间歇温度处理对育成蛋鸡采食及肠道发育的影响
2023-04-10刘增民潘亚丽林海焦洪超赵景鹏王晓鹃
刘增民,潘亚丽,林海,焦洪超,赵景鹏,王晓鹃
间歇温度处理对育成蛋鸡采食及肠道发育的影响
刘增民,潘亚丽,林海,焦洪超,赵景鹏,王晓鹃
山东农业大学动物科技学院/山东省动物生物工程与疾病防治重点实验室,山东泰安 271018
【】研究环境温热对家禽采食的影响,补充蛋鸡饲养过程中的参数缺失,为蛋鸡的正确饲养提供科学依据。【】选取11周龄的伊莎褐蛋鸡360只,分为5个处理组,每组6个重复,每个重复12只鸡。正式试验前将试验蛋鸡分别转入5间智能环控鸡舍预饲1周。采用标准笼养,每笼3只鸡。鸡舍内相对湿度保持在60%,育成期每天光照8 h(9:00 — 17:00光照)。分为对照组和4个处理组,对照组保持基础温度22℃不变;4个处理组采用每日间歇温度处理,即每天10:00 —18:00期间分别进行24℃、26℃、28℃、30℃的温度处理,其余时间恢复到基础温度22℃(升温与降温时间均在1 h以内)。试验期8周。试验蛋鸡自由采食和饮水,每周统计采食量,每两周采集一次样品,每组随机挑选12只鸡,称重后断颈处死,称量腺胃重量,以及十二指肠、空肠和回肠的重量与长度。采集下丘脑、腺胃和十二指肠样品于液氮中速冻,-80℃保存。试验结束前连续3 d分别在热处理期和非热处理期统计采食量。【】在试验的前4周,与对照组T22相比,T30组的采食量显著降低(<0.05);在试验后4周时,T24组的采食量显著高于T28组和T30组(<0.05)。在热处理期,T30组的采食量显著低于T22和T24;在非热处理期,T30组的采食量显著低于T22、T24和T26组(<0.05);各组热处理期的采食量均显著低于非热处理期(<0.05),并且保持T24组采食量最高,T30组采食量最低。通过统计器官指数,发现在16周时,与T22组相比,各处理组的腺胃指数极显著升高(<0.01);18周时,与T22组相比,T30组的空肠指数显著降低,且低于其他处理组(<0.05);20周时,与T22组相比,T24组的腺胃指数、空肠指数和回肠指数显著升高(<0.05),且空肠指数显著高于T30组(<0.05)。检测食欲基因的表达,发现在14周时,与T22组相比,各处理组下丘脑(Neuropeptide Y)的表达量显著升高(<0.05),T30组下丘脑(Agouti-related protein)的表达量显著降低(<0.05),T30组十二指肠(Cholecystokinin)的表达量显著升高(<0.05);20周时,与T22组相比,T24组下丘脑(Cocaine amphetamine-regulated transcript)的表达量显著降低(<0.05),T24组腺胃的表达量显著降低(<0.05)。【】育成期环境温度保持在24℃可以促进蛋鸡胃肠道的发育,提高下丘脑中促食因子的表达,抑制下丘脑抑食因子和腺胃的表达量,有利于蛋鸡的生长发育。而30℃高温处理会对蛋鸡的肠道造成损伤,抑制下丘脑促食因子的表达,同时促进十二指肠抑食因子的表达,从而抑制蛋鸡采食,降低采食量。
蛋鸡;采食;温热;育成期;肠道;下丘脑
0 引言
【研究意义】温度是影响畜禽生产的主要环境因素之一。家禽因其特殊的生理特点,特别容易受到高温环境的影响。采食量与育成期蛋鸡的生长发育密切相关,但目前的研究缺乏育成期蛋鸡最佳采食量所需的温度参数。本文旨在研究环境温热对育成期蛋鸡采食的影响,为蛋鸡适宜的温热参数提供一定的科学依据。【前人研究进展】采食是家禽消化吸收的首要环节,也是其维持生存、生长发育和生产的前提[1]。采食量与家禽生长发育和生产性能密切相关,是评估家禽能量代谢和营养需求的基础。与哺乳动物一样[2-3],家禽的食欲调节系统是一个复杂的信号系统,涉及中枢和周边调节。下丘脑是中枢神经系统调节采食量的重要器官,它在整合调控采食的各种信号并对采食行为做出调整中发挥着关键作用[4-5]。哺乳动物和家禽的下丘脑弓状核都含有在食物摄入的中枢调节中起重要作用的前阿片黑素细胞皮质激素(POMC)和神经肽Y(NPY)/刺鼠相关蛋白(AgRP)[6-7]。中枢给药NPY和AgRP刺激食物摄入,而中枢给药α-黑素细胞刺激激素(α-MSH,一种源自POMC的神经肽)抑制哺乳动物和雏鸡的食物摄入[8-11]。食物剥夺诱导哺乳动物和雏鸡下丘脑中和的表达,并抑制的表达[12-13]。因此,下丘脑、和的mRNA水平可作为食欲的指标。可卡因-苯丙胺调节转录肽(CART)在体内多处区域都有表达,其中下丘脑表达量最高,其次为肠道。研究表明,CART能够通过作用于下丘脑以及胃肠道来降低家禽食欲和饲料消化速率的途径降低采食量[14]。胆囊收缩素(CCK)和肽YY(PYY)已被研究作为肠道激素,对肉鸡和蛋鸡的采食量调节非常重要[15]。这两种基因在进食后将饱腹感信号传递给大脑,导致食欲减弱[16-18]。以上食欲调节因子在中枢神经系统和身体外周组织的交汇处起作用[19],并受到包括环境温度在内的多种因素的影响[20]。家禽属于恒温动物,当环境温度处于一定变化范围内时,家禽可以自我调节产热和散热来维持体温的稳定[21]。由于羽毛厚实、汗腺缺乏和高代谢率,家禽特别容易受到热应激的影响[22]。热应激是影响家禽健康最为主要的环境影响因素之一[23]。研究发现,家禽暴露在高温环境中会改变其生理内稳态,导致免疫紊乱、内分泌和电解质紊乱,导致体重减轻、产卵量减少,甚至死亡率增加[24]。在一定的温度范围内,蛋鸡的采食量随着环境温度的升高而降低,在高温下饲养的蛋鸡的采食量和产蛋量较低[25]。下丘脑视前区的温度调节中心在热应激期间被激活,与采食调控相关的神经递质或脑肠肽水平发生变化,包括NPY、AgRP、POMC和ghrelin等[25-27]。因此,高温会通过调控中枢和外周的食欲相关基因影响蛋鸡的采食量和生产性能。肠道是家禽主要的消化吸收场所,其功能好坏与家禽采食量息息相关。研究报道,急性热应激影响肉鸭的小肠形态,降低空肠绒毛高度[28]。热应激严重损伤肠道黏膜,降低消化吸收能力,影响体重的增加[29]。在热应激期间,家禽降低采食量以降低消化热。当机体处于轻微热应激时,交感神经兴奋,促使胃肠道蠕动减缓,增加食物在消化道内的滞留时间,降低采食量。而当机体处于严重热应激时,血管膨胀充血,使得消化器官中循环血量降低,消化酶分泌减少,削弱消化功能,采食量大幅下降。严重时,还会造成消化器官损伤。热应激会首先导致胃损伤和胰腺炎恶化,显著升高血清中淀粉酶、脂肪酶、白介素、过氧化物酶的浓度[30]。热应激还会导致血清皮质酮水平升高,法氏囊、胸腺、脾脏等免疫器官的比重显著降低,引起肠道损伤[31]。综上所述,家禽采食调控涉及到外源(环境温度)与内源(食欲调控因子、消化器官发育)两个方面,热应激会通过调控食欲基因和消化器官发育来影响采食量。目前,国内外对家禽采食行为的研究已经有比较多的报道,但主要是以肉鸡或产蛋期蛋鸡为试验对象,对蛋鸡的育成期关注较少。此外,相关的长期热应激试验大多是全程高温,这不符合温度的日变化规律。【本研究切入点】因此,本文在蛋鸡育成期设计了间歇温度处理,即每天10:00— 18:00期间进行8 h的温度处理,通过评估采食量、肠道发育及食欲调控因子的表达,为育成期蛋鸡最佳采食量的适宜温热参数提供一定的科学依据。【拟解决的关键问题】本试验从温热这一重要的环境因子入手,探究其对家禽采食行为的影响,旨在补充蛋鸡饲养过程中的参数缺失,为蛋鸡的科学饲养提供一定的理论基础。
1 材料与方法
本试验于2018年在山东农业大学动物生物工程与疾病防治山东省重点实验室完成。试验选用的海兰褐蛋鸡购自青岛奥特种禽场。饲喂所用的饲料均为商品日粮,购自山东众成饲料厂。
1.1 试验动物与试验设计
选取体重相近的11周龄伊莎褐蛋鸡360只,分为5个处理组,每组6个重复,每个重复12只鸡。试验前将试验蛋鸡分别转入5间智能环控鸡舍预饲,环控舍定制于济南科益试验设备有限公司,每间3.75 m× 2.40 m,可独立设定温湿度(控温范围15—40℃,控温精度± 0.5℃,控湿范围50%—80%,控湿精度± 5%)。采用标准笼养,每笼3只鸡。鸡舍内相对湿度保持在60%,育成期每天8 h光照(9:00—17:00光照)。分为对照组(T22)和4个处理组(T24、T26、T28、T30),对照组保持基础温度22℃不变;4个处理组采用每日间歇温度处理,即每天10:00—18:00期间分别进行24℃、26℃、28℃、30℃的温度处理,其余时间恢复到基础温度22℃(升温与降温时间均在1 h以内)。预饲1周,试验处理8周。试验蛋鸡自由采食和饮水,每周统计采食量,每两周采集一次样品,每组随机挑选12只鸡,称重后断颈处死,称量腺胃重量,以及十二指肠、空肠和回肠的重量与长度。采集下丘脑、腺胃和十二指肠样品于液氮中速冻,-80℃保存。试验结束前连续3 d分别在热处理期和非热处理期统计采食量。
1.2 测定指标及方法
(1)采食量:每周统计采食量,计算平均日采食量。试验结束前连续3 d分别在热处理期和非热处理期统计采食量,计算平均每小时采食量。
平均日采食量(g/d/hen)= 耗料量/试验天数/试验鸡数;
平均每小时采食量(g/h/hen)= 耗料量/试验时间/试验鸡数。
(2)器官指数
每组随机挑选12只鸡称重后断颈处死取样,称量腺胃、十二指肠、空肠和回肠的重量。
器官指数(%)=(器官的绝对重量/鸡的体重)×100。
1.3 组织中相关基因mRNA表达水平的测定
用异硫氰二胍盐法提取组织总RNA,提取RNA的浓度和质量分别采用核酸分光光度计和琼脂糖凝胶电泳检测。反转录参照Roche公司的反转录试剂盒说明书操作进行,根据Roche公司的Real-Time PCR试剂盒(04913914001)说明书进行荧光定量检测。引物序列参见表1。以和的表达水平作为内参,采用“平均相对表达量=2-△△Ct”计算基因的相对表达量。
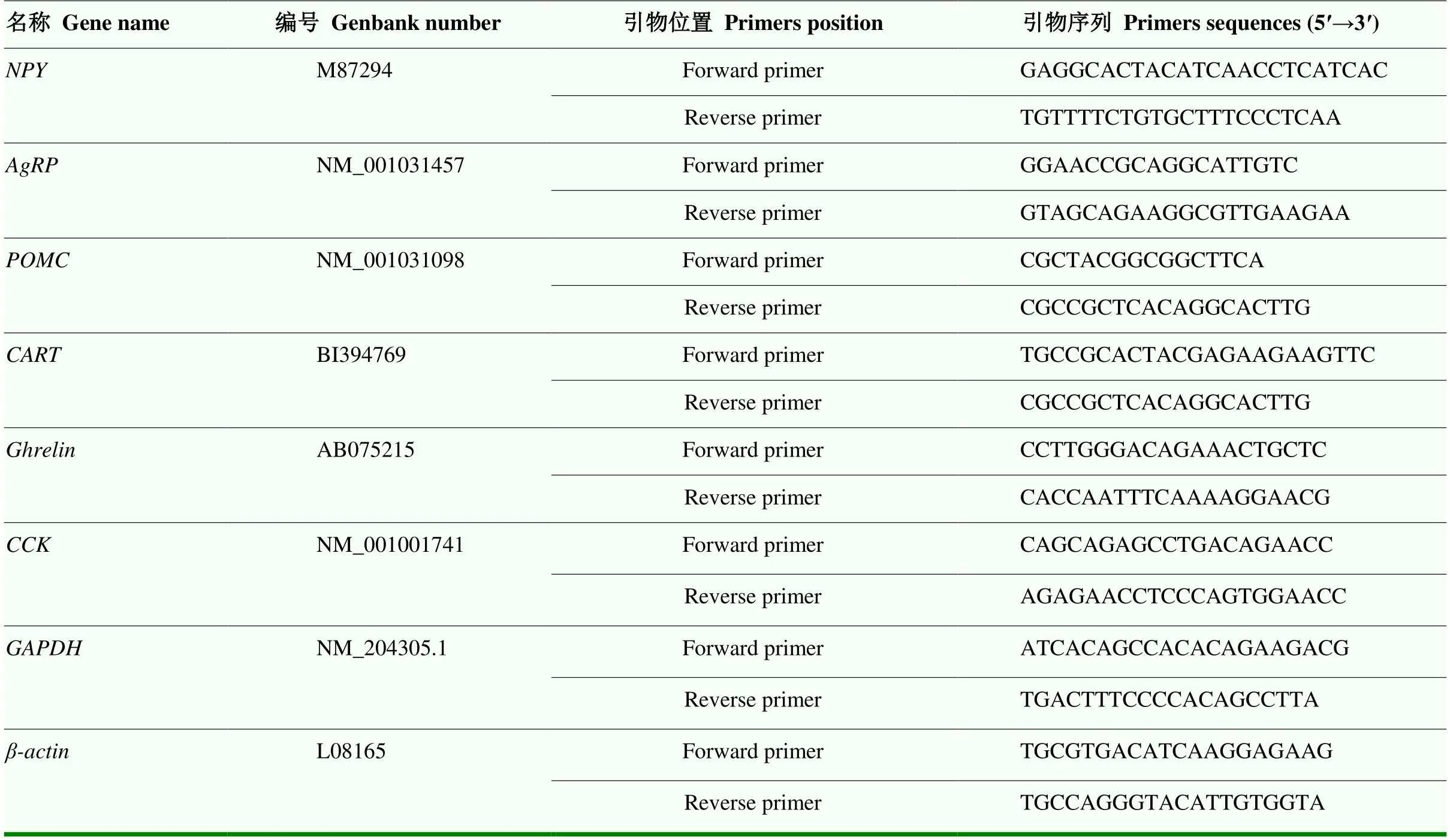
表1 基因特异性引物序列表
1.4 数据分析
试验数据采用 SAS(Version 8e,SAS Institute,1998)统计软件ANOVA程序进行单因子方差分析,Duncan 氏法进行多重比较。试验数据用平均值±标准误(Mean ± SE)表示,<0.05表示处理间差异显著,<0.01 表示处理间差异极显著。
2 结果
2.1 间歇温度处理对育成蛋鸡采食量的影响
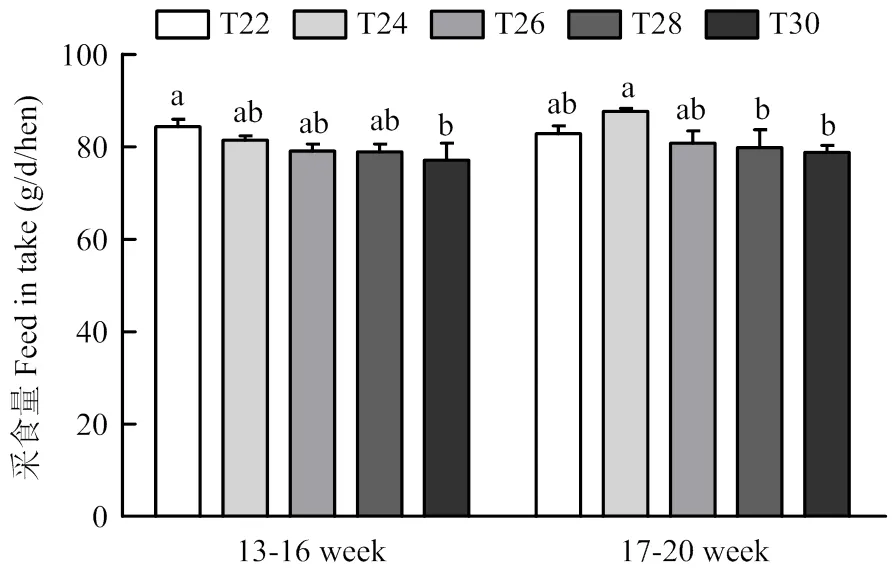
如图1所示,在试验的前4周时,与对照组T22 相比,T30组的采食量显著降低(<0.05);在试验后四周时,T24组的采食量显著高于T28组和T30组(<0.05)。
试验结束前连续3 d分别在热处理期和非热处理期统计采食量。如图2-A所示,无论是在热处理期还是非热处理期,T30组的采食量均为最低。在热处理期,T30组的采食量显著低于T22和T24;在非热处理期,T30组的采食量显著低于T22、T24和T26组(<0.05)。如图2-B所示,各组热处理期的采食量均显著低于非热处理期(<0.05)。
同一图中标不同字母者差异显著,P<0.05。下同
2.2 间歇温度处理对育成蛋鸡消化器官发育的影响
如图3所示,14周时,各组的腺胃指数、十二指肠指数、空肠指数和回肠指数没有显著差异(>0.05)。16周时,与对照组T22相比,各处理组的腺胃指数极显著升高(<0.01);T30组的空肠指数和回肠指数显著低于T28 组(<0.05)。18周时,T26组和T30组的腺胃指数显著低于T28 组(<0.05);T30组的十二指肠指数和回肠指数显著低于T28 组(<0.05);与T22组相比,T30组的空肠指数显著降低,且低于其他处理组(<0.05)。20周时,与T22组相比,T24组的腺胃指数、十二指肠指数和空肠指数显著升高(<0.05),且空肠指数显著高于T30组(<0.05),T26组的回肠指数显著升高,且高于T28 组(<0.05)。其余指标无显著差异(>0.05)。
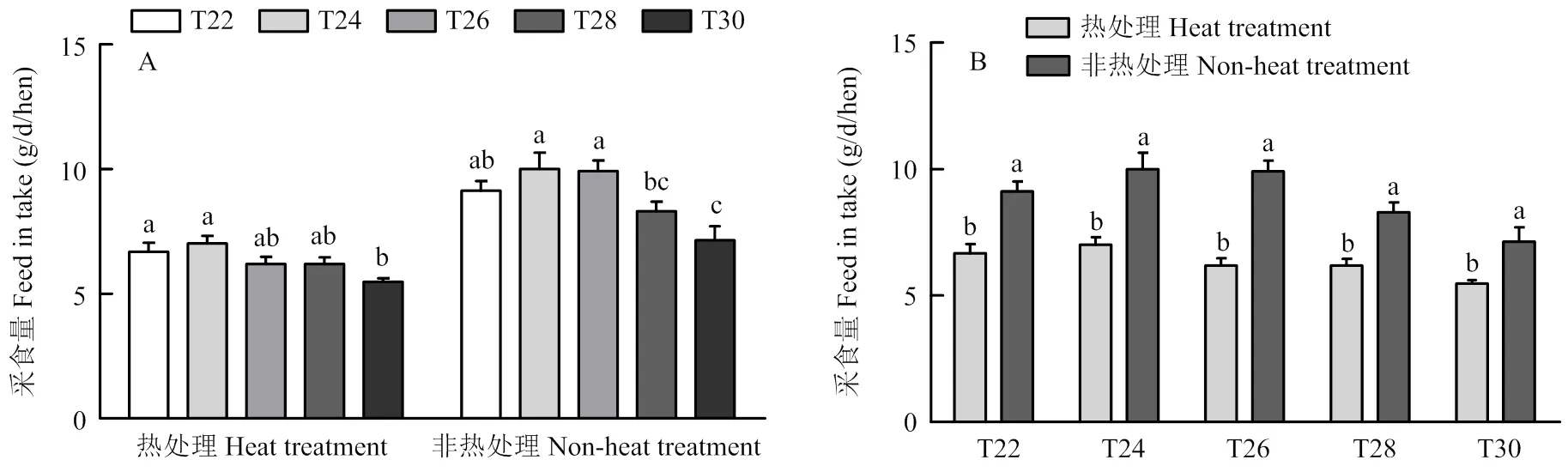
图2 热处理期与非热处理期温度对育成蛋鸡采食量的影响
如表2所示,各组十二指肠、空肠和回肠的长度均无显著差异(>0.05)。
2.3 间歇温度处理对育成蛋鸡食欲基因表达水平的影响
如图4所示,14周时,与对照组T22相比,各处理组下丘脑的表达量显著升高(<0.05),T26组、T28组和T30组下丘脑的表达量显著降低(<0.05),T30组十二指肠的表达量显著升高(<0.05)。16周时,与T22组相比,T30组下丘脑的表达量显著升高(<0.05);T24组下丘脑的表达量显著高于T28组(<0.05)。18周时,T28组下丘脑的表达量显著高于T24 组和T26组(<0.05);与T22组和其他热处理组相比,T28组下丘脑的表达量显著升高(<0.05)。20周时,与T22组相比,T28组下丘脑的表达量显著升高,且高于T26组和T30组(<0.05),T24组、T28组和T30组下丘脑的表达量显著降低(<0.05),T24组和T26组腺胃的表达量显著降低(<0.05);T28组下丘脑的表达量显著高于T26 组和T30组(<0.05)。其余指标无显著差异(>0.05)。
3 讨论
3.1 间歇温度处理对育成蛋鸡生产性能的影响
家禽的适宜温度为16—25℃,据统计,温度在21—30℃之间每升高1℃,采食量下降1.5%,温度在32— 38℃之间每升高1℃,采食量约下降4.6%。在较高的环境温度下,家禽的产热量随着饲料消耗量的降低而降低[32]。当鸡舍内温度上升到一定程度时,家禽采食中枢会受到抑制,促使采食量下降。当外界温度高于自身适宜温度后,家禽会增加呼吸频率,增加采食频次,减少采食持续时间,降低总采食量[33]。目前的长期热应激试验大多是全程高温,这不符合温度的日变化规律。因此本试验设计了间歇温度处理,每天10:00—18:00进行8 h的温度处理。升温与降温时间均在1 h内,符合温度的日变化规律,且避免了因温度控制不善对试验结果产生的干扰影响。在间歇温度处理前4周,T30组的采食量最低;在试验后4周,T24 组采食量最高,T30组采食量仍然最低。主要是因为T24组的体重显著高于其他处理组,而30℃高温处理超出蛋鸡适宜的生活温度,损伤了蛋鸡的消化系统,从而降低了蛋鸡的采食量。而且,我们发现在非热处理期,T30组的采食量仍然最低,表明经过长期高温刺激,蛋鸡的消化系统已经受到损伤。无论在哪个温度处理中,非处理期间的平均每小时采食量均高于处理组,对高温组来说,这是由于环境温度下降促进了采食,而对低温组来说,这可能是由于光照后的采食高峰引起的[34]。
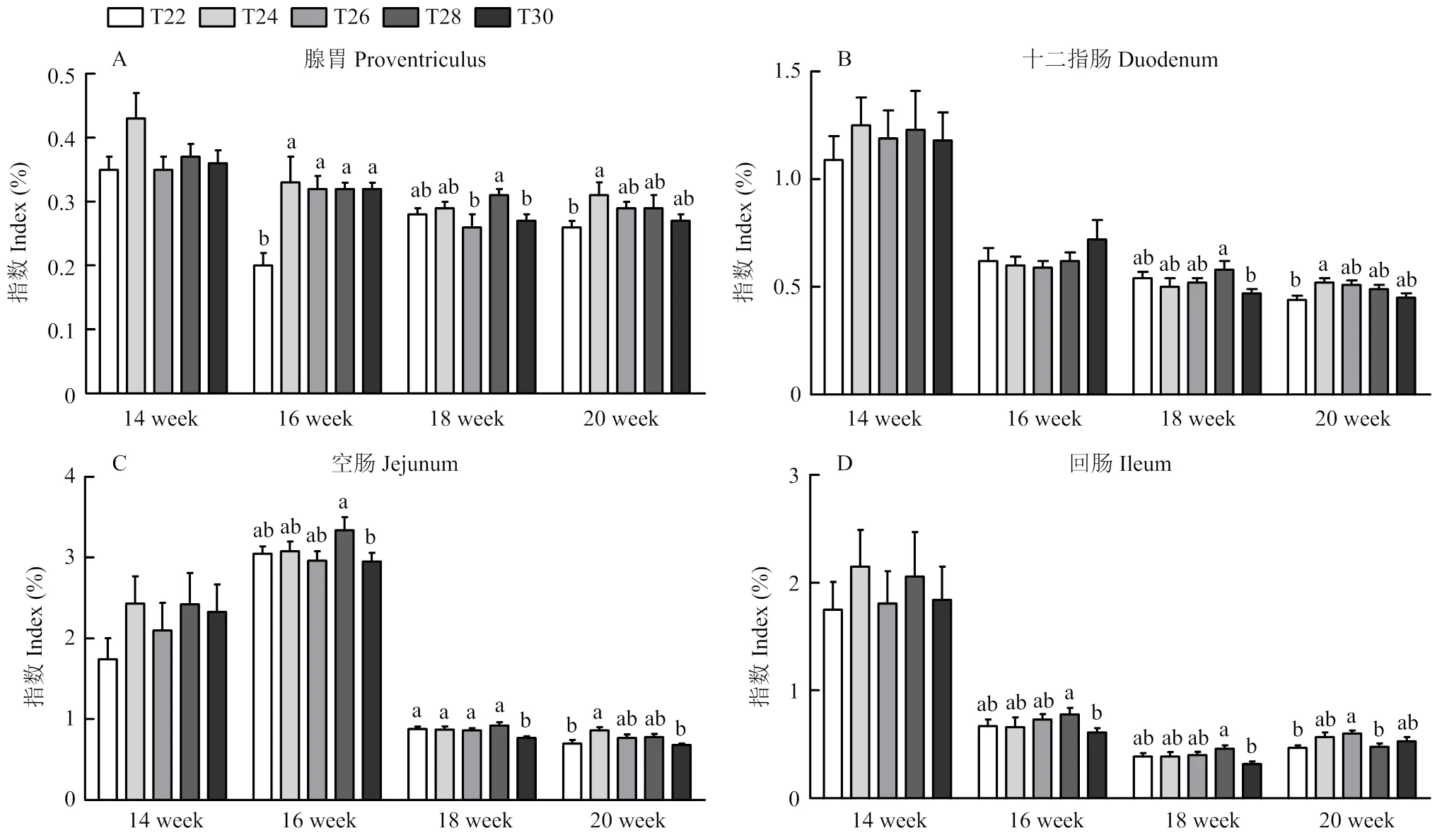
图3 间歇温度处理对育成蛋鸡消化器官指数的影响
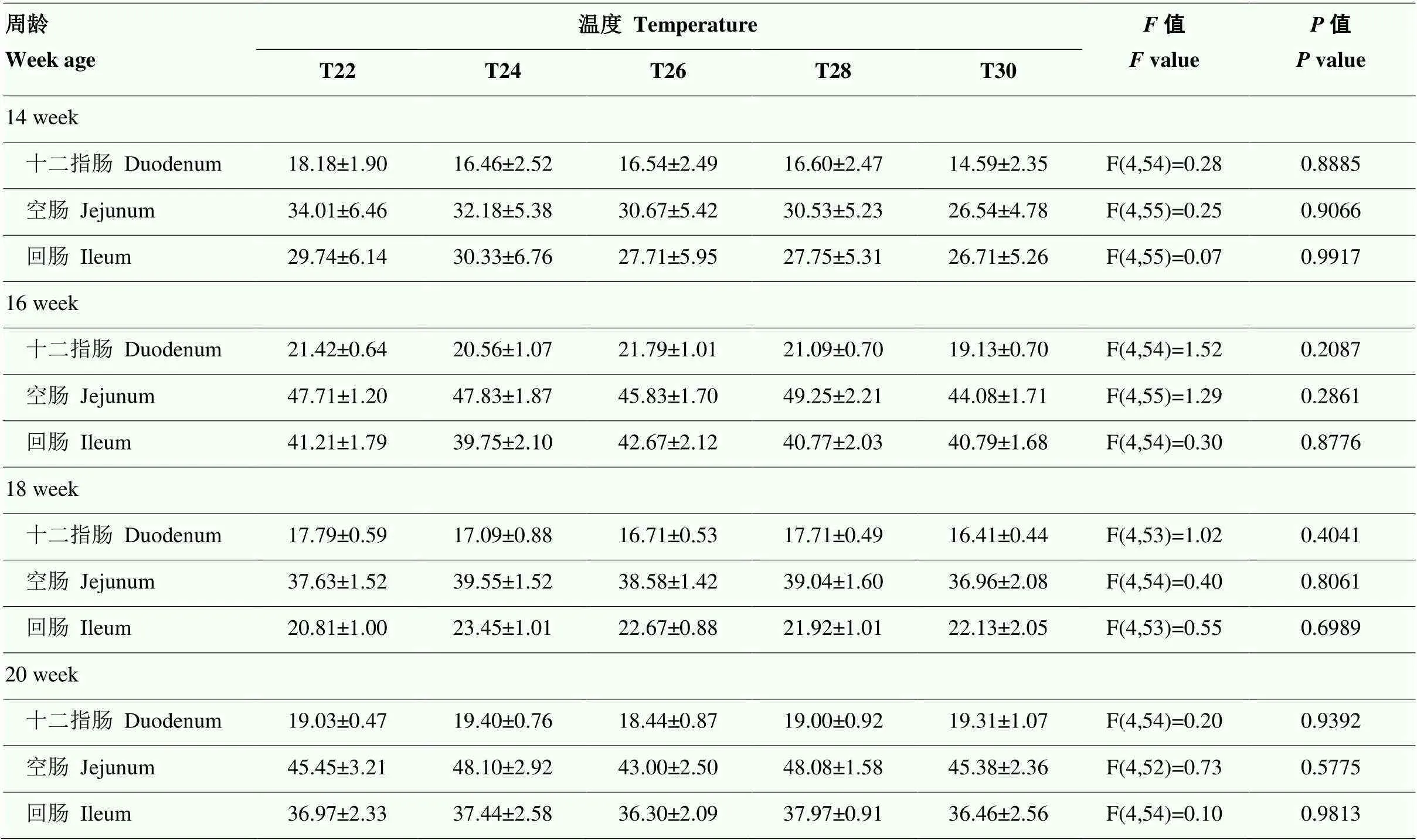
表2 间歇温度处理对育成蛋鸡肠道长度发育的影响
同一行中标不同字母者差异显著,<0.05,n = 12 Means with different letters differ significantly,<0.05, n = 12
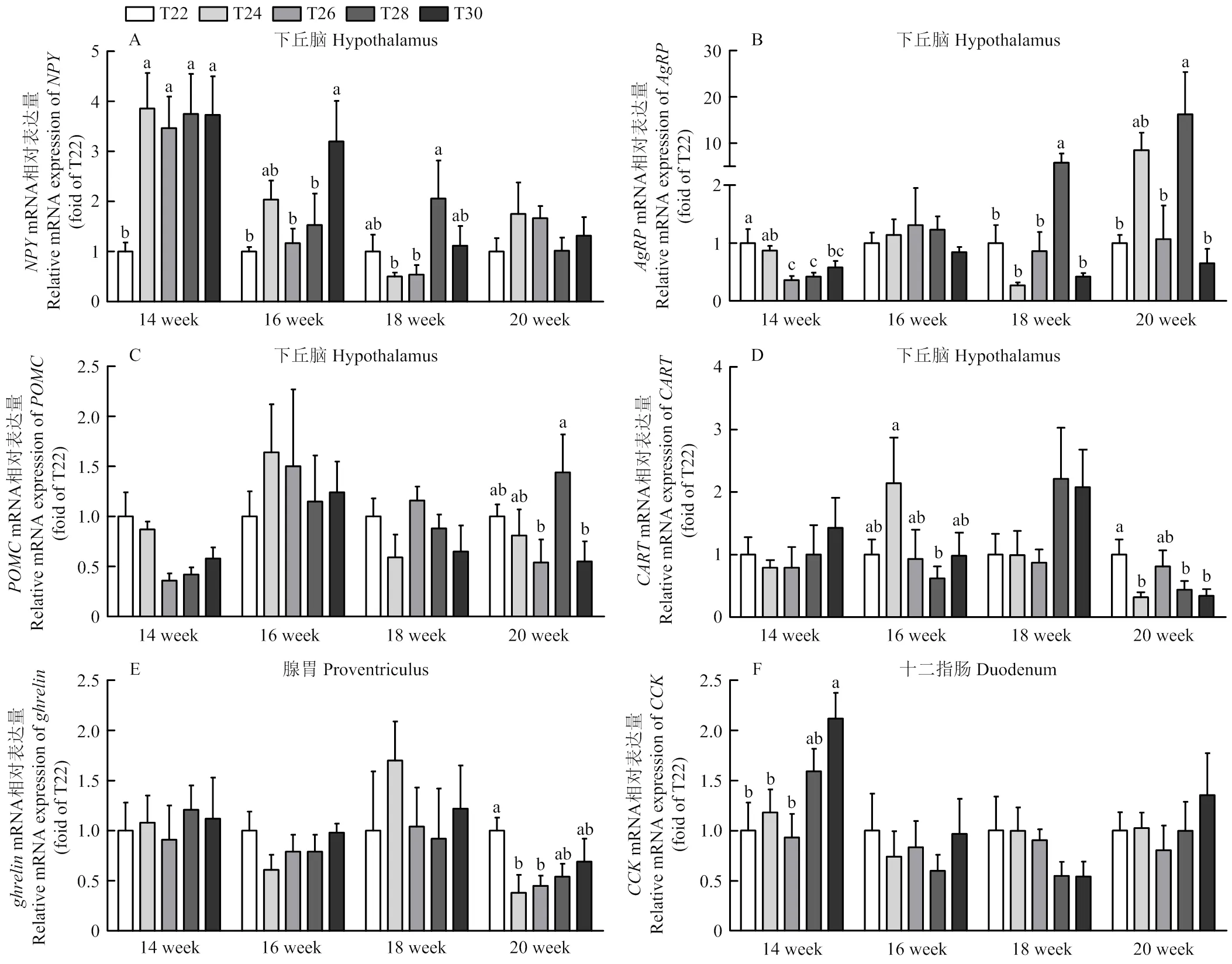
图4 间歇温度处理对育成蛋鸡食欲基因的影响
3.2 间歇温度处理对育成蛋鸡消化器官发育的影响
胃肠道是动物维持生长的消化系统的重要组成部分[35]。热应激、氧化应激和缺氧条件对单胃动物胃肠功能和代谢的负面影响已得到充分证明[36]。研究表明胃肠道对应激源高度敏感,被认为是受热应激影响的主要靶器官之一[37-38]。本试验中,间歇温度处理对肠道的长度没有显著影响。可能是因为常规的热应激试验设定的热应激温度在30—38℃之间,而本试验是为了研究育成期蛋鸡的最佳采食所需温度,设定的温度范围是22—30℃,并未影响到蛋鸡肠道的发育。Mashaly等研究表明,与间歇性热应激相比,持续性热应激对蛋鸡的影响更严重[39]。间歇温度处理4周后,各处理组的腺胃指数均升高,且T28 组的空肠指数和回肠指数显著高于T30组,这表明育成期适当提高环境温度有利于蛋鸡的胃肠道发育。在试验后期,T24组的腺胃指数、十二指肠指数和空肠指数均升高,而T30组的空肠指数显著降低,这表明对育成期蛋鸡进行24℃间歇温度处理,会促进腺胃和肠道的发育,而30℃高温处理会损伤蛋鸡的肠道发育,从而影响采食,使采食量降低。
3.3 间歇温度处理对育成蛋鸡食欲基因表达的影响
食欲受到中枢和外周的调控,下丘脑是各种食欲调节信号的主要整合中心[40]。在家禽中,下丘脑在整合外部环境线索(尤其是应激源)方面起着关键作用,并作出适当的反应来影响采食量[19]。下丘脑神经元可以感知体温的升高,并对负责控制摄食的细胞产生抑制作用。下丘脑既是体温控制中枢,也是采食控制中枢。热信号在传入下丘脑后,不仅会开启家禽的体温平衡机制,而且还会传递到摄食中枢,从而改变家禽采食行为。家禽采食量一般与环境温度呈负相关。一方面外界温热信号可能直接作用于家禽下丘脑食欲调控中枢,另一方面可以削弱消化道活动,导致消化道食物充盈从而抑制食欲。为期7 d的热应激处理能够显著降低蛋鸡采食量,显著升高下丘脑中的和T的表达量,降低下丘脑的表达量[25]。研究表明,急性热应激可以显著增加腺胃、十二指肠和空肠中的表达水平,降低十二指肠的表达水平,而对中枢食欲基因表达无显著影响[41]。从而证明了急性热应激对食欲的调控位点主要在腺胃和肠道上。
本试验属于长期温热刺激,除了可以影响外周食欲基因外,还对中枢食欲基因作用明显。整体来看,24℃温度处理提高了下丘脑促食因子的表达,抑制了下丘脑抑食因子和腺胃的表达。而30℃高温处理抑制了下丘脑促食因子的表达并提高了十二指肠抑食因子的表达。因此,高温处理可能通过抑制中枢促食基因的表达,同时提高外周抑食基因的表达,来影响蛋鸡的采食。环境的温热刺激可能是通过作用于蛋鸡下丘脑和胃肠信号介导,使蛋鸡在高温环境下表现为厌食,适温下采食增加。
4 结论
育成期环境温度保持在24℃可以促进蛋鸡胃肠道的发育,提高下丘脑中促食因子的表达,抑制下丘脑抑食因子和腺胃的表达量,有利于蛋鸡的生长发育。而30℃高温处理会对蛋鸡的肠道造成损伤,抑制下丘脑促食因子的表达,同时促进十二指肠抑食因子的表达,从而抑制蛋鸡采食,降低采食量。综上所述,蛋鸡育成期的饲养温度保持在24℃对其生长发育最为有利。
[1] 余健剑, 束刚, 江青艳. 氨基酸调控畜禽采食的研究进展. 动物营养学报, 2011, 23(6): 908-913. doi:10.3969/j.issn.1006-267X.2011.06.003.
YU J J, SHU G, JIANG Q Y. Recent advances in regulating feed intake by amino acids. Acta Zoonutrimenta Sinica, 2011, 23(6): 908-913. doi:10.3969/j.issn.1006-267X.2011.06.003. (in Chinese)
[2] RICHARDS M P, PROSZKOWIEC-WEGLARZ M. Mechanisms regulating feed intake, energy expenditure, and body weight in poultry. Poultry Science, 2007, 86(7): 1478-1490. doi:10.1093/ps/86.7.1478.
[3] WILLIAMS K W, ELMQUIST J K. From neuroanatomy to behavior: central integration of peripheral signals regulating feeding behavior. Nature Neuroscience, 2012, 15(10): 1350-1355. doi:10.1038/nn.3217.
[4] HUSSAIN S S, BLOOM S R. The regulation of food intake by the gut-brain axis: implications for obesity. International Journal of Obesity, 2013, 37(5): 625-633. doi:10.1038/ijo.2012.93.
[5] HARRIS G C, ASTON-JONES G. Arousal and reward: a dichotomy in orexin function. Trends in Neurosciences, 2006, 29(10): 571-577. doi:10.1016/j.tins.2006.08.002.
[6] MORTON G J, CUMMINGS D E, BASKIN D G, BARSH G S, SCHWARTZ M W. Central nervous system control of food intake and body weight. Nature, 2006, 443(7109): 289-295. doi:10.1038/ nature05026.
[7] BOSWELL T, DUNN I C. Regulation of agouti-related protein and pro-opiomelanocortin gene expression in the avian arcuate nucleus. Frontiers in Endocrinology, 2017, 8: 75. doi:10.3389/fendo.2017. 00075.
[8] TUNG Y C L, PIPER S J, YEUNG D, O’RAHILLY S, COLL A P. A comparative study of the central effects of specific proopiomelancortin (POMC)-derived melanocortin peptides on food intake and body weight in pomc null mice. Endocrinology, 2006, 147(12): 5940-5947. doi:10.1210/en.2006-0866.
[9] TACHIBANA T, SUGAHARA K, OHGUSHI A, ANDO R, KAWAKAMI S I, YOSHIMATSU T, FURUSE M. Intracerebroventricular injection of agouti-related protein attenuates the anorexigenic effect of alpha-melanocyte stimulating hormone in neonatal chicks. Neuroscience Letters, 2001, 305(2): 131-134. doi:10.1016/S0304- 3940(01)01827-4.
[10] SANEYASU T, HONDA K, KAMISOYAMA H, IKURA A, NAKAYAMA Y, HASEGAWA S. Neuropeptide Y effect on food intake in broiler and layer chicks. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 2011, 159(4): 422-426. doi:10.1016/j.cbpa.2011.04.008.
[11] HONDA K, SANEYASU T, HASEGAWA S, KAMISOYAMA H. A comparative study of the central effects of melanocortin peptides on food intake in broiler and layer chicks. Peptides, 2012, 37(1): 13-17. doi:10.1016/j.peptides.2012.06.015.
[12] BERTILE F, OUDART H, CRISCUOLO F, MAHO Y L, RACLOT T. Hypothalamic gene expression in long-term fasted rats: Relationship with body fat. Biochemical and Biophysical Research Communications, 2003, 303(4): 1106-1113. doi:10.1016/S0006-291X(03)00481-9.
[13] FANG X L, ZHU X T, CHEN S F, ZHANG Z Q, ZENG Q J, DENG L, PENG J L, YU J J, WANG L N, WANG S B, GAO P, JIANG Q Y, SHU G. Differential gene expression pattern in hypothalamus of chickens during fasting-induced metabolic reprogramming: Functions of glucose and lipid metabolism in the feed intake of chickens. Poultry Science, 2014, 93(11): 2841-2854. doi:10.3382/ps.2014-04047.
[14] 孙永波, 王亚, 萨仁娜, 张宏福. 家禽采食量调控机制及主要调控因子研究进展. 动物营养学报, 2018, 30(1): 22-29. doi:10.3969/j. issn.1006-267x.2018.01.004.
SUN Y B, WANG Y, SA R N, ZHANG H F. Research progress on regulation mechanism and main regulatory factors of feed intake in poultry. Chinese Journal of Animal Nutrition, 2018, 30(1): 22-29. doi:10.3969/j.issn.1006-267x.2018.01.004. (in Chinese)
[15] RAMIAH S K, ATTA AWAD E, HEMLY N I M, EBRAHIMI M, JOSHUA O, JAMSHED M, SAMINATHAN M, SOLEIMANI A F, IDRUS Z. Effects of zinc oxide nanoparticles on regulatory appetite and heat stress protein genes in broiler chickens subjected to heat stress. Journal of Animal Science, 2020, 98(10): skaa300. doi:10. 1093/jas/skaa300.
[16] HONDA K, SANEYASU T, KAMISOYAMA H. Gut hormones and regulation of food intake in birds. The Journal of Poultry Science, 2017, 54(2): 103-110. doi:10.2141/jpsa.0160100.
[17] WOODS S C. The control of food intake: behavioral versus molecular perspectives. Cell Metabolism, 2009, 9(6): 489-498. doi:10.1016/ j.cmet.2009.04.007.
[18] KEWAN A, SANEYASU T, KAMISOYAMA H, HONDA K. Effects of fasting and re-feeding on the expression of CCK, PYY, hypothalamic neuropeptides, and IGF-related genes in layer and broiler chicks. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 2021, 257: 110940. doi:10.1016/j.cbpa.2021. 110940.
[19] RICHARDS M P, ROSEBROUGH R W, COON C N, MCMURTRY J P. Feed intake regulation for the female broiler breeder: In theory and in practice. Journal of Applied Poultry Research, 2010, 19(2): 182-193. doi:10.3382/japr.2010-00167.
[20] FERKET P R, GERNAT A G. Factors that affect feed intake of meat birds: A review. International Journal of Poultry Science, 2006, 5(10): 905-911. doi:10.3923/ijps.2006.905.911.
[21] 王继强, 龙强, 李爱琴, 张宝彤. 鸡的热应激及抗热应激添加剂的应用研究. 饲料工业, 2008, 29(15): 20-22. doi:10.3969/j.issn.1001- 991X.2008.15.007.
WANG J Q, LONG Q, LI A Q, ZHANG B T. Research on heat stress and application of anti-heat stress additives of broiler. Feed Industry, 2008, 29(15): 20-22. doi:10.3969/j.issn.1001-991X.2008.15.007. (in Chinese)
[22] SONG Z H, CHENG K, ZHENG X C, AHMAD H, ZHANG L L, WANG T. Effects of dietary supplementation with enzymatically treatedon growth performance, intestinal morphology, digestive enzyme activities, immunity, and antioxidant capacity of heat-stressed broilers. Poultry Science, 2018, 97(2): 430-437. doi:10. 3382/ps/pex312.
[23] 常双双, 李萌, 厉秀梅, 石玉祥, 张敏红, 冯京海. 日循环变化偏热环境对肉鸡血清脑肠肽和盲肠菌群多样性的影响. 中国农业科学, 2018, 51(22): 4364-4372. doi:10.3864/j.issn.0578-1752.2018.22.014.
CHANG S S, LI M, LI X M, SHI Y X, ZHANG M H, FENG J H. Effects of the daily cycle variation of the moderate ambient temperatures on the serum brain gut peptide and the diversity of caecal microflora in broilers. Scientia Agricultura Sinica, 2018, 51(22): 4364-4372. doi:10.3864/j.issn.0578-1752.2018.22.014. (in Chinese)
[24] HE S P, YU Q F, HE Y J, HU R Z, XIA S T, HE J H. Dietary resveratrol supplementation inhibits heat stress-induced high-activated innate immunity and inflammatory response in spleen of yellow-feather broilers. Poultry Science, 2019, 98(12): 6378-6387. doi:10.3382/ps/pez471.
[25] SONG Z G, LIU L, SHEIKHAHMADI A, JIAO H C, LIN H. Effect of heat exposure on gene expression of feed intake regulatory peptides in laying hens. Journal of Biomedicine and Biotechnology, 2012, 2012: 484869. doi:10.1155/2012/484869.
[26] ROMANOVSKY A A. Thermoregulation: some concepts have changed. Functional architecture of the thermoregulatory system. American Journal of Physiology Regulatory, Integrative and Comparative Physiology, 2007, 292(1): R37-R46. doi:10.1152/ajpregu.00668.2006.
[27] ITO K, BAHRY M A, HUI Y, FURUSE M, CHOWDHURY V S. Acute heat stress up-regulates neuropeptide Y precursor mRNA expression and alters brain and plasma concentrations of free amino acids in chicks. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 2015, 187: 13-19. doi:10.1016/ j.cbpa.2015.04.010.
[28] 李燕,吴斌,许啸.急性热应激对樱桃谷肉鸭小肠形态的影响//生态环境与畜牧业可持续发展学术研讨会暨中国畜牧兽医学会学术年会和全国畜牧兽医青年科技工作者学术研讨会会议. 2012:2.
LI Y, WU B, XU X. Effect of acute heat stress on the small intestine morphology//Symposium on Ecological Environment and Sustainable Development of Animal Husbandry, Annual Academic Meeting of China Animal Husbandry and Veterinary Society and National Symposium on Young Scientific and Technological Workers of Animal Husbandry and Veterinary Medicine. 2012: 2. (in Chinese)
[29] 胡艳欣, 肖冲, 佘锐萍, 张发明, 郭延军, 罗冬梅, 刘凤华. 热应激对猪肠道结构及功能的影响. 科学技术与工程, 2009, 9(3): 581-586. doi:10.3969/j.issn.1671-1815.2009.03.012.
HU Y X, XIAO C, SHE R P, ZHANG F M, GUO Y J, LUO D M, LIU F H. Effect of heat stress on structure and function of pigs intestines. Science Technology and Engineering, 2009, 9(3): 581-586. doi:10. 3969/j.issn.1671-1815.2009.03.012. (in Chinese)
[30] COSEN-BINKER L I, BINKER M G, NEGRI G, TISCORNIA O. Influence of stress in acute pancreatitis and correlation with stress-induced gastric ulcer. Pancreatology, 2004, 4(5): 470-484. doi: 10.1159/000079956.
[31] QUINTEIRO-FILHO W M, RIBEIRO A, FERRAZ-DE-PAULA V, PINHEIRO M L, SAKAI M, SÁ L R M, FERREIRA A J P, PALERMO-NETO J. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poultry Science, 2010, 89(9): 1905-1914. doi:10.3382/ps. 2010-00812.
[32] SAHIN K, ONDERCI M, SAHIN N, GURSU M F, KHACHIK F, KUCUK O. Effects of lycopene supplementation on antioxidant status, oxidative stress, performance and carcass characteristics in heat- stressed Japanese quail. Journal of Thermal Biology, 2006, 31(4): 307-312. doi:10.1016/j.jtherbio.2005.12.006.
[33] BARRETT N W, ROWLAND K, SCHMIDT C J, LAMONT S J, ROTHSCHILD M F, ASHWELL C M, PERSIA M E. Effects of acute and chronic heat stress on the performance, egg quality, body temperature, and blood gas parameters of laying hens. Poultry Science, 2019, 98(12): 6684-6692. doi:10.3382/ps/pez541.
[34] WANG X J, LIU Z M, ZHAO J P, JIAO H C, LIN H. Dusk feeding in laying hens is shifted by light program via involvement of clock genes. Journal of Animal Physiology and Animal Nutriton (Berl). 2021, doi: 10.1111/jpn.13528. Online ahead of print. doi: 10.1111/ jpn.13528.
[35] ZHAO P P, ZHANG K X, GUO G Y, SUN X, CHAI H L, ZHANG W, XING M W. Heat shock protein alteration in the gastrointestinal tract tissues of chickens exposed to arsenic trioxide. Biological Trace Element Research, 2016, 170(1): 224-236. doi:10.1007/s12011-015- 0462-9.
[36] HE J N, MA L X, QIU J L, LU X T, HOU C C, LIU B, YU D Y. Effects of compound organic acid calcium on growth performance, hepatic antioxidation and intestinal barrier of male broilers under heat stress. Asian-Australasian Journal of Animal Sciences, 2020, 33(7): 1156-1166. doi:10.5713/ajas.19.0274.
[37] LIU C P, CHAUDHRY M T, ZHAO D, LIN T, TIAN Y B, FU J. Heat shock protein 70 protects the quail cecum against oxidant stress, inflammatory injury, and microbiota imbalance induced by cold stress. Poultry Science, 2019, 98(11): 5432-5445. doi:10.3382/ps/pez327.
[38] VARASTEH S, BRABER S, AKBARI P, GARSSEN J, FINK- GREMMELS J. Differences in susceptibility to heat stress along the chicken intestine and the protective effects of galacto-oligosaccharides. PLoS ONE, 2015, 10(9): e0138975. doi:10.1371/journal.pone. 0138975.
[39] MASHALY M M, HENDRICKS G L 3rd, KALAMA M A, GEHAD A E, ABBAS A O, PATTERSON P H. Effect of heat stress on production parameters and immune responses of commercial laying hens. Poultry Science, 2004, 83(6): 889-894. doi:10.1093/ps/83.6.889.
[40] HE X F, LU Z, MA B B, ZHANG L, LI J L, JIANG Y, ZHOU G H, GAO F. Chronic heat stress alters hypothalamus integrity, the serum indexes and attenuates expressions of hypothalamic appetite genes in broilers. Journal of Thermal Biology, 2019, 81: 110-117. doi:10. 1016/j.jtherbio.2019.02.025.
[41] LEI L, HEPENG L, XIANLEI L, HONGCHAO J, HAI L, SHEIKHAHMADI A, YUFENG W, ZHIGANG S. Effects of acute heat stress on gene expression of brain-gut neuropeptides in broiler chickens (domesticus). Journal of Animal Science, 2013, 91(11): 5194-5201. doi:10.2527/jas.2013-6538.
Effects of Intermittent Different Temperature on Feeding and Intestinal Development of Growing Laying Hens
LIU ZengMin, PAN YaLi, LIN Hai, JIAO HongChao, ZHAO JingPeng, WANG XiaoJuan*
College of Animal Science and Technology, Shandong Agricultural University/Shandong Provincial Key Laboratory of Animal Biotechnology and Disease Control and Prevention, Tai’an 271018, Shandong
【】The objective of this study was to study the effects of ambient temperature on feeding and intestinal development of poultry, and to supplement the absent temperature parameters for laying hens rearing, so as to provide a certain scientific basis for the correct feeding of laying hens.【】A total of 360 Issa brown laying hens aged 11 weeks were selected and divided into 5 treatment groups with 6 replicates per group and 12 hens per replicate. The experimental laying hens were transferred to 5 intelligent environmental control chicken houses for 1 week of pre-trial and 8 weeks of formal experiment, with 3 chickens per cage. The relative humidity in the chicken house was kept at 60%, and the light was kept for 8 h (9:00-17:00) every day during the prelay period. The temperature of the control group was kept unchanged at 22℃, and the four treatment groups were carried out in a manner of daily intermittent, including 24℃, 26℃, 28℃, and 30℃ at 10:00-18:00 every day, respectively, and changed to the base temperature 22℃ for the rest of the time, the heating and cooling time were within 1 h. The experiment lasted for 8 weeks. The experimental laying hens were free to eat and drink, the feed intake was counted weekly, and the samples were collected once every two weeks. Twelve hens in each group were randomly selected and weighed, and then killed by neck cutting. The weight of glandular stomach, the weight and length of duodenum, jejunum and ileum were weighed. Hypothalamus, glandular stomach and duodenum samples were frozen in liquid nitrogen and stored at -80℃. Feed intake was calculated in heat treatment period and non-heat treatment period for 3 days before the end of experiment.【】Compared with T22 group, the feed intake in T30 group was significantly decreased at 13-16 week (<0.05); the feed intake in T24 group was significantly higher than that in T28 and T30 groups at 17-20 week (<0.05). During the heat treatment period, the feed intake of T30 group was significantly lower than that in T22 and T24 groups (<0.05). The feed intake of T30 group was significantly lower than that in T22, T24 and T26 groups during the non-heat treatment period (<0.05). The feed intake in the heat treatment period was significantly lower than that in the non-heat treatment period (<0.05), and the highest feed intake was maintained in T24 group and the lowest in T30 group. Compared with T22 group, the glandular gastric index was significantly increased at 16 week (<0.01). At 18 week, compared with T22 group, the jejunum index in T30 group was significantly lower than that in other groups (<0.05). At 20 week, compared with T22 group, the glandular stomach index, jejunum index and ileum index in T24 group were significantly increased (<0.05), and the jejunum index was significantly higher than that in T30 group (<0.05). Compared with T22 group, the expression of(Neuropeptide Y) in hypothalamus of all treatment groups was significantly increased at 14 week (<0.05). The expression of(Agouti-related protein) in hypothalamus of T30 group was significantly decreased (<0.05), and the expression of(Cholecystokinin) in duodenum of T30 group was significantly increased (<0.05). At 20 week, compared with T22 group, the expression of(amphetamine-regulated transcript) in hypothalamus of T24 group was significantly decreased (<0.05), and the expression ofin glandular stomach of T24 group was significantly decreased (<0.05).【】These results indicated that ambient temperature at 24℃ during the growing period could promote the development of gastrointestinal tract, increase the expression ofin hypothalamus, and inhibit the expression ofandin hypothalamus, which was beneficial to the growth and development of laying hens. However, the high temperature treatment at 30℃ damaged the intestinal tract of laying hens, inhibited the expression of hypothalamus feeding promoting factor, and promoted the expression of duodenal feeding inhibiting factor, thus inhibiting feeding intake and reducing feed intake of laying hens.
laying hens; feed intake; temperature; prelay period; intestine; hypothalamus

2021-12-12;
2022-05-27
国家重点研发计划(2018YFE0128200)、山东省重点研发计划(2019JZZY020602)、国家现代农业产业技术体系建设专项资金(CARS- 40-K09)、山东省“双一流”奖补资金、泰山学者项目(201511023)
刘增民,E-mail:1325865598@qq.com。潘亚丽,E-mail:3229482556@qq.com。刘增民和潘亚丽为同等贡献作者。通信作者王晓鹃,E-mail:wangxj@sdau.edu.cn
(责任编辑 林鉴非)
