Total flavonoids of Scutellaria barbata improving podocyte injury induced by high glucose through Smad4/PKM2/HIF-1α pathway
2023-03-28YANDongGUChangruiZHANGXiang
YAN Dong, GU Chang-rui, ZHANG Xiang
1.Heilongjiang Academy of traditional Chinese Medicine, Harbin 150000, China
2.Heilongjiang Ruijing Diabetes Hospital, Harbin 150000, China
Keywords:
ABSTRACT
1.Introduction
Diabetes nephropathy (DN) is one of the most common complications of diabetes and the main cause of chronic kidney disease and end-stage renal disease.Its occurrence is related to hyperglycemia, hypertension and other factors, but the exact mechanism is still unclear [1].Studies have found that hyperglycemia can cause the accumulation of advanced glycation end products(AGEs), inflammation and oxidative stress, increase the production of proinflammatory cytokines and oxidative stress products,damage membrane protein, lipid, carbohydrate oxidation and DNA,apoptosis and necrosis of renal tubular epithelial cells, accumulation of extracellular matrix, cause renal dysfunction, and finally lead to DN[2].At present, the conventional methods for treating DN are mainly to control blood glucose and block the renin angiotensin aldosterone system, but they cannot eliminate the risk of DN[3].Therefore, how to understand the pathogenesis of DN development more comprehensively and seek new and effective treatment measures are particularly important for the treatment of DN.
Podocytes, as a highly specialized and terminally differentiated tubular epithelial cell, are the main cells constituting the glomerular filtration barrier.The typical feature of early DN is the function and structure damage of podocytes, which also leads to the damage of selective glomerular filtration function and the formation of proteinuria [4-5].The production of reactive oxygen species (ROS)induced by continuous hyperglycemia will further lead to the damage of antioxidant defense system, oxidative stress and inflammation[6].Therefore, new therapies with antioxidant and anti-inflammatory properties have protective effects on the treatment of DN.
Scutellaria barbata, also known as Scutellaria tenuifolia and Scutellaria parvifolia, is a dry whole herb of the aboveground part of Scutellaria barbata, a plant of the Labiatae family.It was first seen in the Collection of Medicinal Relics and is one of the commonly used Chinese herbal medicines in clinical practice.Total flavonoids of Scutellaria barbata (TF-SB) is the main active ingredient in Scutellaria barbata extract, which has anti-inflammatory, antioxidant,immunomodulatory and antimutagenic effects, especially has a certain scavenging effect on oxygen free radicals [7].In addition,the study also confirmed that TF-SB has a significant therapeutic effect on hemorheology and glycolipid metabolism of diabetes rat models, which can increase serum insulin and reduce the level of glycosylated hemoglobin [8].The mechanism of TF-SB on DN induced podocyte injury has not been reported.As an important signal cascade molecule in cells, Smad4 has been proved to play an important role in podocyte injury.It can inhibit the accumulation of hypoxia inducible factor-1α (HIF-1α) by inhibiting dimer M2 pyruvate kinase (PKM2) to form tetramer PKM2 translocating into cytoplasm [9-10].We previously showed that TF-SB and Smad4 have good binding activity through molecular docking technology.In this study, we observed the effect of TF-SB on high glucose induced podocyte injury and Smad4/PKM2/HIF-1α to further clarify the mechanism of TF-SB in DN treatment.
2.Materials and methods
2.1 Preparation of TF-SB
Scutellaria barbata was purchased from the Herbal Medicine Bureau of Xiang'an Hospital of Heilongjiang Academy of traditional Chinese Medicine.TF-SB was prepared according to the reference[11].Scutellaria barbata (1 kg) was weighed, soaked in 80% ethanol at a ratio of 1:15 for overnight, heated under reflux at 70 ℃ for 1 h, extracted twice, combined with the extract, decompressed and recovered until it had no alcohol taste.Calcium carbonate was added to the recovered solution, fully stirred for 2 h, centrifuged to remove the precipitation, took the supernatant, added concentrated hydrochloric acid and adjusted to pH 2.0, fully stirred, after standing for 12 h, centrifugation, discarding the supernatant, and drying, 0.05 kg TF-SB powder was obtained, and the TF-SB mass fraction was 72.5% by UV spectrophotometry.Take an appropriate amount of extract, prepare 10 mg/mL mother liquor with methanol, and dilute it with culture medium to the required concentration.
2.2 Reagents and instruments
RPMI-1640 culture medium was purchased from Gibco Company of America (product No.: 31800022); Glucose was purchased from Sigma (product No.: G8270); Recombinant mice γ Interferon (γ interferon, IFN- γ) was purchased from Roche Company in the United States (product number: 11276905001); Fetal bovine serum(FBS), CCK8, interleukin 1β (IL-1β), tumor necrosis factor(TNF-α), Monocyte chemoattractant protein 1 (MCP-1), adenine triphosphate (ATP) and ROS detection kit were purchased from Shanghai Beyotime (product No.: C0234, C0037, PI301, PT512,PC130, S0026 and S0033S); Annexin V-FITC kit was purchased from Nanjing Keygentec Bio (product No.: KGA08); Smad4, PKM2,HIF-1 , Histone H3 and GAPDH were purchased from CST(product numbers 46535, 4053, 36169, 4499 and 97166 respectively);HRP labeled goat anti rabbit secondary antibody was purchased from Abcam (product number: ab6728); ECL chemiluminescence detection kit, reverse transcription and fluorescence quantitative detection kit were purchased from Thermo company (product No.:A38554, 4387391 and 4385612).Flow cytometry was purchased from BD (BD FACSCeresta); The full-automatic multi-function microplate reader was purchased from Thermo Fisher (Varioskan LUX).The automatic fluorescent quantitative PCR system was purchased from Roche (LightCyler® 480).The electrophoresis instrument was purchased from Bio Rad (Powerpac HC).
2.3 Cell culture
Mouse kidney foot cells (MPC-5) were purchased from ATCC(product No.HTX2304).With 10% FBS and IFN-γ, the MPC-5 cells were resuscitated in RPMI-1640 medium and cultured in 33℃, 5% CO2constant temperature and humidity incubator.When the degree of cell fusion reaches 80%~90%, the cells shall be subcultured and replaced without IFN-γ MPC5 cells were cultured in 37 ℃ and 5% CO2incubator for 10~14 days.Synchronize before the formal experiment and change the cell culture medium to be IFN free- γ continue to culture for 24 h.
2.4 Cell grouping and drug intervention
MPC-5 cells were divided into the control group, model group and TF-SB group.The control group was treated with conventional medium for 24 h, the model group was intervened with medium containing 30 mmol/L glucose for 24 h, and the TF-SB group was treated with 200 μg/mL of TF-SB was intervened together for 24 h.
2.5 Index detection
2.5.1 CCK-8
MPC-5 cells in logarithmic growth period to 1×105/mL was inoculated in 96 well plate, divided into conventional medium and high sugar medium, and incubated in 37 ℃, 5% CO2incubator for 24 h.Different concentrations of TF-SB (0, 25, 50, 100, 200, 400 μg/mL) after treating cells for 0, 6, 12, 24, 48 and 72 h, take 10 μL of CCK8 working solution was added to the plate and incubated for 2 h.The absorbance (OD) value at 450 nm was measured with the microplate reader.
In addition, MPC-5 cells from each group after intervention in Section 1.4 were taken as 1×105/mL into 96 well plate, take 10 μL of CCK8 working solution was added to the plate and incubated for 2 h.The absorbance (OD) value at 450 nm was measured with the microplate reader, and the cell viability was calculated.Cell viability(%)=(ODExperiment-ODblank)/(ODControl-ODblank) × 100%.
2.5.2 ELISA
MPC-5 cells from each group after intervention in Section 1.4 to 1×104/mL was inoculated into a 24 well plate to collect the supernatant of cell culture, and the contents of IL-1β, TNF-α,MCP-1 and ATP in the supernatant were determined by the microplate reader in strict accordance with the steps in the instructions of the ELISA kits.
2.5.3 Apoptosis detection
MPC-5 cells from each group after intervention in Section 1.4,wash them twice with PBS, collect cell precipitation, and add 100 μL Binding buffer suspension cell, add 5 μL FITC marked Annexin V and 5 μL PI, mix well, incubate at room temperature for 15 min,add 400 μL Binding buffer, cell apoptosis was detected by flow cytometry.
2.5.4 Reactive oxygen species detection
MPC-5 cells from each group after intervention in Section 1.4 to 6×105/mL was inoculated in 6-well cell culture plate, and DCFHDA fluorescent probe was used to dye the cells at 37 ℃ in dark for 20 min; PBS was washed twice, DCF fluorescence was detected by flow cytometry, and ROS level in cells was analyzed.
2.5.5 RT-PCR detection
Total RNA was extracted from MPC-5 cells of each group after intervention in Section 1.4, and cDNA was reverse transcribed.The operation was strictly in accordance with the instructions of the reverse transcription kit.The cDNA was used as the template for PCR amplification.The reaction conditions are 94 ℃: 30 s, 94 ℃:5 s; 50~60 ℃: 15 s; 72 ℃: 10 s.There are 34 cycles in total, and the primer sequence is shown in Table 1.With GAPDH as internal parameter, pass 2-ΔΔCtmethod was used to calculate the relative expression level of genes in cells.
2.5.6 Western blot detection
MPC-5 podocytes in each group were lysed on ice with RIPA buffer, homogenized at 3 000 r/min, centrifuged for 5 min, collected and measured the protein concentration.Take 15 μg protein loading,polyacrylamide gel electrophoresis, membrane transfer, 5% skim milk blocking for 1h, cell protein sample dripping Smad4 (1:1 000),PKM2 (1:1 000), HIF-1 α (1:1 000) and GAPDH (1:1 000) primary antibodies, incubate overnight at 4 ℃, add HRP labeled sheep anti rabbit IgG (1:2 000), incubate at room temperature for 1 h, drip the hypersensitive ECL developer, and let it stand at room temperature for 2 min.Use the gel imaging system for scanning analysis.Use GAPDH as the internal reference to calculate the relative expression of the target protein.In addition, the cytoplasm and nuclear protein are separated by the kit, and PKM2 (1:1 000) and Histone H3 (1:2 000) primary antibodies are added respectively.After treatment according to the above steps, Histone H3 is used as the internal reference of nuclear protein, and GAPDH is used as the internal reference of cytoplasmic protein to calculate the relative amount of PKM2 protein.
2.6 Statistical processing
The experimental data involved in this experiment were statistically analyzed by SPSS 23.0 software.Among them, the test meeting the homogeneity of variance was processed by One way ANOVA, and P<0.05 was taken as the basis for statistical difference of data.
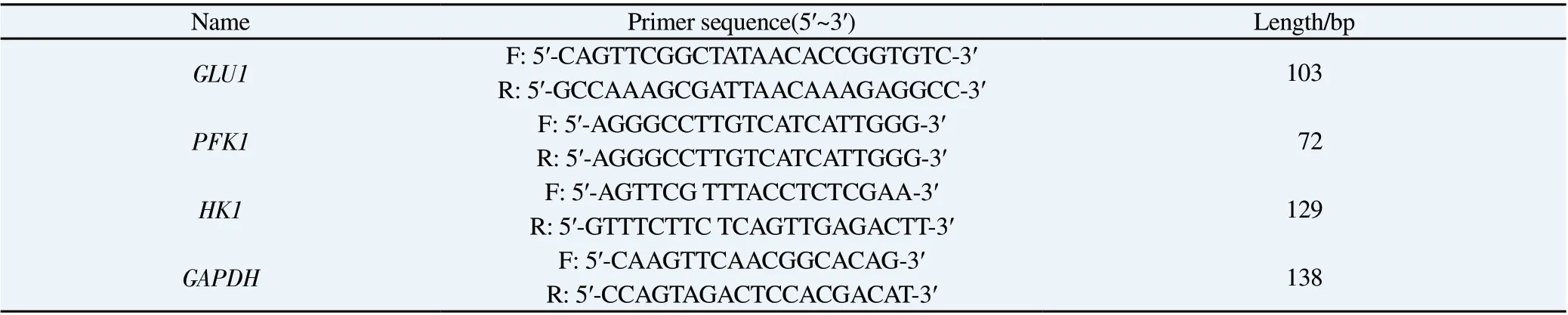
Tab1 PCR Primer sequence of each gene
3.Results
3.1 Effect of TF-SB on MPC-5 cell viability
We used CCK8 test to evaluate the cytotoxicity of TF-SB.As shown in Table 2, compared with TF-SB group with zero concentration, the concentration of TF-SB increases to 400 μg/mL,the activity of MPC-5 cells was significantly reduced, so 0~200 μg/mL of TF-SB concentration of is a safe concentration for MPC-5.To further clarify the effective concentration of TF-SB on MPC-5,different concentrations of TF-SB were added to the MPC-5 cell model induced by high glucose for treatment.The results showed that high glucose could significantly inhibit the activity of MPC-5 cells compared with blank MPC-5 cells (P<0.01); Compared with the high glucose group, with the increase of TF-SB concentration,the activity of MPC-5 cells increased significantly, and the difference was statistically significant (P<0.01).When TF-SB concentration reaches 400 μg/mL, the activity of MPC-5 cells decreased (Table 3).
As shown in Table 4, 200 μg/mL was added to the MPC-5 cell model induced by high glucose with the prolongation of the intervention time, the activity of MPC-5 cells was significantly increased with the intervention of TF-SB, and the difference was statistically significant (P<0.01).However, 24 h MPC-5 cell activity is the best, so 200 μg/mL TF-SB intervention for 24 h.
Tab3 Effect of different concentrations of TF-SB on the activity of MPC-5 cells induced by high glucose(n=3,)
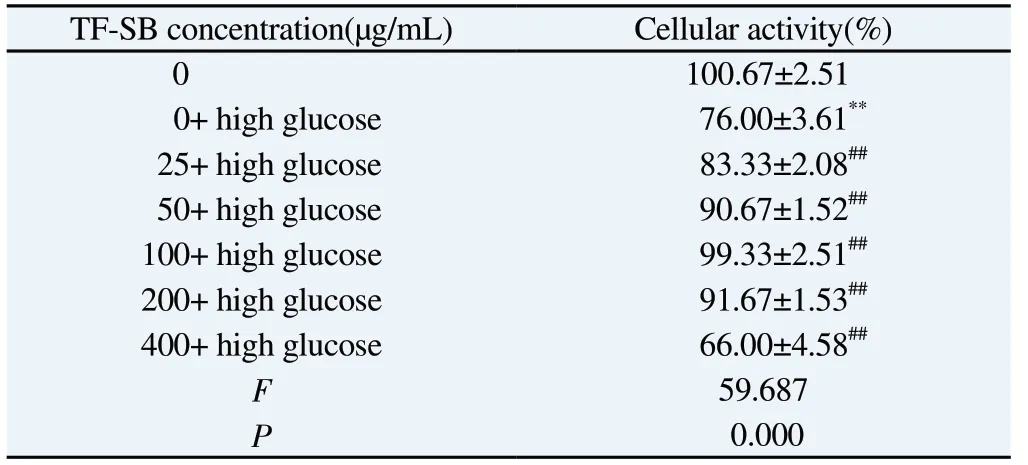
Tab3 Effect of different concentrations of TF-SB on the activity of MPC-5 cells induced by high glucose(n=3,)
Note: Compared with 0 concentration group, *P<0.05, **P<0.01; Compared with 0 concentration+ high glucose group, #P<0.05, ##P<0.01.
?
Tab4 Effects of 200 μg/mL TF-SB at different times on the activity of MPC-5 cells induced by high glucose(n=3,)
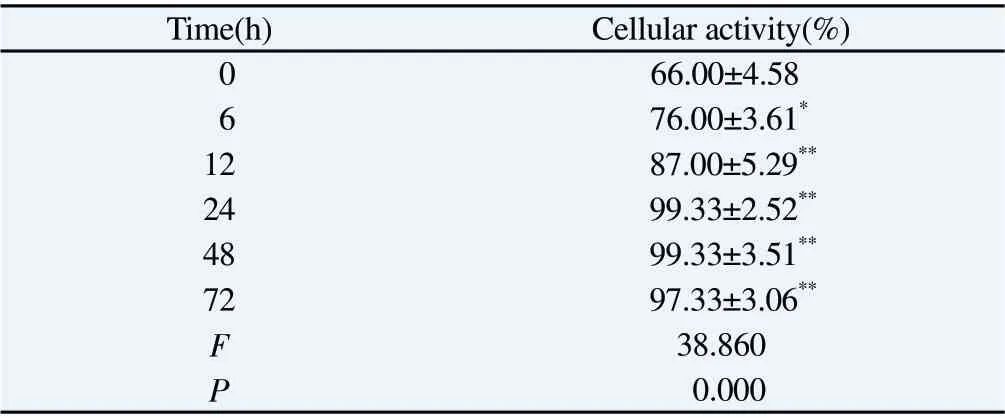
Tab4 Effects of 200 μg/mL TF-SB at different times on the activity of MPC-5 cells induced by high glucose(n=3,)
Note: Compared with 0 concentration group, *P<0.05, **P<0.01.
Time(h) Cellular activity(%)0 66.00±4.58 6 76.00±3.61*12 87.00±5.29**24 99.33±2.52**48 99.33±3.51**72 97.33±3.06**F 38.860 P 0.000
3.2 Effect of TF-SB on ATP and cytokine content of MPC-5 cells
Compared with the control group, the ATP content of MPC-5 cells in the model group decreased significantly (P<0.01); Compared with the model group, the content of ATP in TF-SB group was significantly higher (P<0.01).
Compared with the control group, the contents of IL-1β, TNF-α and MCP-1 in MPC-5 cells in the model group were significantly increased (P<0.01); Compared with model group, the contents of IL-1β, TNF-α and MCP-1 in TF-SB group decreased significantly(P<0.01).See Table 5 for details.
3.3 Effect of TF-SB on MPC-5 cell apoptosis and ROS level
Compared with the control group, high glucose exposure significantly increased the level of apoptotic cells and ROS, the difference was statistically significant (P<0.01).; Compared with the model group, TF-SB intervention could significantly inhibit the level of apoptotic cells and ROS, and the difference was statistically significant (P<0.01).See Table 6 for details.
3.4 Effect of TF-SB on the expression of glycolytic gene in MPC-5 cells
Compared with the control group, the expression abundance of GLU1, PKF1 and HK1 in MPC-5 cells in the model group decreased significantly (P<0.01); Compared with the model group,the expression abundance of GLU1, PKF1 and HK1 in TF-SB group increased significantly (P<0.01).See Table 7 for details.
3.5 Effects of TF-SB on MPC-5 cells Smad4/PKM2/HIF-1αsignal pathway related proteins expression
As shown in Figure 2, compared with the control group, the expression of Smad4 protein in MPC-5 cells and PKM2 in the nucleus of the model group significantly increased, and the expression of PKM2 in the cytoplasm significantly decreased, with a statistically significant difference (P<0.01), while the expression of total PKM2 protein had no significant change, with no statistically significant difference (P>0.05); Compared with the model group,the expression of Smad4 and PKM2 in the nucleus of TF-SB group decreased significantly, while the expression of PKM2 in the cytoplasm increased significantly (P<0.01).
Compared with the control group, the protein expression of HIF-1α in MPC-5 cells in the model group was significantly increased(P<0.01); Compared with model group, The protein expression of HIF-1α in TF-SB group was significantly decreased (P<0.01).See Table 8 for details.
Tab5 ATP, IL-β, TNF- α and MCP-11 in each group content(n=3,)
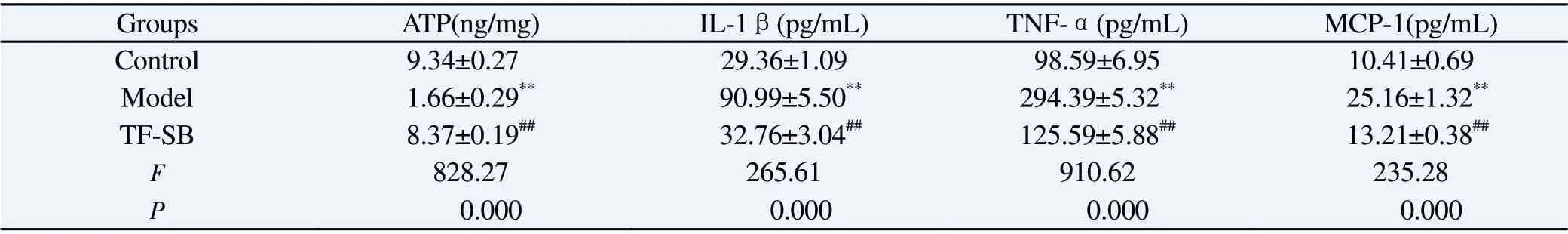
Tab5 ATP, IL-β, TNF- α and MCP-11 in each group content(n=3,)
Note: Compared with control group, *P<0.05, **P<0.01; Compared with model group, #P<0.05, ##P<0.01.
Groups ATP(ng/mg) IL-1β(pg/mL) TNF-α(pg/mL) MCP-1(pg/mL)Control 9.34±0.27 29.36±1.09 98.59±6.95 10.41±0.69 Model 1.66±0.29**90.99±5.50**294.39±5.32**25.16±1.32**TF-SB 8.37±0.19##32.76±3.04##125.59±5.88##13.21±0.38##F 828.27 265.61 910.62 235.28 P 0.000 0.000 0.000 0.000
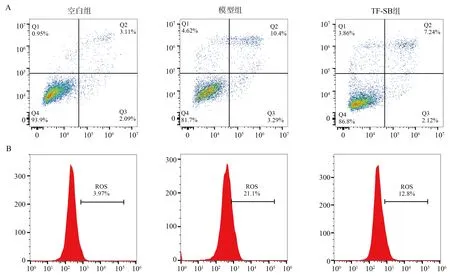
Fig1 Comparison of apoptosis and ROS levels in each group
Tab6 Comparison of apoptosis and ROS level in each group(%, n=3,)
Tab6 Comparison of apoptosis and ROS level in each group(%, n=3,)
Note: Compared with control group, *P<0.05, **P<0.01; Compared with model group, #P<0.05, ##P<0.01.
?
Tab7 Comparison of GLU1, PKF1 and HK1 mRNA expression levels among groups(n=3,) )
Tab7 Comparison of GLU1, PKF1 and HK1 mRNA expression levels among groups(n=3,) )
Note: Compared with control group, *P<0.05, **P<0.01; Compared with model group, #P<0.05, ##P<0.01.
Groups GLU1 mRNA PKF1 mRNA HK1 mRNA Control 1.00±0.00 1.00±0.00 1.00±0.00 Model 0.46±0.05**0.22±0.04**0.23±0.03**TF-SB 0.73±0.04##0.70±0.05##0.76±0.04##F 159.35 366.63 583.65 P 0.000 0.000 0.000
Tab8 Comparison of expression of Smad4/PKM2/HIF-1 signal pathway related proteins in each group(n=3,)

Tab8 Comparison of expression of Smad4/PKM2/HIF-1 signal pathway related proteins in each group(n=3,)
Note: Compared with control group, *P<0.05, **P<0.01; Compared with model group, #P<0.05, ##P<0.01.
Group Smad4/GAPDH Cytoplasmic PKM2/总PKM2 Nuclear PKM2/H3 Total PKM2/GAPDH HIF-1 /GAPDH Control 0.42±0.03 0.66±0.06 0.14±0.06 0.66±0.06 0.16±0.03 Model 0.92±0.03**0.13±0.03**0.76±0.06**0.13±0.03**0.73±0.02**TF-SB 0.49±0.03##0.48±0.03##0.24±0.03##0.48±0.03##0.39±0.02##F 262.67 126.69 117.32 126.69 478.98 P 0.000 0.000 0.000 0.000 0.000
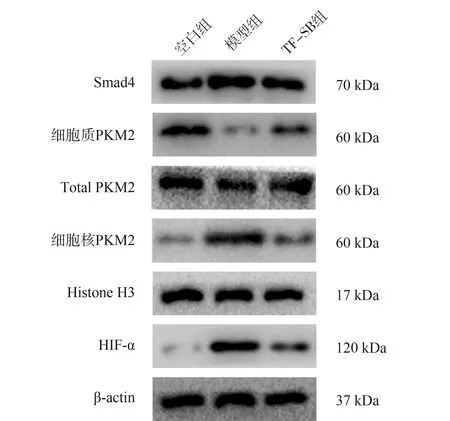
Fig2 Smad4/PKM2/HIF-1 in each group Comparison of expression of signal pathway related proteins
4.Discussion
Diabetes is one of the major diseases threatening human health.It is characterized by persistent hyperglycemia and is characterized by insulin resistance (IR) and/or pancreatic islets β A metabolic disease characterized by dysfunction or loss of cells [12].DN is an important driving factor for microvascular complications in diabetes patients.Its chronic inflammation can not only promote the activation of macrophages and destroy the glomerular filtration barrier, but also cause glomerular hypertrophy, continuous accumulation of extracellular matrix, podocyte damage, tubular damage and tubulointerstitial fibrosis [13].The results of this study confirmed that the content of IL-1β, TNF-α and MCP-1 in MPC-5 cell injury model induced by high glucose increased significantly,and TF-SB intervention can significantly reduce the content of IL-1β, TNF-α and MCP-1 in MPC-5, to achieve the effect of inhibiting inflammation.
All cells in the kidney need ATP to maintain their corresponding functions, and mitochondria mainly produce ATP through the electron transport chain, thus playing the role of antioxidation.Low concentration of ROS makes the function of mitochondria in a stable state, while high concentration of ROS has a toxic effect on cells and mitochondria, leading to the release of cytochrome C and inducing apoptosis.It was found that the abnormal activity of mitochondria would lead to an increase in the accumulation of toxic glucose metabolites, thus aggravating the damage of podocytes [14-16].Glycolysis is a key link in the glucose metabolism process.Glucose can produce pyruvate through glycolysis outside the mitochondria,and then enter the mitochondria to convert into acetyl coenzyme A,further promoting the cycle of tricarboxylic acid and generating ATP[17].GLUT1, PKF1 and HK1 are important glycolysis related genes in cells.Our results in this study confirmed that the expression levels of GLUT1, PKF1 and HK1 in MPC-5 cell injury model induced by high glucose were significantly reduced.TF-SB intervention can significantly increase the expression levels of GLUT1, PKF1 and HK1, inhibit the accumulation of ROS and promote the production of ATP, thereby improving the function of mitochondria, improving podocyte injury and inhibiting apoptosis.
HIF-1 as one of the key regulators of mammalian hypoxia response, it is not only involved in the pathogenesis of diabetes, but also involved in the occurrence and development of macrovascular and microvascular complications of diabetes [18].PKM2, as a rate limiting enzyme in the glycolysis process, usually translocate to the nucleus in the form of dimer to activate HIF-1α It also upregulates the expression of a series of glycolysis related genes, thereby enhancing glycolysis activity [19].Similarly, PKM2 plays a central role in the function of glomerular mitochondria.The dimerized PKM2 can translocate to the nucleus through its non-standard protein kinase activity, participate in the regulation of inflammatory genes, and affect the pathogenesis of DN [20].The results of this study indicate that TF-SB intervention can inhibit Smad4 and HIF-1α Protein expression, thereby inhibiting PKM2 protein entry into the nucleus.
To sum up, TF-SB can improve podocyte injury induced by high glucose, promote the activity of podocyte mitochondria and induce glycolysis, thereby inhibiting the secretion of inflammation.Its mechanism is probably related to inhibition of Smad4/PKM2/HIF-1α Activation of signal pathway.However, the current study also has many shortcomings.Due to the lack of inhibitor products targeting Smad4, the current study did not set up an inhibitor control group to verify the mechanism of TF-SB.In the next experiment,we will use the control experiment of inhibitors in vivo and establish MPC-5 cell lines with Smad4 silencing and overexpression in vitro to further analyze the target of TF-SB.
Tab2 Effect of different concentrations of TF-SB on MPC-5 cell activity(n=3,)
Note: Compared with 0 concentration group,*P<0.05,**P<0.01.
TF-SB concentration(μg/mL) Cellular activity(%)0 100.33±2.08 25 106.00±3.00 50 104.97±1.53 100 100.67±4.16 200 101.33±3.21 400 81.33±4.73**F 22.105 P 0.000
[18] Ren R, Guo J, Shi J, et al.PKM2 regulates angiogenesis of VR-EPCs through modulating glycolysis, mitochondrial fission, and fusion.J Cell Physiol 2020, 235(9): 6204-6217.
[19] Zhang Z, Deng X, Liu Y, et al.PKM2, function and expression and regulation.Cell Biosci 2019, 9: 52.
[20]Zahra K, Dey T, Ashish, et al.Pyruvate kinase M2 and cancer: The role of PKM2 in promoting tumorigenesis.Front Oncol 2020, 10: 159.
杂志排行
Journal of Hainan Medical College的其它文章
- Mechanism of ROS-NLRP3 signaling pathway in rats with acute pancreatitis
- Effects of CUMS combined with CRS on hippocampal glial cells and synaptic plasticity in depressed mice
- Effect of electroacupuncture at pericardium meridian on D-ser and NMDAR in acute phase of MCAO rats
- Serum anti-blood type IgG/IgM binding and cytotoxicity to pig PBMC and RBC
- Effect of Cx32 over-expression on cell proliferation, migration, and invasion of hepatocellular carcinoma cell line Huh7 and its mechanism
- METTL14 upregulates m6A modification of pri-miR-141 inhibiting ZEB1 to promote proliferation and inflammation of lung fibroblasts
