=0.003; LMH:P<0.001). At M6, the BCVA of the FD group (0.86±0.44LogMAR) was worse than that of FS (0.44±0.30LogMAR;P=0.002) and LMH (0.34±0.27LogMAR;P<0.001) groups. Representative OCT images of patients with different types of MTM were shown in Figure 3.

Table 2 Anatomical and functional outcomes in eyes that underwent vitrectomy

Figure 3 Representative optical coherence tomography images of well-defined anatomical and functional outcomes after vitrectomy in different myopic traction maculopathy types. Case 1 (A-B): OCT images of an eye with FD from a 41-year-old woman. Case 2 (C-D): OCT images of an eye with FS from a 43-year-old woman. Case 3 (E-F): OCT images of an eye with LMH from a 57-year-old woman. OCT: Optical coherence tomography; FD: Foveal detachment; FS: Foveoschisis; LMH: Lamellar macular hole.
CorrelationbetweenFovealDetachmentandBestCorrectedVisualAcuityTo evaluate the prognosis of eyes with FD, we divided the FD group into the limited subgroup (n=50) and the extensive subgroup (n=20; Table 3). The extensive FD subgroup reported a thicker CFT (811.00±252.26μmvs.492.46±189.36μm,P<0.001), a lower incidence of ILM detachment (5.0%vs.28.0%,P=0.034) and a thicker nasal PSH (218.80±134.15μmvs.130.90±138.38μm,P=0.024) compared to the limited FD subgroup. No significant difference was found regarding temporal PSH (P=0.333) and the incidence of ERM (P=0.914) between subgroups. Correlation analysis showed that an extensive FD was associated with a lower incidence of ILM detachment (rs=-0.25,P=0.034), a larger nasal PSH (rs=0.27,P=0.023), and a thicker CFT (rs=0.56,P<0.001). However, an extensive FD was not significantly associated with the presence of ERM (rs=0.01,P=0.916) and temporal PSH (rs=0.12,P=0.336; Figure 2B). Considering the 37 FD eyes that underwent vitrectomy, 28 of them were classified in the limited subgroup and 9 in the extensive subgroup (data not shown). In eyes with extensive FD, the BCVA increased from 1.02±0.47 to 0.74±0.33LogMAR (P=0.028). In eyes with limited FD, the BCVA increased from 1.52±0.42 to 1.20±0.58LogMAR (P<0.001). The extensive FD subgroup was associated with a worse BCVA at M0 (P=0.013) and M6 (P=0.030) than the limited FD subgroup. As shown in Table 4, we further analyzed the correlation between baseline parameters and BCVA at M6 in FD eyes that underwent vitrectomy. The results showed that an extensive FD (standardβ=-0.295,P=0.042) and BCVA at M0 (standardβ=0.669,P<0.001) were risk factors for a worse BCVA at M6.

Table 3 Comparison of baseline characteristics between eyes with limited foveal detachment and extensive foveal detachment (n=70)

Table 4 Correlation between baseline parameters and best corrected visual acuity at 6mo after surgery in eyes with foveal detachment that underwent vitrectomy (n=37)
DISCUSSION
A consensus has not been reached yet on the pathogenesis of MTM. We speculate that MTM represents a series of manifestations including two different pathogenic factors: the preretinal traction from the vitreoretinal interface and the subretinal traction from the sclera[4,24]. ILM and ERM may potentially affect the preretinal traction[24-25].
The longitudinal traction on the ILM may contribute to the development of FD and FS. In our study, we found that the incidence of ILM detachment in FD and FS groups was higher than that in the LMH group. Of interest, there was a significant correlation between eyes with FD or FS and the presence of ILM detachment. Consistently with our study, Fujimotoetal[26]indicated that the longitudinal traction generated from ILM detachment may be transmitted through multiple columnar structures to the outer retina, leading to the development of FD. Fangetal[27]found that ILM detachment was associated with MTM progression, with the resolution of MTM being accomplished after ILM was disrupted. Therefore, the inward force caused by ILM detachment might be a risk factor for retinal splits or FD. At the same time, the tangential traction from ERM might result in the development of LMH. We showed that the incidence of ERM was higher in the LMH group, with the presence of ERM being positively associated with the presence of LMH. As suggested by previous studies, progressive myofibroblast proliferation at the vitreal side of ILM played a significant role in ERM formation[25,28]. Russelletal[25]found that preretinal tissuesfrom eyes with MTM were histopathologically indistinguishable from idiopathic ERM, suggesting that cellular contraction of ERM contributes to the development of MTM. Moreover, the progressive deformation of the sclera and posterior staphyloma could increase the stretching perpendicular to the choroid-RPE-retina complex[5,29]. However, we found no significant differences in nasal and temporal PSH among the FD, FS, and LMH groups. Our results illustrated that different preretinal MTM-inducing forces caused different forms of MTM: the longitudinal traction was the leading cause of FD and FS whereas the tangential traction predominately led to LMH. Thus, we speculated that preretinal factors had a dominant relevance to different forms of MTM.
Although the surgical indications are still controversial, vitrectomy combined with ILM peeling is considered as an effective treatment for MTM[12,19]. However, its therapeutic effects on different types of MTM remain unclear. Kimetal[17]showed that CFT was significantly decreased after surgery in MTM eyes. In our study, a complete anatomical recovery was achieved by all patients except for the LMH group, with no apparent change of CFT, which probably due to its thinner CFT at baseline. Consistently with our findings, previous studies showed that vitrectomy with ILM peeling was associated with satisfactory visual improvements in MTM eyes[16,18]. In this study, the LMH group reported the best postoperative BCVA, followed by the FS group and the FD group. However, it is still uncertain whether vitrectomy is necessary for functional recovery in FD patients. Kumagaietal[20]showed that BCVA was improved significantly in the FD group but not in the no-FD group after vitrectomy. Hirakata and Hida[21]and Ikunoetal[22]reported that eyes with FD gained more visual acuity than eyes without FD. Conversely, Hattorietal[16]and Kimetal[17]reported that postoperative BCVA was better in the no-FD group than the FD group. Duanetal[18]and Pengetal[19]reported a worse visual acuity in the FD group than the no-FD group. Consistently with the last two studies, we showed that the FD group had the worst BCVA before and after vitrectomy.
Since the precise cause of inconsistent efficacy remains unknown, we hypothesize that the extent of FD was a critical factor impacting surgical outcomes. We showed that the limited FD group was significantly associated with a low incidence of ILM detachment. Eyes with an extensive FD that underwent vitrectomy were associated with a worse BCVA at M0 and M6. The multivariate linear regression analysis showed that an extensive FD was an independent risk factor for a poor BCVA at M6, suggesting that releasing the strong traction from ILM may be therapeutic to treat the limited FD.In addition, we found that the PSH was higher in the extensive FD subgroup than the limited FD subgroup, especially on the nasal side, which might account for the poor efficacy of vitrectomy for FD because vitrectomy could not change the morphology of the sclera. However, the visual function of both subgroups significantly improved after vitrectomy. Therefore, the combination of vitrectomy with ILM peeling remains an important treatment option for MTM patients.
This study is affected by limitations. We performed a cross-sectional, retrospective study with a limited number of patients and a predefined follow-up. However, we included only eyes of sufficient quality to make valid judgments on the outcomes of MTM. Eyes with full-thickness macular holes or macular retinal detachment were excluded from our research. Further studies discussing postoperative surgical complications will be of interest.
In conclusion, our study demonstrated that there were different pathogenic mechanisms in MTM, with preretinal factors playing a major role. FS and FD were associated with ILM detachment, suggesting that their presence may be related to the longitudinal traction at the vitreoretinal interface. The presence of LMH was significantly associated with the occurrence of ERM, indicating the relevance of the tangential traction in the progression of LMH. Vitrectomy combined with ILM peeling improved functional and anatomical outcomes of MTM patients. In eyes with FD, extensive FD may carry a poor prognosis, suggesting that clinicians should make early interventions for patients with FD to improve surgical outcomes.
