Do PON1-Q192R and PON1-L55M polymorphisms modify the effects of hypoxic training on paraoxonase and arylesterase activity?
2023-04-10OyYigittrkFrukTurgyServetldDuzsoylurkemAyrsBl
Oy Yigittürk,Fruk Turgy,*,Servet Kızıld˘g,Du özsoylu,Görkem Ayrs Blı
a Faculty of Sport Sciences,Department of Sport Health Sciences,Ege University,Izmir 35040,Turkey
b Faculty of Medicine,College of Vocational School of Health Services,Dokuz Eylul University,Izmir 35330,Turkey
c Faculty of Medicine,Institute of Health Science,Department of Medical Biology and Genetics,Dokuz Eylul University,Izmir 35330,Turkey
Abstract Background:Low levels of antioxidant paraoxonase 1(PON1)enzyme activity,PON1-Q192R polymorphism(a glutamine(Q)to arginine(R)substitution at position 192),PON1-L55M polymorphism(a leucine(L)to methionine(M)substitution at position 55),and oxidized low-density lipoprotein(oxLDL)are risk factors for coronary heart disease.Aerobic exercise improves PON1 activity,but the effects of hypoxic exercise are yet unclear.The aim of this study was to determine the effects of hypoxic underwater rugby training on PON1 activity and oxLDL levels and the role of the mentioned polymorphisms.Methods:Serum PON1 and arylesterase activities(ARE),PON1,PON3,and oxLDL protein levels(by using the enzyme-linked immunosorbent assays)were determined in an athletic group(42 trained male underwater rugby players;age=21.7±4.2 years,mean±SD)and a control group(43 sedentary men;age=23.9±3.2 years).The polymorphisms were determined from genomic DNA samples.Results:PON1 activity(25.1%,p=0.052),PON3(p<0.001),and oxLDL(p<0.001)of the athletic group,including most genotype groups,were higher than those of the control group.In comparison to the controls,PON1 activity levels(p=0.005)of the PON1-Q192R homozygote QQ genotype group and PON1 activity levels(30%,p=0.116)of the PON1-L55M homozygote LL genotype group were higher,whereas ARE activity values of athletic R allele carrier(Rc=QR+RR)(p=0.005)and LL group(p=0.002)were lower than the control genotype groups related to their polymorphisms.Conclusion: Hypoxic training can cause(1)significant oxidative stress,including oxLDL,and an antioxidant response(increase in PON1 activity and PON3),(2)differences in the activity of PON1 and ARE,which are modified by PON1-Q192R and PON1-L55M polymorphisms,respectively,and(3)improvements in PON1 activity of QQ and LL groups.However,hypoxic training can cause a disadvantage of LL and Rc groups for ARE.
Keywords: Hypoxic training;Paraoxonase;Polymorphism;Underwater rugby
1. Introduction
Coronary heart disease(CHD)is the leading cause of death in the world.1Low levels of paraoxonase (PON) family enzymes(PON1,PON2,and PON3)are accepted as a risk factor for CHD. PONs have antioxidant, anti-inflammatory, and antiatherosclerotic properties2,3that prevent oxidation of lowdensity lipoprotein (LDL) and high-density lipoprotein(HDL).4,5Oxidized LDL(oxLDL)causes endothelial dysfunction and cholesterol accumulation, resulting in foam cell formation, which causes coronary artery disease together with other risk factors.6PON1 hydrolyzes the formation of oxLDL and oxidized lipids on it. Therefore, low PON1 and oxLDL levels are important predictors of coronary artery disease.7
The PON1 enzyme is capable of hydrolyzing both paraoxon(PON1 enzyme activity,PON1 activity)and phenylacetate(arylesterase activity (ARE)).8Compared with PON1, PON3 has higher lactonase and limited ARE activity and no PON1 activity.9,10Both PON1 and PON3 hydrolyze lipid peroxides as oxLDL.The antioxidant and anti-inflammatory properties of HDL are partly due to PON1 and PON3 on it.2,8,10PON1 and PON3 are synthesized primarily in the liver and carried by HDL in circulation. PON2 has been reported to be synthesized in almost all tissues,including the liver,mitochondria,and muscle,and its antioxidant role performs at the intracellular level.4In physiological conditions,PON2 concentration is lower than the others.In addition,recombinant human PON1 and PON3 have the capacity to delay LDL oxidation in vitro,but it is reported that PON1 is more effective than PON3 in this respect.11However,another study reported that rabbit serum PON3 is more effective than PON1 in protecting LDL from oxidation.10
PON1 activity has been found to be significantly lower in individuals with CHD.12PON1 has 2 common polymorphisms in the coding region:a glutamine(Q)to arginine(R)substitution at position 192 (Q192R) and a leucine (L) to methionine(M) substitution at position 55 (L55M).4It has been reported that these polymorphisms have phenotypic differences in the protection of LDL against oxidation. Furthermore, these 2 polymorphisms cause interindividual variation in PON1 enzyme concentration and activity.4Additionally,PON1 activity has been shown to be modulated by such factors as the mentioned polymorphisms of PON1, various nutritional and pharmacological molecules and some pathophysiological events, such as inflammation and oxidative stress.13Human serum PON1 activity is inactivated by oxLDL and preserved by antioxidants.14Therefore, the anti-inflammatory capacity of HDL is mainly impaired in diseases such as type 2 diabetes mellitus,which is partly attributable to decreased PON1 activity, the degree of hyperglycemia and low-grade chronic inflammation.15In addition, PON1 gene polymorphism is related to several diseases such as CHD,type 2 diabetes mellitus, and Parkinson’s disease caused by oxidative stress.13It has been found that HDL from QQ/MM homozygotes was more effective at protecting LDL compared to RR/LL homozygotes.16Similarly,it has been also reported that people with Q allele have a more antiatherogenic lipid and lipoprotein profile than those with R allele.3,17,18
These findings show how important it is to keep the activity and protein levels of PON1 and PON3 at high levels.Another factor that affects PON1 and PON3 activity or levels is exercise.19-22It is known that both aerobic20,21and anaerobic exercise22improve PON1 activity, but the effects of hypoxic exercise training on PON1 and PON3 are unclear.In addition,while PON1-Q192R polymorphism plays a modifier role in the effect of aerobic exercise on PON1 activity,20the role of PON1-L55M polymorphism in this regard is unclear. Hypoxia or intermittent hypoxia is an oxidative stress source and a risk factor for coronary artery disease23because intermittent hypoxia can cause peroxidation of LDL and endothelial dysfunction.oxLDL is related to oxidative stress and sleep-disordered breathing with intermittent hypoxia.7Similarly,obstructive sleep apnea and nocturnal desaturated chronic obstructive pulmonary disease also increase the levels of lipid peroxidation and decrease PON1 activity.24Underwater rugby(UWR) training also involves intermittent hypoxia training,and it has been shown that UWR games cause oxidative stress.25Thus,it may also lead to an increase in oxLDL.UWR is played under water at a depth of 5 m, and a match takes 30-40 min. The energy used during a match is dominantly provided by anaerobic glycolysis, and this may lead to metabolic acidosis and oxidative stress. Intermittent hypoxia can lead to reductive stress,which also results in increased reactive oxygen species production by the mitochondrial electron transport system. Furthermore, in these conditions, the xanthine oxidase and nicotinamide adenine dinucleotide phosphate oxidase path are also activated,which leads to more reactive oxygen species production.26
Therefore, the oxidative stress that occurs during hypoxic training can cause atherosclerosis by oxidizing LDL and decreasing levels of PON1 activity, PON1, and PON3 enzymes. Furthermore, the above-mentioned polymorphisms may also modify the effects of hypoxic training,although this is not yet completely clear. Therefore, the aim of the present study was to investigate the effects of UWR training on PON1 and PON3 enzymes and the role of the above-mentioned PON1 polymorphisms in welltrained male athletes.
2. Materials and methods
2.1. Study groups
In this study, participants included an athletic group(AG) that consisted of 42 active, well-trained male UWR players with 4-5 years of sports experience (age = 21.7 ±4.2 years, mean ± SD). A control group (CG) consisted of 43 sedentary males (age = 23.9 ± 3.2 years) who had physical characteristics similar to those in the AG. CG individuals had not regularly exercised for at least 3-4 months. The selection criteria for both groups included (1)being male, (2) having a body mass index of less than 30,(3) being 16-40 years old, (4) not having any disease or disability, and (5) not regularly smoking cigarettes, using alcohol, or taking any drugs or antioxidant substances. Participants were asked not to modify their diets the week before the tests and not to train hard 3 days prior to the tests. Participants who were found to be healthy after filling out an anamnesis form and taking biochemical tests were enrolled in the study. The aim of the study, possible benefits, test procedures, and possible risks were explained to the participants and to their parents verbally and in writing; and written consent was obtained from the participants (or their parents if participants were <18 years old). The University Medical Faculty’s Ethics Committee approved the study, which was conducted according to the committee’s guidelines.
2.2. Training details
AG participants performed a total of 15 h of UWR training 5 days a week, 3 h/day, on average. UWR training sessions included high-intensity swimming, technical-tactical exercise and 4-5 h/week of underwater training.Additionally,strength training was performed 2 days a week during the morning.On 2 other days during the week,aerobic,and anaerobic endurance,and speed training were carried out on the track and field area.The present study was conducted 1 month before the major UWR championship.
2.3. Physical measurement methods
Measurements of height and body weight were taken with the participants wearing shorts, without shoes with electronic scale (Seca 769; Seca GmbH, Hamburg, Germany). Body mass index was calculated from height and body weight.
2.4. Critical speed test
Critical speed as an indicator of endurance level (aerobic endurance capacity) was determined using participants’maximal 50 m(y1)and 100 m(y2)freestyle swimming speeds.The swimming time(s)for 50 m(x1)and 100 m(x2)was recorded.Critical speed(Eq.1)was then calculated using a modification of the method described by Wakayoshi et al.27Those measurements conducted with both group.
2.5. Analysis of blood samples
Postprandial serum samples were collected at least 3 days after the critical tests were conducted.Heparinized blood samples were immediately centrifuged at 2000 g for 10 min, and the plasma samples were used for thiobarbituric acid reactive species(TBARS)analysis.
The other blood samples were incubated for 20 min at room temperature, and then they were centrifuged at 2000×g for 15 min.The serum was then separated from the whole blood.The serum and plasma samples were stored at -80˚C until the biochemical assays were performed as a single batch. Biochemical parameters were determined within a month after obtaining the serum samples. Total cholesterol, HDL-cholesterol (HDL-C)LDL-cholesterol(LDL-C),and triglyceride(TG)levels were determined by an auto-analyzer, model Roche COBAS C501 (Roche Diagnostics, Basel, Switzerland), via standard enzymatic-colorimetric methods. LDL-C levels were calculated by means of the following widely used formula(Eq.2):28
2.6. Analysis of PON1,PON3,and oxLDL protein concentrations
Concentrations of PON1, PON3, and oxLDL were determined using enzyme-linked immunosorbent assays by measuring absorbance at 450 nm on a microplate reader (Diareader ELX800G;Dialab GmbH,Vienna,Austria).A commercial kit(Sunred Biological Technology, Shanghai, China) was used for analyzing PON1.Two different kits(Elabscience Biotechnology, Wuhan, China) was used for analyzing PON3 and oxLDL. Serum concentrations of PON1 and PON3 enzymes and oxLDL were determined by means of standard curves constructed with purified PONF and oxLDL proteins in a single batch.The coefficients of variation of PON3 and oxLDL were less than 10%.
2.7. Determination of PON1 activity and ARE activity
Measurements of serum PON1 activity and ARE activity were conducted manually based on the method used by Eckerson et al.29Levels were measured with spectrophotometry(UV-1700;Shimadzu,Kyoto,Japan)using paraoxon as PON1 substrate and phenylacetate as ARE substrate.
2.8. Determination of TBARS
The measurement of plasma TBARS, which is used as an indicator of lipid peroxidation, was performed spectrophotometrically (UV-1700; Shimadzu) using the method described by Meijer et al.30
2.9. Genotyping
PON1-Q192R and PON1-L55M polymorphisms were determined by using the polymerase chain reaction restriction fragment length polymorphism (PCR-RFLP) analysis method.Genomic DNA samples were isolated from peripheral blood by using a commercial kit(NucleoSpin®Tissue,Macherey-Nagel,Germany).For PCR analysis,the reaction mixture was made of 0.2 μg template DNA, 200 μmol/L deoxyribonucleotide triphosphate mix, 3 μL of 10×Taq reaction buffer (Mg2+-free),primers (0.13 μmol/L for PON1-Q192R, 0.16 μmol/L for PON1-L55M), MgCl2(3.33 mmol/L for PON1-Q192R and 2.5 mmol/L for PON1-L55M) and 1.25 units of Taq DNA polymerase in a final volume of 30 μL. The sequences of the primer pair were 5′-TATTGTTGCTGTGGGACCTGAG-3′(forward) and 5′-CACGCTAAACCCAAATACATCTC-3′(reverse) for PON1-Q192R, and 5′- GAAGAGTGATGTATAGCCCCAG-3′(forward) and 5′- TTTAATCCAGAGCTAATGAAAGCC-3′(reverse) for PON1-L55M. Using a thermal cycler (TC-3000G; Techne, Burlington, NJ, USA) the PCR was initiated with a denaturation by 1st heating the samples for 5 min at 95˚C.A total of 38 cycles of denaturation(30 s at 95˚C), annealing (30 s at 56˚C), primer extension (40 s at 68˚C) was repeated, and followed by the last extension for 5 min at 68˚C in order to amplify the polymorphic fragments of the PON1-Q192R. The PCR was initiated with a denaturation by first heating the samples for 5 min at 95˚C. A total of 40 cycles of denaturation (30 s at 95˚C),annealing (30 s at 65˚C), primer extension (30 s at 68˚C) was repeated and followed by the last extension for 5 min at 68˚C in order to amplify the polymorphic fragments of the PON1-L55M.
PCR products of PON1-Q192R and PON1-L55M were digested with 2 units of BspPI(Alwl)restriction enzyme(at 55˚C for 2 h)and 5 units of NlaIII(Hin1II)restriction enzyme(at 37˚C for overnight),respectively.The digested products were separated with 2% NuSieve agarose (Lonza, Basel, Switzerland) gel electrophoresis system (EasyCast B1; Thermo Fisher Scientific,Waltham, MA, USA) and observed under an ultraviolet light through ethidium bromide. For PON1-Q192R polymorphism,nondigested PCR products (99 base pair (bp)) represent 192Q allele, while fragments (66 bp and 33 bp) indicate 192R allele.For PON1-L55M polymorphism, nondigested PCR products(171 bp) represent 55L allele, while fragments (127 bp and 44 bp)indicate 55M allele.Due to the small number of RR and MM homozygote genotype groups, the R carrier (Rc=QR+RR),similarly to the M carrier (Mc=LM+MM), were combined to form carrier groups in order to make the statistical analysis safer.
2.10. Statistical analysis
The data were analyzed using SPSS for Windows (Version 23.0; IBM Corp., Armonk, NY, USA). Descriptive data were presented as mean±SD.The normality of variables was evaluated with the Shapiro-Wilk test.When the data did not show normal distribution, the differences between the groups were tested with the non-parametric Mann-Whitney U test.On the other hand,if the data were distributed normally,the differences were tested with the independent t test.Multivariate analysis of variance for repeated measures was used to test the interactions between the effects of exercise on the measured parameters and PON1 polymorphisms. For correlation analysis,Spearman and Pearson’s test was used.Allelic frequencies were estimated by gene counting. Allelic and genotype frequencies were analyzed by Fisher’s exact or x2test. The x2test was employed for the agreement of genotypic frequencies with Hardy-Weinberg expectations. A p value of less than 0.05 was considered significant.
3. Results
The critical speed of AG values were higher than the CG(p < 0.001), whereas age and TG value was lower (p=0.005 and p=0.011, respectively). PON3 level (p < 0.001), oxLDL(p < 0.001), and TBARS (p=0.013) levels of the AG were higher than those of the CG. Although PON1 activity of the AG was higher than that of the CG(25.1%,p=0.052),no significant difference was found between the AG and CG for PON1 level(Table 1).
3.1. PON1-Q192R polymorphism
PON1 activity (p=0.005) and PON3 (p < 0.001) and oxLDL (p=0.002) levels of the athletic QQ (AQQ) homozygote genotype group were higher than those of the controls.Only the increase in PON1 activity was related to the polymorphism,F(1,81)=4.792,p=0.031.PON3 level(p<0.001)and oxLDL levels (13.6%, p=0.051) of the athletic R carrier(ARc=QR+RR)group were higher than the control R carrier(CRc=QR+RR) group (p=0.005), whereas ARE activity(p=0.005)values of ARc were lower than the control and the decrease in ARE activity was related to the polymorphism,F(1,81)=8.231, p=0.005. The ARE activity of the AQQ group was higher than that of the ARc (p=0.020) (Fig. 1).When the CGs were compared in themselves, the PON1 protein level (23%, p = 0.557) and PON1 activity (46%,p=0.346)of the CRc group were higher than those of the control QQ homozygote (CQQ) group. As expected, the PON1 activity of the CRc group was 46%(p=0.346)higher than the CQQ group, whereas in the athletes, the PON1 activity of the AQQ group was 29% (p=0.080) greater than the ARc. There was no significant difference between AQQ and ARc groups for PON1 value in the AG,and the value of Rc in the CG was 22%(p=0.959)higher than the QQ.
Critical speed values of the AQQ and ARc groups were higher than those of the controls(p<0.001 and p=0.001,respectively),whereas age of the AQQ and ARc groups were lower than those of the controls(p=0.046 and p=0.036,respectively).The TBARS level of the AQQ group was higher than that of the control(p=0.016). The TG level of ARc was lower than the control(p=0.040).When the CG were compared in themselves,the bodymass index of the CQQ group was higher than that of the CRc group(p=0.029)(Table 2).
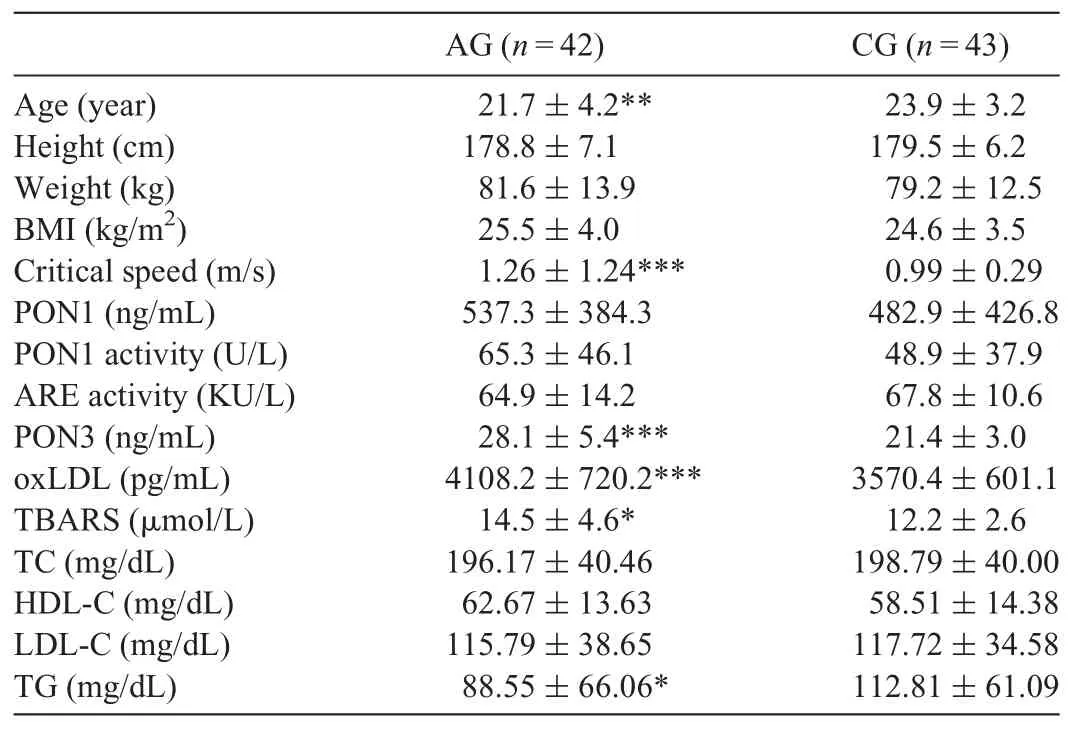
Table 1 Physical and physiological features, PON1, activities of PON1 and ARE,PON3,oxLDL,TBARS,and serum lipid and lipoprotein levels of athletic and CGs(mean±SD).
3.2. PON1-L55M polymorphism
PON1 protein (18%, p = 0.275) and PON1 activity (30%,p = 0.116) of the athletic LL (ALL) homozygote group were higher than the control LL (CLL) group. PON3 level(p=0.001)of the ALL group were higher than the CLL homozygote group, whereas the ARE activity (p=0.002) of the ALL group was lower than that of the CLL group (Fig. 1).Only the decrease in the ARE activity of the ALL group was related to PON1-L55M polymorphism, F(1,81)=6.196,p=0.015.PON3 and oxLDL(p<0.001)levels of the athletic M carrier(AMc=LM+MM)group were higher than those of the control M carrier (CMc=LM+MM) group. When genotype groups were compared in themselves, ARE activity(p=0.033) and oxLDL (p=0.003) values of the CLL group was higher than those of the CMc group. PON1 protein level(20%, p=0.858) and PON1 activity (16%, p=0.750) of the CMc group were higher than levels of the CLL group.
The age of the AMc group was lower than the CMc group(p=0.015). The critical speed of the ALL and AMc groups were higher than those of the controls (p=0.001 and p <0.001,respectively).The HDL-C value of the AMc group was higher than that of the CMc group(p=0.029),whereas the TG value was lower than the CMc group(p=0.016)(Table 3).
Moreover, no statistically significant difference was found between the 2 groups in terms of Q192R and L55M genotype distribution or their alleles frequencies (Table 4). The correlations between physical and physiological parameters and biochemical parameters of the AG and CG were given as follows(Table 5).
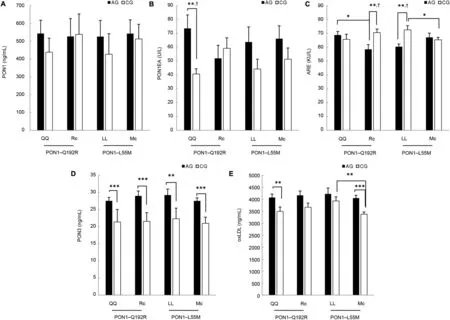
Fig.1. Demonstration of PON1 protein level(A),PON1 activity level(B),ARE activity level(C),PON3 level(D),and oxLDL level(E)between athletic(solid black column)and control(solid white column)genotype groups.QQ,RR,LL,and MM are homozygote groups.LM and QR are heterozygote groups of the mentioned polymorphisms.*p<0.05,**p<0.01,***p<0.001. †Related to their polymorphisms.AG=athletic group;ARE=arylesterase;CG=control group;L=leucine; M=methionine; Mc=M carrier (LM+MM) group; oxLDL=oxidized LDL; PON1=paraoxonase 1 protein; PON3=paraoxonase 3 protein;PONIEA=PON1 enzyme activity;Q=glutamine;R=arginine;Rc=R carrier group(QR+RR).
4. Discussion
The main findings of the present study are that PON1 activity (25.1%, p=0.052), PON3 level (p=0.001), oxLDL level(p < 0.001), and TBARS level (p = 0.013) of the AG were higher than those of the CG (Table 1). The differences in the increase of PON1 activity and the decrease of ARE activity of the athletic QQ and LL homozygote groups compared to control genotype groups were related to PON1-Q192R and PON1-L55M polymorphism, respectively (Fig. 1). In addition,the aerobic fitness levels of all exercise groups were significantly higher than those of the controls(Tables 1,2,and 3).
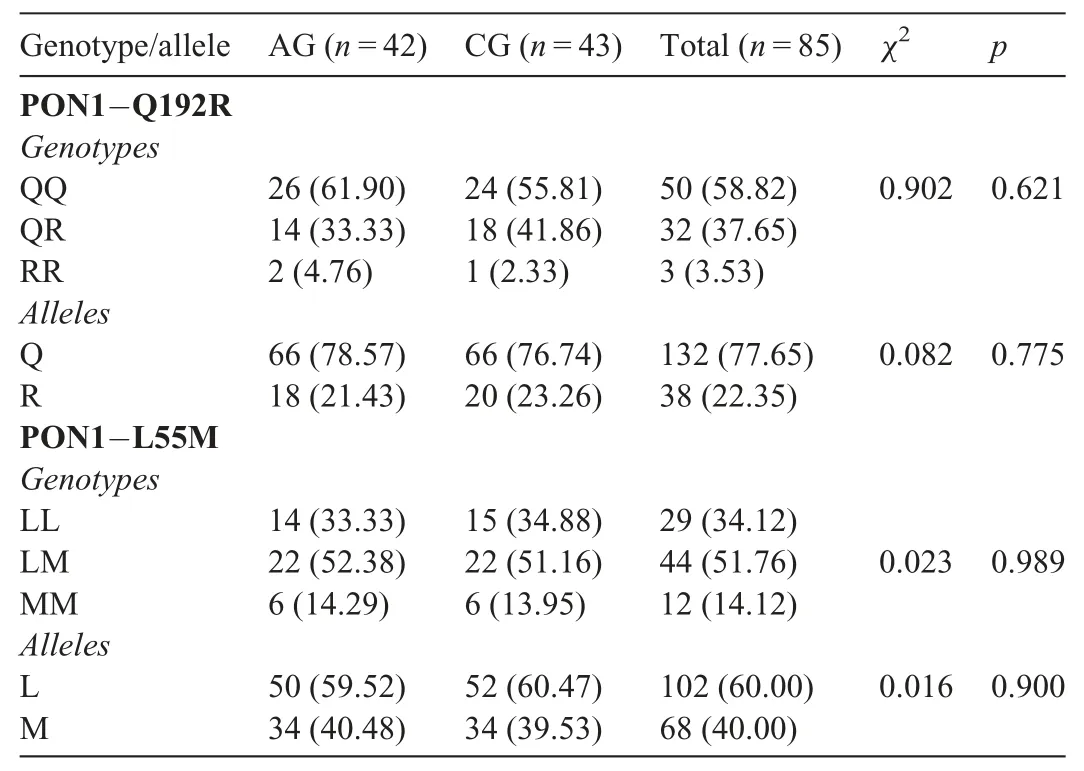
Table 4 Genotypes and alleles frequency of PON1-Q192R and PON1-L55M polymorphisms in athletic and CGs.
UWR is an anaerobic and full-contact sport played at the bottom of a pool. UWR players are exposed to physiological stress such as hypoxia, hydrostatic pressure, and heat. It was found in 1 study that the UWR games caused both oxidative stress and the antioxidant response in both male and female UWR players after the game.25The higher level of TBARS and oxLDL compared to the controls in our study also indicate that hypoxic training can cause oxidative stress. On the other hand, as previous literature has shown, PON1 activity and the protein levels of PON1 and PON3 may have increased in the athletes in response to the oxidative stress.25This can be based on the finding that long-term hypoxic training(in humans)23,31and acute hypobaric hypoxia (in rats)32increased antioxidant enzyme proteins, including PON1 as the tolerance to hypoxia in humans.23,31In another human study,it has been shown that the increase in PON1 activity constituted an important defensive line to meet the oxidative stress that occurs during maximal exercise.32Similarly, Romani et al.33(who documented,for the 1st time, the presence of PON3 in rat serum) showed that 10 weeks of moderate aerobic(treadmill)exercise trainingsignificantly increased blood PON3,rather than PON1,protein levels(which is similar to the findings in our study)but did not change PON1 expression or PON1 activity(unlike the findings in our study). As has been shown, repeating physical movements can cause training adaptations for the antioxidant system’s improvement.34
Hypoxic exercise training increased PON3 levels,as well as PON1 activity, in our study, which is the 1st human study in the literature for PON3. There was a significant positive relationship between PON3 and oxLDL in both the AG and CG(Table 5). Thus, these relationships may indicate that PON3 was expressed as a response to oxLDL oxidation during training and resting. Furthermore, it has been reported that PON3 may also be the 1st line of defense against oxidative stress inrats.33Therefore, the increase in PON3,33as well as in PON1 activity,may have occurred as an antioxidant response to oxidative stress. However, it has also reported that serum PON1 activity is inactivated by oxLDL and preserved by antioxidants.14These findings indicate that PON333and PON1 activity20may have been more resistant to oxidative stress in our study. But the fact that the athletes had higher oxLDL levels than the controls shows that the oxidative stress cannot be fully neutralized.In our study,it was found that PON1 activity of the athletes was higher than that of the controls,whereas ARE activity was lower. The oxidative stress in our study was below a threshold value for an increase in PON1 activity in addition to being more resistant to oxidative stress.35PON3 is also an HDLassociated enzyme with biological activity similar to PON1 protein,but it is not regulated by oxidized lipids.9Therefore,PON3 may be more efficient10during oxidative stress.This feature of PON3 may be one of the reasons for a more pronounced increase in both aerobic36and the hypoxic training.
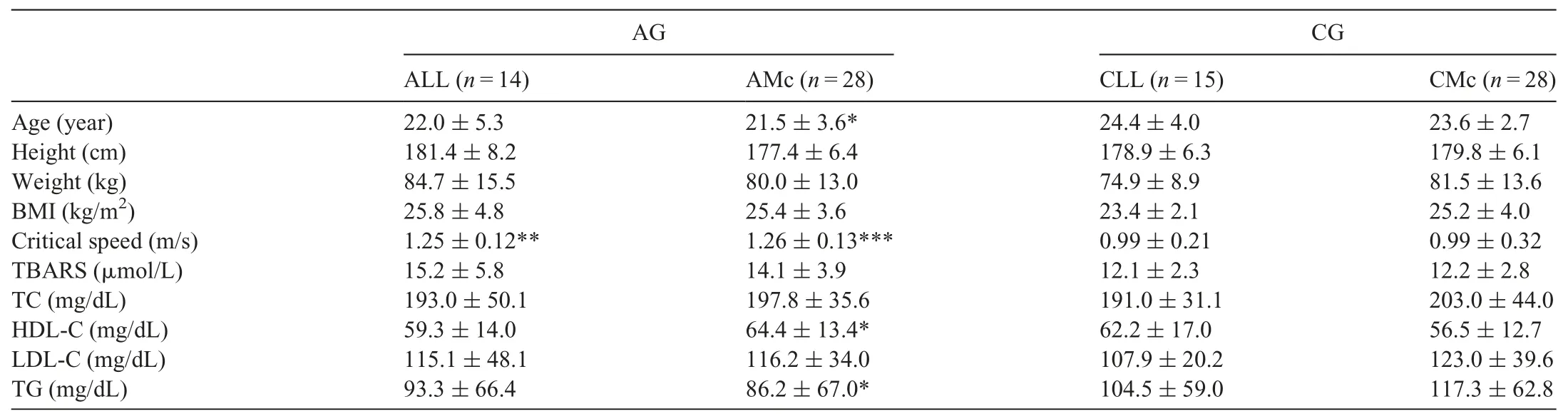
Table 3 Physical and physiological features, TBARS and lipids and lipoproteins levels of athletic and CGs, and the comparisons of the PON1-L55M genotype groups(mean±SD).
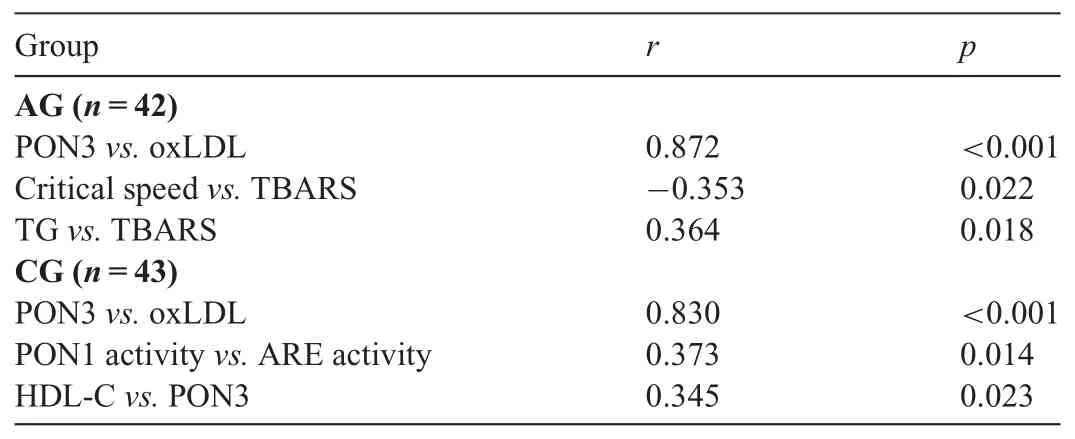
Table 5 Correlations between physical and physiological parameters and biochemical parameters of the athletic and CG.
As we observed, if we had only measured ARE activity instead of PON1 activity,the results could be different.Therefore, the use of different methods to measure PON1 activity may have also affected the results of our study.The reason for the decrease in ARE activity is unclear,but some speculations can be made.Both PON1 activity and ARE activity are different activities related to the same PON1 enzyme. As has been reported in the literature,the ARE activity response to the hypoxic exercise stress could also be due to the difference in hydrolysis of phenylacetate because hydrolysis rate of ARE is 1000 times faster than that of paraoxon,37which can cause hypersensitivity against oxidative stress and inflammation in hypoxic conditions.36However, additional research is needed to clarify this.In contrast to what we found in our study,Rector et al.38found that approximately 6 months of aerobic exercise and caloric restriction significantly decreased PON1 protein level, PON1 activity, and oxLDL level in sedentary and obese individuals,but ARE activity did not change.Mahdirejei et al.39showed that endurance training significantly increased PON1 level in obese men, whereas resistance training did not produce a significant change in PON1 protein levels, which is similar to findings in our study. The differences between our study and Mahdirejei et al.’s39study are due to factors such as intensity, duration and type of exercise, age and training levels of the participants, diet and study design,and related polymorphisms.40Since there is no similar study in the literature examining the effects of hypoxic exercise training on combined PON1 and PON3 proteins in healthy subjects,our discussion is limited to the examples above.
As a result, although it has hypoxic and oxidative stress characteristics, it can be said that UWR training has an improving effect on PON3 level, PON1 activity, and TG levels,but not on ARE activity levels.
4.1. The role of PON1-Q192 polymorphism
The decrease in ARE of the Rc group was related to PON1-Q192R polymorphism in the present study. Tomás et al.20found that regular aerobic exercise increased PON1 activity and decreased oxLDL levels in the QQ participants(as in our study),while it decreased PON1 activity in the Rc group(unlike in our study). In our study, although PON1 activity did not decrease significantly in the Rc group, ARE activity decreased as a result of the training(Fig.1).It has been reported that both aerobic20-22and anaerobic training22increased PON1 activity in the QQ group and decreased in the Rc group, and these changes were related to the indicated polymorphism or the phenotype.The findings in our study was compatible with these studies except for the Rc group. In vitro studies, it has been shown that the Q group provides a better protection against oxidative stress than the R group.20,40These similarities can be explained by the specified phenotypic features of this polymorphism. But in our study, the decrease in ARE activity of the ARc group was associated with the polymorphism.This is interesting because ARE is not bound to PON1-192 polymorphism,8but mutations in the PON gene family or other genes on the 7q chromosome may cause the indicated interactions.20This may also be explained with similar reasons. Although PON1 activity (45.5%, p=0.346) and PON1 protein levels (22.0%,p=0.557)of the CRc group were higher than those of the CQQ group,these parameters of the athletic Rc genotype group were lower than both the control and the AQQ groups,which shows that the CRc group is badly affected by the training for these parameters,as well as for ARE.
But, unlike in the present study, it was reported in the another study41that the changes in blood activities of PON1 and ARE in rugby players after a maximal exercise depended on age,body composition,and training experience and that the effect of PON1-Q192R polymorphism on PON1 changes caused by maximal exercise remained debatable.Although the athletes in the present study had 4-5 years of training experience,there was no significant relationship between the differences in activities of PON1 and ARE with the specified parameters mentioned. The differences found in terms of PON1-Q192R polymorphism between the 2 studies may be due to the differences in the ethnicity and number of participants.
Our results indicate that PON1 activity of the QQ group is positively affected by the hypoxic training, whereas the Rc group can be badly affected by the hypoxic training for ARE activity. As has been found elsewhere in the literature,20our findings indicate that PON1-Q192R polymorphism modifies the effect of hypoxic exercise on PON1 activity, but not for the Rc group.
4.2. The role of PON1-L55M polymorphism
PON1 activity (30%, p=0.116) and PON3 (p=0.001)levels of the ALL group were higher than the CLL group,whereas ARE (p=0.002) was lower. Only the difference in ARE activity was related to PON1-L55M polymorphism,which is similar to the Rc group for PON1-Q192R.In addition,the ARE activity of the CLL group was higher than that of the CMc group(Table 3).Activities of PON1 and ARE of the same enzyme have different effects in protecting LDL from oxidation.9Although the ARE activity of the CLL group was higher than the CMc group,the value of ALL was lower than its control and AMc. Thus, it can be said that prolonged exposure to the hypoxic exercise leads to a decrease in ALL,which creates an additional atherosclerotic risk for this genotype group.
Our findings show that PON1-L55M polymorphism modifies the effect of hypoxic exercise on ARE.These findings are the first in the literature, although our study findings are limited given that there is no other study in the literature on the mentioned polymorphism.
4.3. The effect of UWR training on classical CHD risk factors(blood lipids and lipoproteins)
In the present study, there was no significant difference between AG and CG in terms of HDL-C,whereas the TG level of the AG was lower than that of the CG (p=0.011). It is reported that regular exercise lowers plasma TG levels and increases HDL concentration18,42,43and that increased HDL is associated with PON1 activity.44In the present study,although PON1 activity of the AG was higher than that of the CG(p=0.052), there was no significant relationship between PON1 activity and HDL-C. However, HDL-C was correlated with PON3 only in the CG (Table 5). PON1 and PON3 have been associated with HDL.9,45Nevertheless, in our study HDL-C in the AG did not increase as expected. Thus, it is likely that changes in PON1 activity are independent of HDL-C changes. These findings indicate that the increase in PON3 with hypoxic exercise training may be caused by factors independent of HDL-C.For example,the increase may also be attributed to the increased amount of free PON3 in circulation.
Bailey et al.46showed that plasma total cholesterol, HDL,and LDL decreased significantly after 4 weeks of cycling exercise training (3 days per week, 20-30 min per session,70%-85% maximum heart rate) under both normoxic and hypoxic conditions. Although both studies were hypoxic, the results of our study were different from the results in Bailey et al.46Factors such as severity, duration, type and design of the exercise may have played a role in creating the differences.In our study, TG values in the ARc and AMc groups were lower than those in the controls (p=0.040 and p=0.016,respectively). HDL-C levels of the AMc group were higher than those in the controls(p=0.029),which was not related to its polymorphisms, unlike the findings in Sentí et al.18and similar to those finding by Tomás et al.20In our study, it is likely that UWR training also improved HDL by increasing TG catabolism and lipoprotein lipase activity.47
In another study similar to ours, no significant difference was found between the genotype groups of the PON1-L55M polymorphism for classic CHD risk factors.48In the literature, there is no study investigating the role of related polymorphisms in the effect of hypoxic training on blood lipids and lipoproteins. As far as we know, this is the first study using a Turkish population on this subject in the literature.
5. Conclusion
Hypoxic training caused significant oxidative stress(oxLDL and TBARS) but also causes an antioxidant response(an increase in PON1 activity and PON3).PON1-Q192R and PON1-L55M polymorphisms modified the effects of hypoxic training on activities of PON1 and ARE,respectively.Hypoxic training can improve PON1 activity of the QQ and LL groups,but it can cause a disadvantage for the LL and Rc groups in terms of ARE activity.
Acknowledgment
We would like to thank the participants in our study and Science and Technology Centre unit of Ege University for its financial support(No.33.102.2014.0001).
Authors’contributions
FT and OY participated in the design of the study,contributed to data collection and data reduction/analysis of biochemical parameters, and interpreted the results; GAB participated in the physical and physiological measurements used in the study;SK and Dö contributed to the determination of the polymorphism and interpretation of results.All authors contributed to the manuscript writing.All authors have read and approved the final version of the manuscript,and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
杂志排行
Journal of Sport and Health Science的其它文章
- Physical activity,COVID-19,and respiratory comorbidities:The good,the bad,and the ugly
- Population physical activity legacy from major sports events:The contribution of behavior change science
- Endoscopic debridement for non-insertional Achilles tendinopathy with and without platelet-rich plasma
- Six-year trends and intersectional correlates of meeting 24-Hour Movement Guidelines among South Korean adolescents:Korea Youth Risk Behavior Surveys,2013-2018
- Physical fitness before and during the COVID-19 pandemic:Results of annual national physical fitness surveillance among 16,647,699 Japanese children and adolescents between 2013 and 2021
- The effects of plyometric jump training on lower-limb stiffness in healthy individuals:A meta-analytical comparison
