Mesenchymal stem cells in ischemic tissue regeneration
2023-03-17RenataSzydlak
Renata Szydlak
Renata Szydlak,Chair of Medical Biochemistry,Faculty of Medicine,Jagiellonian University Medical College,Kraków 31-034,Poland
Abstract Diseases caused by ischemia are one of the leading causes of death in the world.Current therapies for treating acute myocardial infarction,ischemic stroke,and critical limb ischemia do not complete recovery.Regenerative therapies opens new therapeutic strategy in the treatment of ischemic disorders.Mesenchymal stem cells(MSCs)are the most promising option in the field of cell-based therapies,due to their secretory and immunomodulatory abilities,that contribute to ease inflammation and promote the regeneration of damaged tissues.This review presents the current knowledge of the mechanisms of action of MSCs and their therapeutic effects in the treatment of ischemic diseases,described on the basis of data from in vitro experiments and preclinical animal studies,and also summarize the effects of using these cells in clinical trial settings.Since the obtained therapeutic benefits are not always satisfactory,approaches aimed at enhancing the effect of MSCs in regenerative therapies are presented at the end.
Key Words:Ischemia;Mesenchymal stem cells;Regenerative medicine;Stem cell therapy;Clinical applications
INTRODUCTION
Ischemia of an area is defined as insufficient blood supply to specific tissues and various organs or individual parts of the body(e.g.,limbs).Reduced blood perfusion causes inadequate transport of oxygen and nutrients to tissue-resident cells,leading to ischemia and ultimately damaging tissues and organs.The leading cause of tissue ischemia is the narrowing or blockage of the lumen of an artery,most often due to the formation of atherosclerotic plaques,thrombus,or spasms of a specific artery[1].Disruption of blood flow to particular organs or parts of the body may be chronic developing over several months or years or acute occurring suddenly(e.g.,during exercise)and usually takes a more rapid course often with a worse prognosis.Ischemia resulting from atherosclerotic lesions is most commonly found in the heart muscle,lower extremities,kidney,and brain[1-4].Examples of ischemic diseases caused by the narrowing of the coronary and cerebral arteries that cause high morbidity and mortality in patients are myocardial infarction and ischemic stroke(Figure 1)[5].Ischemia may entail several functional changes at the level of individual cells that build tissues,causing their dysfunction and death due to necrosis or apoptosis[6].
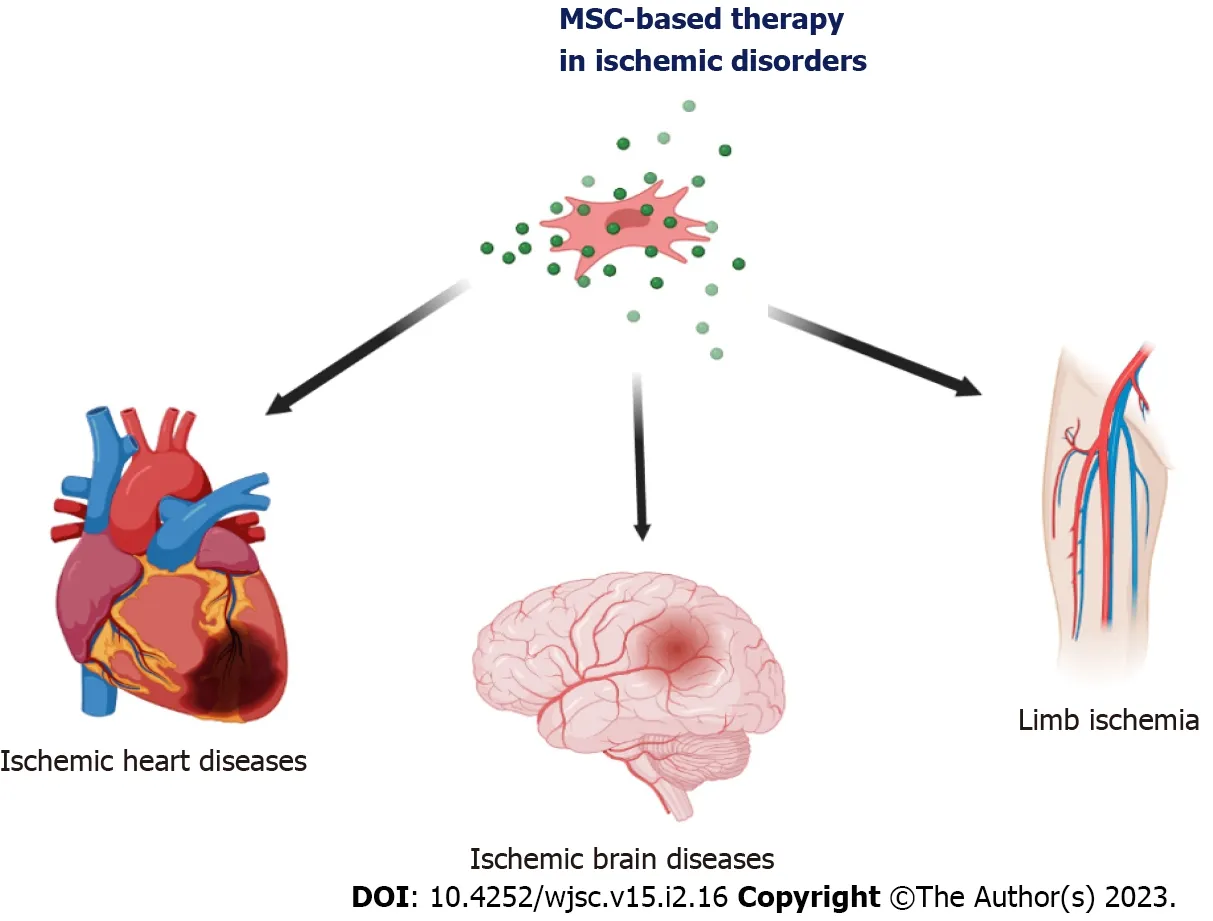
Figure 1 Mesenchymal stem cell therapy in ischemic diseases.The mesenchymal stem cells can be use as biological drugs in the treatment of ischemic disorders of various organs,including brain ischemia in ischemic stroke,heart ischemia in acute myocardial infraction,Chronic ischemic cardiomyopathy,ischemic heart failure,and chronic lower extremity ischemia.MSC:Mesenchymal stem cell.
Standard treatments for ischemia include invasive and pharmacological control and treatments for the effects of ischemia in the damaged tissues.These methods emphasize improving the quality of and extending the patient’s life,but they cannot fully reverse the effects of tissue ischemia in case of people suffering from congenital heart disease[7],ischemic heart failure[8],acute limb ischemia[9],critical limb ischemia[10],and ischemic stroke[11].Even with the substantial progress of therapeutic strategies,these approaches do not provide the expected clinical benefits for all patients.Therefore,novel treatment pathways while replacing or supporting classic therapeutic approaches should continue to be investigated[12-14].
Currently,great hopes are placed on regenerative treatment,i.e.,therapies based on cellular preparations,including using various progenitor and stem cells(SCs)and their products[8,12,15].Over the past decade,growing effort has been directed to the regenerative properties of SCs in relation to the biological treatment strategy of substitution of damaged cells in the tissue with new ones[16].It is also believed that SCs may be involved in the neovascularization of ischemic tissues[17,18].
In 1970,Friedensteinet al[19]discovered an exceptional type of cell that has been extensively researched over the years for its potential use in regenerative medicine to treat ischemic damage[20].These cells,called mesenchymal SCs(MSCs),reside in both young and adult donors[e.g.,umbilical cord(UC),Wharton’s jelly(WJ)amniotic fluid,UC blood(UCB),placenta,adipose tissue(AT),bone marrow(BM),dental pulp(DP),and others],which has been a particular and exciting source of SCs for many years,mainly for autotransplantation and allotransplantation.Regardless of the tissue source,a cell that meets the criteria set out by the International Society for Cell Therapy(Table 1)may be qualified as MSCs[21].
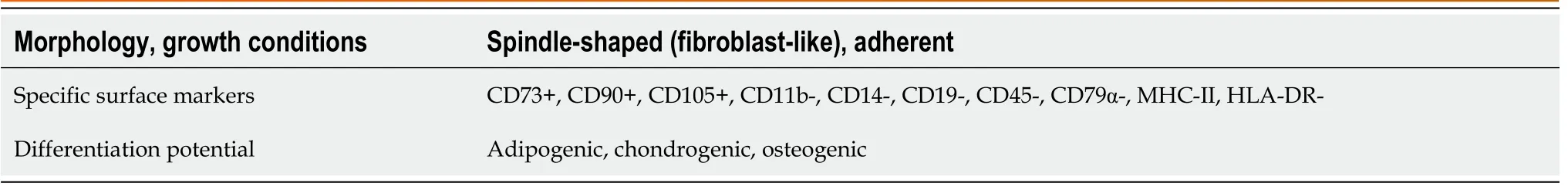
Table 1 Minimal criteria for defining mesenchymal stem cells
They now account for the most popular SC population used in clinical trials worldwide(clinicaltrials.gov).MSCs derived from birth-related tissues have more promise due to better proliferative capacity compared with MSCs obtained from adult tissues.They are safe in terms of both sourcing and ethical aspects[22,23].Due to several specific characteristics,these cells are essential candidates for regenerative therapies for ischemic tissues.Moreover,it is possible to use these cells to manufacture ready-to-use SC-based medicinal products.This review described the potential therapeutic mechanisms of MSCs in the context of ischemic disorder treatment.Exemplary clinical trials and procedures enhancing the therapeutic effect of MSCs were also discussed.
PATHOGENESIS OF ISCHEMIC DISEASES AND MSC-BASED THERAPY
The leading cause of ischemic disorders is the chronic inflammatory disease of the arteries,called“atherosclerosis”.It can be caused by many risk factors,including aging,high blood pressure,diabetes,hypercholesterolemia,and smoking[24,25].In the course of atherosclerosis,pathological damage and dysregulation of the endothelium lining the blood vessel wall,accumulation of lipids,smooth muscle cells,leukocytes,and foam cells are observed.Also,there is an aggregation of platelets in the lumen of the blood vessels,which leads to the formation of plaques narrowing the lumen of the vessels[26,27].Moreover,in atherosclerotic lesions,increased expression of matrix metalloproteinases(MMP)and their participation in weakening the vascular wall through the degradation of the extracellular matrix(ECM)have been demonstrated[28].
To compensate for the degradation of the ECM,smooth muscle cells migrate from the outer layers of the artery wall to the inner lining of the sheath to increase the collagen secretion rate[25].It often causes undesirable remodeling as macrophages secrete cytokines such as tumor necrosis factor(TNF)-α,interleukin(IL)-1β,and IL-6 to induce smooth muscle cell apoptosis[25].The unbalanced degradation rate of the ECM caused by increased collagen production results in the formation of atherosclerotic plaques with a thin fibrous collagen cap[29].Injecting MSCs at this stage can modulate immune cell function,MMP activity,and the secretion of proinflammatory cytokines and restore collagen homeostasis[30].In addition,due to hemodynamic changes and high shearing stresses in atherosclerotic plaques,ruptures and bleeding may occur,increasing platelet recruitment,coagulation processes,and thrombus formation[31].Due to the secretion of proangiogenic factors and the ability to differentiate in the endothelium,MSCs can promote angiogenesis to restore blood flow to ischemic tissues for tissue regeneration and organ function restoration[32].
KEY PLAYERS IN ISCHEMIC TISSUE REGENERATION
Over the past decade,scientists and clinicians often discuss the regenerative properties of SCs in the context of biological treatment approaches.These strategies involve the replacement of damaged tissue cells with new SCs,including ischemic myocardium[16,33].SCs are also considered cells that can participate in the neovascularization of ischemic tissues,which may also be associated with the improvement of the function of this organ[18,34,35].
One type of cell studied for years for their potential future use in regenerative medicine to treat myocardial damage or improve perfusion in limb muscle tissue is the mesenchymal/stromal SC(MSC)isolated from the BM,AT,birth-associated tissues,and other sites.These cells were found in both young and adult donors and have been an essential and exciting source of SCs,primarily for autotransplantation.They now account for the most often used SC population in clinical trials worldwide(clinicaltrial.gov)[18,36,37].
The history of studies on MSCs began in the 1970s when Friedensteinet al[19]observed colonyforming unit-fibroblasts(today known as MSCs).These cells constituted a fraction of adherent cells in the BM[19].Moreover,their previous studies showed that subpopulations of BM cells could differentiate to other cell types,i.e.,osteoblasts[38].These discoveries initiated an increase in interest in MSCs and the search for these cells in other tissues as well.
One of the primary and best-known sources of MSCs is BM[39,40].Cells with similar morphology and biological characteristics can also be found in other tissues collected from adult donors,such as peripheral blood,AT,DP,and fetal tissues such as UCB and WJ[41].
BM-MSCs show morphological and phenotypic similarities to AT-MSCs and WJ-MSCs.These markers include CD29 +,CD44 +,CD73 +,CD90 +,CD105 +,CD166 +,human leukocyte antigen(HLA)-ABC +,CD11b-,CD14-,CD19a-,CD34-,CD45-,CD79-,and HLA-DR[42,43].However,the source tissues from which MSCs are isolated differ in the content of these SCs.For example,the number of MSCs in the BM is lower than in AT[43,44].The BM contains from 0.0017% to 0.02% of MSCs among all mononuclear cells,while in AT,these cells constitute 5.0% to 25.6% of all cells,which is the so-called stromal-vascular fraction obtained from this tissue[44-46].The number of colony-forming unit-fibroblast colonies isolated from the same number of plated BM or AT cells is several times higher from AT than BM[42].Also,WJ-MSCs cells have a higher frequency of colony-forming unit-fibroblasts than BM-MSCs cells[47].Considering the differentiation potential of BM-MSCs,AT-MSCs,and UC-MSCs,these cells show a comparable capacity to differentiate into osteoblasts,chondrocytes,and adipocytes[42,48],confirming their mesodermal origin.
MSCs derived from various tissues can also be differentiatedin vitrointo phenotypically similar cells,including cardiomyocytes[49-53],vascular endothelial cells(ECs)[54,55],and nerve cells[56].However,it has been shown that the effectiveness of such differentiation is variable and depends on the tissue origin[49,51,53,56].Within one culture of MSCs,cells may be more or less predisposed to differentiate to a specific phenotype of the mature cell[57].Despite the morphological and antigenic similarity between MSCs from different source tissues,the results of world studies showed that BM-MSCs may offer a different expression profile of many genes compared to placenta-derived MSCs[57],which suggests that MSCs obtained from BM,WJ,and AT may differ in terms of their molecular composition and their ability to differentiate.
The International Society for Cellular Therapy,to clarify the nomenclature and define the requirements of human MSCs and facilitate the comparison of test results between laboratories around the world,proposed three minimum criteria for characterizing human MSCs[21].
Due to numerous world studies showing the relatively wide potential of MSCs to differentiate into various types of tissues,these cells constituting a population obtained from mature tissues have for years interested scientists in the context of their potential use in tissue regeneration[58-60].
MSC MECHANISMS OF ISCHEMIC TISSUE REGENERATION
Multipotent MSCs show many features desirable from the point of view of their potential use in the regeneration of damaged tissues,not only in autologous but also in allogeneic transplants.Therefore,over the last decade,many studies have been undertaken to explain the mechanisms of action of these cells replaced by tissue damage.Figure 2 presents several essential mechanisms of action contributing to ischemic tissue regeneration.
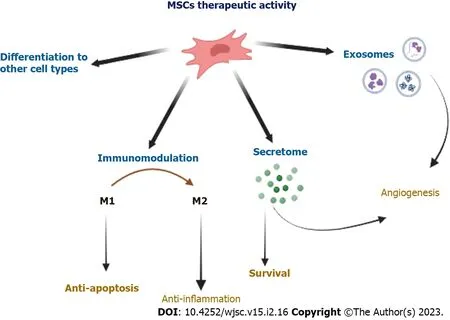
Figure 2 Proposed therapeutic mechanisms of mesenchymal stem cells,including differentiation,immunomodulation,secretion of paracrine factors,and secretion of microvesicles.Mesenchymal stem cells(MSCs)can repair and/or rescue injured cells via differentiation into replacement cell types including endothelial cells,and cardiomyocytes.The immunomodulatory activity of MSCs includes the suppression of macrophage polarization to M1,though favors M2 polarization.MSCs can secrete a number of factors that stimulate the regeneration of damaged cells and tissues[e.g.,vascular endothelial growth factor(VEGF),insulin-like growth factor 1(IGF-1),hepatocyte growth factor(HGF),stromal cell-derived factor-1]and having a beneficial effect for neovascularization processes(e.g., VEGF,IGF-1,HGF,fibroblast growth factor-2,platelet-derived growth factor-BB,placental growth factor),inhibiting the pathological remodeling of ischemic tissues[e.g.,matrix metalloproteinase 2(MMP-2),MMP-9].The exosomes secreted by MSCs contain cytokines and growth factors,signaling lipids,mRNAs,and regulatory microRNAs which can be responsible for the therapeutic effects.MSC:Mesenchymal stem cell.
Differentiation of MSCs
It was initially suggested that MSCs administrated into the area of tissue damage are able to differentiate into desired cell types,including muscle-building cardiac mesenchymal cells(CMCs),vascular smooth muscle cells,and ECs[59,61,62].It was shownin vivothat MSCs injected to the heart muscle had a phenotype similar to differentiating cells,including CMCs and ECs[63-65].Pochonet al[66]confirmed that WJ-MSCs differentiated into CMCsin vitro,expressing CMC markers and spontaneous throbbing,which might be evidence of their terminal maturation into specialized cells capable of playing their proper functions.Although MSCs show the potential to differentiate to CMCsin vitro,the fundamental issue is to restore damaged tissuein vivo.Effective delivery and retention must be emphasized here because if cells do not reach the target tissue,they cannot exert any therapeutic effect[60].Even if studies have shown that MSCs can differentiate into various types of myocardial cells,an increasing number of studies show that this is not a main mechanism for their regenerative activity in the cardiovascular system.
Several articles now contain information on the neural differentiation of MSCs from various sourcesin vitro[67-69].In the described studies,the prevailing view suggests that MSCs,derived from immature tissues due to their plastic properties,can differentiate more effectively into cells with neural phenotypes,showing the presence of typical linear markers[70].Positive results ofin vitroneural differentiation of MSCs were obtained in experiments using specific chemical compounds,growth factors,coculturing with mature neurons,or the culture of three-dimensional aggregates[71,72].Another study showed that neural differentiation of MSCs can be induced by a conditioned medium derived from cultures of olfactory ensheathing cells or Schwann cells[73].Sotthibundhuet al[74]discovered that stimulation of autophagy MSCs could improve the efficiency of SC differentiation and the formation of neural-like cells.
Interaction of MSCs with other cells
MSCs can communicate with neighboring cells through direct cell-cell interactions,including gap junctions and tunneling nanotubes[75].Moreover,MSCs are able to transport mitochondria through nanotubes and thus obtain cardiological protection by regaining the respiratory chain in myocytes[76].To allow damaged tissue to regenerate,MSCs communicate with other cells in the damaged areas to recruit other types of SCs.For example,studies have demonstrated that CMCs can re-enter the cell cycle after supplementation with specific cytokines secreted by MSCs(e.g.,transforming growth factor-β)[77].MSCs can also keep other cells to active migration to the area of tissue damage,as demonstrated by the trafficking of hematopoietic SCs to the damaged myocardium[78].
Immunomodulatory properties of MSCs
Despite the reported low rate of retention of MSCs in ischemic heart muscle after their injection,the results of many experiments showed improvement in the functional heart parameters,like inhibition of adverse tissue remodeling myocardium and left ventricle ejection fraction(LVEF)[33].So,this begs the questions:Is the improvement in injured organ function following the administration of MSCs results only from the implantation and ability of MSCs to differentiate into specific cell types or is another mechanism also involved in this process?
No doubt,that in order to answer this question,other studies,which have focused on the immunomodulatory properties of MSCs,should be mentioned here[79-81].The remarkable ability of MSCs to produce an enormous number of soluble factors,such as anti-inflammatory cytokines,enables them to modulate the immune system response[82,83].For example,MSCs secrete the cytokines,IL-4 and IL-10,which inhibit the proliferation of T cells,the growth factor,hepatocyte growth factor,which inhibits the proliferation of CD4+ T cells,and transforming growth factor-β1,which with prostaglandin E2 inhibits the inflammation process[84-86].Moreover,they encourage the maturation of monocytes towards anti-inflammatory macrophages type M2[79,87].
In addition,the most extraordinary attribute of MSCs is an immunological privilege.MSCs are known to be capable of avoiding and suppressing immune responses[88,89].Most MSCs show the low expression of HLA class I and a lack of HLA class II markers.Due to this feature,they do not cause an immune conflict between the transplant recipient and the injected cells.Additionally,MSCs possess HLA-G,which is a key factor in the elimination of the fetus rejection by the maternal immune system[90,91].Because of the high expression of HLA-G,MSCs can modulate the tolerance of the immune system and it has a very beneficial effect on acceptance of the transplant.
Paracrine effects of MSCs
Another reported mechanism of therapeutic activity of MSCs can be attributed to the secretion of paracrine factors,including several cytokines,growth factors,and chemokines,that may regulate many regenerative processes at the MSCs implantation site[92].Proangiogenic molecules produced by MSCs involve,among others,the protein fibroblast growth factor-2[92-94],platelet-derived growth factor[95],and an extremely proangiogenic vascular endothelial growth factor,supporting the proliferation of vascular smooth muscle cells and ECs as well as the migration and the new blood vessel structure formation[96].
On the other hand,molecules that promote ECM remodeling involve MMP1,MMP2,MMP9,a family of enzymes that degrade ECM structure,and TNF-α and the activator plasminogen that leads to ECM protein impairment[78,97].A distinct category of molecules produced by MSCs are the factors responsible for MSCs survival,proliferation,and migration to the area of tissue injury,which involve fibroblast growth factor-2 supporting the proliferation of vascular smooth muscle cells and ECs,stromal cell-derived factor-1 reducing apoptosis and regulating cell migration,insulin-like growth factor-1 controlling cell differentiation and growth and inhibiting apoptosis,and a secreted frizzled-related protein-2 supporting CMC survival at the conditions of low oxygen availabilityin vivo[77,98].
Exosomes secretion
Extracellular vesicles are biological nanoparticle structures containing bioactive molecules,including protein,and nucleic acids.They can influence other cells and participate in intercellular communication over long distances[99].Many studies on tissue regeneration mechanisms demonstrated that extracellular vesicles released by SCs can deliver bioactive molecules to target cells,which may influence the function of those cells,including the process of damaged tissue regeneration[100].
PRECLINICAL STUDIES:ANIMAL MODELS OF TISSUE ISCHEMIA
Most of the molecular and cellular mechanisms that affect the therapeutic potential of MSCs in the therapy of ischemic tissues were initially identified in animal models.The capacity of MSCs to survive in the recipient after administration and the ability to differentiate into Ecs and CMCs has been proven in acute myocardial infarction in a mini-swine model[65].In this paper,Zhanget al[65]also confirmed the migratory activity of MSCs towards inflammation,inhibition of CMC apoptosis,stimulation of cardiac SCs,reduction of fibrosis,myocardium reverse remodeling,and enhancement of LVEF.It has been demonstrated that MSCs derived from UCB(UCB-MSCs)can reduce the acute myocardial infarction size by ≥ 50% and enhance LVEF[101].The observed therapeutic effect may be due to the ability of MSCs to secrete bioactive factors.In turn,thanks to their immunosuppressive properties and the paracrine effect,MSCs can alleviate inflammation and ischemic heart disorders,contributing to the reduction of infarct size and improving LVEF through a paracrine effect[102].
The therapeutic efficacy of UC-MSCs and heart function improvement has been demonstrated[103].Intravenous administration of MSCs has improved LVEF contractility,function,perfusion,and reverse remodeling[18].The transplantation of MSCs in a rat model of acute myocarditis can reduce inflammation by decreasing the infiltration of an inflammatory cell,reducing CMC death,and remodeling adverse myocardium[104,105].Based on the results of animal studies,hopes are high regarding enhancing many heart functions,such as reduction of scar tissue,myocardium reverse remodeling,increase in cardiac contractility,improvement of ejection fraction,and increase in heart perfusion.However,there is still a need for long-term observation of the effects of injecting MSCs to ensure the safety and efficacy of therapy.
In preclinical studies on transgenic animals of models of neurological diseases,significant functional improvement was observed after MSC cell transplantation[106].The use of these cells may be related to their direct action,i.e.,replacement of damaged cells as a result of neural differentiation or to an indirect influence positively influencing the endogenous regenerative processes of the organism.In addition,preclinical studies on animals have confirmed the neuroprotective properties of MSC transplants,which may be linked to their production of numerous growth,anti-inflammatory,and anti-apoptotic factors important for neurons[107].
CLINICAL TRIALS:MSC AS MODERN THERAPEUTIC AGENTS IN ISCHEMIA
Clinical trials testing MSCs in regenerative therapy are growing(ClinicalTrials.gov).Regenerative therapies based on MSCs for several ischemic disorders are now carefully examined.The remedial effects obtained are promising and prove that the transplantation of MSCs may be beneficial in the treatment of many diseases[108,109].The concept of clinical application of MSCs may improve the health of patients suffering from many cardiovascular diseases[110].So far,a few dozen studies have been registered in the international database of clinical trials to assess the safety and effectiveness of MSCs administration in the treatment of ischemic diseases.
The most popular source of therapeutic MSCs used in clinical practice is BM.The first MSC-based biological drug,AMI HeartiCellgram®,used in myocardial infarction therapy was also based on BMMSCs.Leeet al[111]described the manufacturing procedure for this drug,and Kimet al[112]proved its effectiveness in a clinical experiment.The therapeutic benefits included the restoration of normal systolic heart function and the reduction of post-infarction scar tissue[113,114].
The pioneer pilot clinical study applying WJ-MSCs in treating ischemic disorders was performed by Musialeket al[115]in 2015.This group has shown the safety of injected MSCs as an off-the-shelf product,percutaneous allogeneic SC therapy in human acute myocardial infarction.Later observation proved no clinical adverse events in the treated tissue,except for a local rise in body temperature of 1 patient[115].Currently,Musialeket al[115]are examining the safety and effectiveness of the“CardioCell” drug(WJ-MSC-based biological therapeutic)in a phase II/III randomized,placebocontrolled,double-blind clinical trial in several ischemic disorders[i.e.,acute myocardial infarction(EudraCT Number:2016-004662-25),chronic ischemic heart failure(EudraCT Number:2016-004683-19),and non-option critical limb ischemia(EudraCT Number:2016-004684-40)][116,117].
A clinical trial with MSCs has shown the enhancement in heart muscle function in cases of heart failure.For example,Bartolucciet al[118]have shown that intravenous administration of UC-MSCs improved LVEF,functional status,and standard of living.Also,exosomes released by UC-MSCs can alleviate the effect of acute myocardial ischemic injury[119].Scientists confirmed that the injection of UC-MSCs exosomes can greatly improve contractile heart function and minimize myocardial fibrosis.These bioactive bubbles protected heart cells from death and supported EC migration and angiogenesis.UC-MSCs have also been applied in a clinical study for the treatment of chronic coronary occlusion[17].Also in this trial,the improvement the heart function and better left ventricular ejection fraction were reported[17].
So far,several clinical trials of ischemic stroke have shown that transplanting MSCs into patients with successful reperfusion therapy reduces the volume of lesions after stroke and promotes the regeneration of neurological function.This success is shown by improvements in human functional,behavioral,and sensorimotor assessments,such as the Barthel Index,Modified Rankin Scale,European Stroke Scale,Fugl-Meyer Scale,and National Institutes of Health Stroke Scale[120,121].MSCs participate in the regeneration of ischemic tissues and organs with beneficial effects,as outlined above(Table 2).The therapeutic activity is presumed to include immunomodulation,cardioprotective effects,activation of endogenous repair processes,and tissue remodeling.
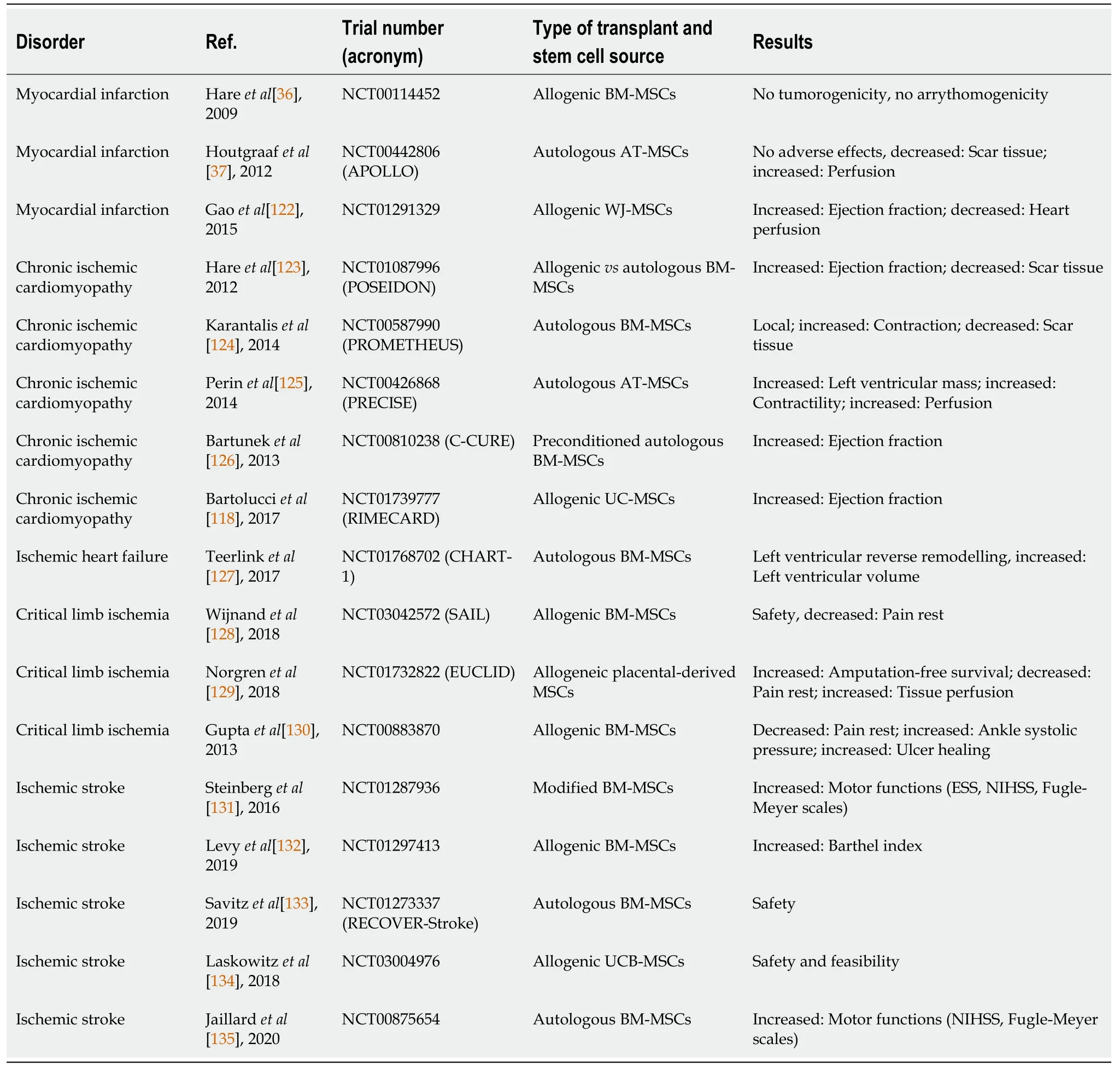
Table 2 Selected clinical trials of mesenchymal stem cell-based therapy in ischemic diseases
IMPROVING THE EFFECTIVENESS OF MSC-BASED THERAPY
MSCs can supply alternative therapy in the treatment of many disorders,but many studies have demonstrated that depending on the method of isolation,expansion,and delivery we can obtain cells with distinct functional features.The therapeutic benefits of MSC-based therapy involve paracrine activity,immunomodulation,and enhanced function of the damaged organ.However,not all patient responses to treatment are satisfactory;therefore this approach requires a deep understanding of the therapeutic actions of MSCs after injection into the recipient.The therapeutic efficacy of MSCs is affected by many factors,including the method of MSCs cultivationin vitro,the metabolic activity of the MSCs,the number of injected cells,the patient’s genetic sensitivity,and the stage of the disease[136,137].
The selection of the appropriate source of therapeutic MSCs depending on the disease is crucial as more and more data show source-dependent variations in therapeutic activity such as levels of released trophic proteins or different differentiation capacities.There is much disagreement as to the therapeutic efficiency of MSCs derived from different tissues(fetal and adult sources).Therefore,extensive studies are desirable to obtain consistent data about remedial effects.
Another problem that is much debated is the type of transplant,i.e.,allogeneic or autologous.The results obtained from clinical trials showed no difference between the therapeutic effects of allogeneic and autologous MSCs in the treatment of ischemic cardiomyopathy[138].The undoubted advantage of autologous transplants is the lack of burdening the cells with other diseases because in such cases the donors are healthy volunteers.Autologous SCs obtained from the patient do not have this privilege,which may limit the therapeutic effectiveness.In addition,it is an important issue to obtain the right dose of autologous cells.The autologous transplant requires the collection of appropriate tissue from the patient,isolation of SCs,and obtaining the necessary dose of therapeutic cells,which is a challenge as the disease and the patient’s age may contribute to the reduction of the proliferative activity of MSCs[139,140].
To resolve these issues,scientists introduced allogeneic sources of SCs that can be used to produce ready-to-use biological products.The use of allogeneic SCs shortens the waiting time for a transplant.Allogeneic cells from young and healthy donors are used to produce biological drugs of quality that canbe stored frozen.The medicinal product prepared in this way,if necessary for clinical intervention,can be thawed at any time and administrated to the patient.
MSCs applied in the clinic as therapeutic agents must be carefully prepared,according to the good manufacturing practice and good clinical practice standards,with the established quality control system.The manufacturing process should be properly optimized in terms of therapy requirements to reach a sufficient remedial effect.MSCs applied in the clinic as therapeutic agents must be carefully prepared,according to the good manufacturing practice and good clinical practice standards,with the established quality control system.The manufacturing process should be properly optimized in terms of therapy requirements to reach a sufficient remedial effect.Here it should be emphasized that we can prepare therapeutic cells to treat different disorders,such as graftvshost disease,myocardial infarction,Crohn’s disease,and others,however,the therapeutic benefits obtained may not be satisfactory.Therefore,there is still a need for a more sophisticated approach to obtaining highly effective biological medicines.The use of an appropriate approach to the production of therapeutic MSCs for the treatment of a particular disease should contribute to the achievement of satisfactory results.
Additionally,to adopt therapeutic MSCs for demanding conditions in the host body,preconditioning methods can be applied.This approach requires the presence of additional adverse factors(such as low oxygen availability or proinflammatory cytokines)duringin vitroculture.Bernardo and Fibbe[141],during the production of therapeutic MSCs,added proinflammatory cytokines to the culture medium,the concentration of which increased after acute myocardial infarction.In this study,an anti-inflammatory response of MSCs was observed within 24-48 h based on the analysis of the composition of the culture medium.The use of selected cytokines in MSCs cultures for the treatment of acute myocardial infarction enhanced anti-inflammatory secretory activity and therapeutic efficacy.
CONCLUSION
The remarkable ability of MSCs to regenerate damaged body parts to regain lost function is promising in many disorders,including ischemic diseases.Three properties of MSCs render them optimal for ischemic tissue repair and regeneration:(1)Immunomodulatory and immunoregulatory capacity beneficial to ameliorate abnormal immune responses;(2)Soluble and insoluble paracrine factorgenerating potential;and(3)Endothelial differentiation.
While MSCs have several advantages,there are still many challenges to overcome.The unique immunomodulatory properties of MSCs are essential to their function,but the mechanisms of the immune regulation of MSCs have not been elucidated.Many factors can influence the therapeutic potential of MSCs,such as donor age,isolation and culture method,induction factors,oxygen concentrations,mechanical stimuli,and others.Hence,optimizing the culture conditions of MSCs may be an effective way to improve the therapeutic potential of MSCs for successful tissue repair.
FOOTNOTES
Author contributions:Szydlak R wrote the paper.
Conflict-of-interest statement:The author reports having no relevant conflicts of interest for this article.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial(CC BYNC 4.0)license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is noncommercial.See:https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Poland
ORCID number:Renata Szydlak 0000-0003-2760-2952.
S-Editor:Wang JJ
L-Editor:Filipodia
P-Editor:Wang JJ
