Potential Impacts of Exogenous Nitrogen Enrichment on Distribution and Transfer of Nitrogen in Plant-Soil System of Suaeda salsa Marsh in the Yellow River Estuary, China
2023-03-17HUXingyunSUNZhigaoYULinyingandCHENBingbing
HU Xingyun SUN Zhigao , YU Linying and CHEN Bingbing
Potential Impacts of Exogenous Nitrogen Enrichment on Distribution and Transfer of Nitrogen in Plant-Soil System ofMarsh in the Yellow River Estuary, China
HU Xingyun1), 2),3), SUN Zhigao1), 2),3),*, YU Linying1), 2),3), and CHEN Bingbing1), 2),3)
1) Fujian Provincial Key Laboratory for Subtropical Resources and Environment, Fujian Normal University, Fuzhou 350007, China 2) Key Laboratory of Humid Subtropical Eco-Geographical Process (Fujian Normal University), Ministry of Education, Fuzhou 350007, China 3) Institute of Geography, Fujian Normal University, Fuzhou350007, China
To determine the potential impacts of exogenous nitrogen (N) enrichment on distribution and transfer of N inmarsh in the Yellow River Estuary, the variations of N in plant-soil system during the growing season were investigated by field N addition experiment. The experiment included four treatments: NN (no N input treatment, 0gNm−2yr−1), LN (low N input treatment, 3.0gNm−2yr−1), MN (medium N input treatment, 6gNm−2yr−1) and HN (high N input treatment, 12gNm−2yr−1). Results showed that N additions generally increased the contents of total nitrogen (TN), ammonia nitrogen (NH4+-N) and nitrate nitrogen (NO3−-N) in different soil layers and the increasing trend was particularly evident in topsoil. Compared with the NN treatment, the average contents of TN in topsoil in the LN, MN and HN treatments during the growing season increased by 10.85%, 30.14% and 43.98%, the mean contents of NH4+-N increased by 8.56%, 6.96% and 14.34%, and theaverage contents of NO3−-N increased by 35.73%, 45.99% and 46.66%, respectively. Although exogenous N import did not alter the temporal variation patterns of TN contents in organs, the N transfer and accumulation differed among tissues in different treatments. With increasing N import, both the N stocks in soil and plant showed increasing trend and the values in N addition treatments increased by 9.43%–38.22% and 13.40%–62.20%, respectively. It was worth noting that, compared with other treatments, thein the MN treatments was very likely to have special response to N enrichment since not only the period of peak growth was prolonged by about 20 days but also the maximum of TN content in leaves was advanced by approximately one month. This paper found that, as N loading reached MN level in future, the growth rhythm ofand the accumulation and transference of N in its tissues would be altered significantly, which might generate great impact on the stability and health ofmarsh ecosystem.
nitrogen import; nitrogen transfer; plant-soil system;; Yellow River Estuary
1 Introduction
Nitrogen (N) is one of the most essential nutrients in marsh ecosystem, which plays important roles in driving many ecological processes such as participating in the synthesis of N-containing compounds (protein, nucleic acids, chlorophyll and enzyme) and controlling the metabolism of plants and microorganisms (Jeppesen., 2011; Alldred., 2017; Hester., 2018). Generally, the distribution of mineral N in soils (Bai., 2012; Chen., 2020) and the transfers of N in tissues of plant (Cott., 2018; Tegeder and Masclaux-Daubresse, 2018) can influence the stability of marsh ecosystem. The deficiency of N usually induces the decline of photosynthetic rates and enzyme metabolism activities in plants,which ultimately reduces their primary productivity (Uribelarrea., 2009; Boussadia., 2010; Mu., 2021). However, as the exogenous N was imported into marsh, the growth of primary producers, the metabolism of microorganisms and the stoichiometric balance with other biogenicelements (.., carbon, phosphorus and sulfur) would be greatly disturbed (Iversen., 2010; Hoyos-Santillan., 2018; Chen., 2020; Craig.,2021). Previous studies have demonstrated that appropriate N addition generally elevated the contents of available N in soils by stimulating net N mineralization (Luce., 2016; Hu., 2020) or nutrient release from decomposing litters (Gerdol., 2007; Vivanco and Austin, 2011; Hoyos-Santillan., 2018; Hu and Sun, 2021) and enhanced the photosynthetic rate by increasing the stomatal conductance and chloroplast contents in plant leaves (Liang., 2020; Zangani., 2021). By com- parison, excessive N input usually caused negative effects on marsh ecosystem such as the acidification of soils (To- massen., 2004; Tian and Niu, 2015), the increase of nitrate (NO3−) leaching and nitrous oxide (N2O) emission (Magill., 2000; Szukics., 2009; Davis., 2015), and the decline of biomass and biodiversity (Isbell., 2013; Tilman., 2014).
Coastal marsh is one of the most sensitive ecosystems that respond to global change and human activities, in which the material-energy exchanges frequently and significantly occur between continental river water and marine salt water (Moffett., 2015; Renzi., 2019). In intertidal zone, the formation of large numbers of aerobic and anaerobic interfaces, to some extent, induces the N biogeochemical processes to be more complicated (Vivanco., 2015; Steinmuller., 2018). With the rapid development of industrialization, urbanization and agricultural practices in coastal regions, large quantities of pollutants containing N are discharged into estuary. The import of exogenous N not only can improve the productivity of ecosystem to a certain extent (Martina., 2016), but also can cause many environmental problems such as eutrophication (Deegan., 2012), nutrient im- balance (Lu., 2018) and substantial greenhouse gas release (Hershey., 2021). All these produce profound effects on the habitation of flora and fauna in coastal marsh (Howarth, 2008). Over the past three decades, there has been an increasing interest in discussing the influences of N enrichment on the ecological processes (.., biological N fixation, N transformation and N release from decomposing litters) of forest ecosystem (Szu- kics., 2009; Vivanco and Austin, 2011; Hao., 2016), grassland ecosystem (Sirulnik., 2007; Hammelehle., 2018; Tognetti., 2021) and farmland ecosystem (Silgram and Chambers, 2002; Pandey., 2018; Pareja-Sánchez., 2020), while information on the related studies in coastal mash ecosystem is still very lacking.
The Yellow River is well known as the largest sediment-laden river in the world. Every year, approximately (1.71–3.12)×105t of artificial pollutants in the Yellow River basin were transported into estuary, including (1.84–2.82)×104tons of N nutrient (China Oceanic Information Network, 2019). In the past 30 years, the atmospheric N deposition in the Yellow River estuary also showed an increasing trend and the values increased from 1–2gm−2yr−1to 3–4.5gm−2yr−1(Ti and Yuan, 2010), which approximated the critical load of N deposition (4.0 gm−2yr−1) in this region (Duan. 2002). The intertidal zone in the Yellow River Estuary ranges from 4km to > 10km, and, in a seaward direction,is the most prevalent halophyte which occupies more than 70% of the total vegetation area in intertidal zone (Sun., 2017). As a pioneer plant, it can tolerate many environmental stresses such as high salinity, sediment burial and tidal inundation. As affected by both terrestrial N input and atmospheric N deposition, themarsh acted as a sink of exogenous N, which might produce great impact on the biogeochemical processes of biogenic elements (particularly for N) (Jiang., 2012). However, information on the influences of exogenous N import on these critical processes inmarsh remains scarce.
In this paper, thesimulating experiment of N addition was conducted inmash of the Yellow River Estuary and the effects of N enrichment on distribution and transfer of N in plant-soil system were investigated in the growing season. It was hypothesized that exogenous N enrichment might greatly alter the distribution and transfer of N in plant-soil system ofmarsh. Objectives of this study were: 1) to explorethe in- fluences of N enrichment onvariations of N contents in soils during the growing season; 2) to determine the va- riations of N accumulation and transfer in tissues of plant with increasing N additions;and 3) to illustrate the potential impacts of N enrichment on variations of N stock in plant-soil system ofmarsh.
2 Materials and Methods
2.1 Study Region
This study was conducted in intertidal zone of the nor- thern Yellow River Estuary, located in the nature reserve of Yellow River Delta (37˚40´–38˚10´N, 118˚ 41´–119˚16´E) in Dongying City, Shandong Province, China (Fig.1a). The nature reserve is of typical continental mon- soon climate with the distinctive seasons. The annual evaporation is 1928.2mm and the annual precipitation is 551.6mm, with about 70% of precipitation occurring between June and August. The annual mean temperature is 12.1℃ and the frost-free period is 196 days. The tide in intertidal zone is irregular semidiurnal tide and the mean tidal range is 0.73–1.77m. The soils are dominated by intrazonal tide soil and salt soil, and the typical halophytes in intertidal zone are composed of,and. As the most prevalent plant in the intertidal zone,generally germinates in late April, blooms in July, matures in late September and completely dies in late November (Gu, 1999).
2.2 Study Methods
2.2.1 Field experiment of N addition
The purecommunity in high tidal flat was selected for the fieldN addition experiment (Fig.1b). The study area could be slightly submerged at high tide which generally occurred twice every month (Xie, 2017). On 10 April 2014, three experimental plots (50m×50m) were randomly laid along the contour line (with the similar elevation), and, at each plot, four subplots (5m×10m) were randomly laid. The distance between two subplots was over 15m and the PVC fences were set around the subplot to prevent the N from being lost. The PVC boards (1.5m) were embedded approximately 1.0m into soil and the fence height above soil surface was 0.5m. The PVC fences could also prevent the subplots from being submerged at high tide and avoid the mutual impacts of different N treatments.
Combined with the existed data and considering the synthesis influences of terrestrial N input (2.5–3.5gNm−2yr−1) and atmospheric N deposition (3.0–4.5gNm−2yr−1), the background N input

Fig.1 Geographical location of the experimental plot (a) and sketch of the in situ nitrogen enrichment experiment (b). NN, no N input treatment; LN, low N input treatment; MN, medium N input treatment; HN, high N input treatment.
amount in the study region was determined to be 6.0gNm−2yr−1. The field experiment designedfour N addition levels: 1) Control treatment (NN), no additional N was imported which represented the background N input amount ofmarsh; 2) Low N input level (LN), 1.5NN (9.0gNm−2yr−1, additional N input amount was 3. 0gNm−2yr−1) which simulated the increasing of N loading with a low level in future; 3) Medium N input level (MN), 2.0NN (12.0gNm−2yr−1, additional N import amount was 6.0gNm−2yr−1) which simulated the increasing of N loading with a medium level in future; and 4) High N input level (HN), 3.0NN (18.0gNm−2yr−1, additional N input amount was 12.0gNm−2yr−1) which simulated the increasing of N loading with a high level in future. The experiment was conducted in the growing season of(from April to November). The N addition started on 21 April and ended on 27 October. The designed N addition amounts were divided into 8 portions and then were added to the corresponding subplot for 8 times (21 April, 6 May, 31 May, 24 June, 18 July, 15 August, 8 September and 27 October). According to the data set provided by the related studies in the Yellow River Estuary,NH4+-N was the main body of terrestrial N import and atmospheric N deposition (China Oceanic Information Network, 2011; Yu., 2014). Thus, the actual N chemical component added in this experiment was urea. At each time, the exogenous N (Urea: CO(NH2)2; Purity: 99.5%), dissolved in 20L distilled water, was uniformly imported into each subplot.The subplots with no additional N input were imported into equal amount of distilled water.
2.2.2 Sampling and chemical analyses
Becausegenerally germinated in the last ten days of April and the seedlings at this period were very small, the plants were not sampled on 6 May. The plants were firstly sampled on 31 May as the N addition lasted for 40days. Thereafter, additional 7 sampling campaigns (25 June, 18 July, 15 August, 8 September, 5 October, 27 October and 21 November) were conducted according to the growth rhythm of. In this paper, the eco-phy- siological period ofwas divided into three stages: 1) initial growth stage (from May to June); 2) vigorous growth stage (from July to October); and 3) late growth stage (November). Aboveground and belowground biomasses were determined at each subplot (3–4 replications) using quadrat method (50cm×50cm). The aboveground part of plants was clipped near the ground, and stem, leaf and standing litter were separated in the laboratory. Roots in the quadrat were dug out and washed carefully. At the late stage, the fruits (seeds) and small leaves were tightly connected and difficult to be separated, so the leaves at this stage were referred to as leaves (+fruits). All plant samples were washed thoroughly with deionized water and then were oven-dried. Since the substantial roots ofwere distributed in 0–15cm depth (Mou, 2010; Sun., 2013), the N nutrient transferred between soil and plant mainly occurred in this depth. In this study, the soils were sampled as the N addition lasted for 15d (6 May), 40d (31 May), 64d (24 June), 88d (18 July), 116d (15 August), 140d (8 September), 189d (27 October) and 214d (21 November). At each sampling campaign, three columnar samples (0–20cm) were obtained from the same position with plant samples. After the columnar samples were extracted, they were divided at a 10cm interval.
All plant samples were ground into fine powder after the determination of dry weight. Soil samples were air- dried, ground and sieved through a 100-mesh nylon sieve. The samples of plant and soil were analyzed for total nitrogen (TN) contents by an element analyzer (Elementar Vario Micro, Elementar, German; Range: 0–100%; Accuracy: ±0.02%). After the soils (5g) were extracted with 25mL of 2molL−1KCl solution,the ammonia nitrogen (NH4+-N) and nitrate nitrogen (NO3−-N)in solution were determined by a sequence flow analyzer (San++SKALAR, Netherlands).
2.2.3 Parameter calculations
The N stock (T, gm−2) in soil was calculated according to Batjes. (1996):

whered(gm−3) is the soil bulk density of thelayer;N(mgg−1) is TN content in thelayer; andhis the soil depth (10cm).
The N concentration quotients for roots/stems (), root/leaves (), and stems/leaves () were calculated according to Dahmani-Muller. (2000):


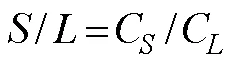
whereC,CandCare TN contents (mgg−1) in root, stem and leaf, respectively.
The N stocks in tissues (root, stem, leaf and standing litter) of plant (N, gm−2) were calculated according to Li and Redmann (1992):

whereC(gm−2) is TN content in thepart; andB(gm−2) is biomass of thepart.
The accumulation factors of N (N) in tissues of plant were calculated according to Mou. (2011):
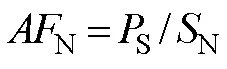
whereS(mgg−1) is TN contents in tissues of plant; andN(mgg−1) is TN contents in soils (0–20cm).
2.3 Statistical Analysis
The results were presented as means over the replications, with standard deviation (S.D). Data analysis was performed using SPSS Version 22.0 Statistical Software Package (SPSS Inc., Chicago, USA).The Shapiro-Wilk test was applied to identify the normality of data before the related statistical analyses were conducted. The analysis of variance (ANOVA) test was applied to determine if TN, NH4+-N and NO3−-N contents in soils or TN contents in plants during the growing season differed significantly among N input treatments (<0.05). If ANOVA showed significant differences, multiple comparison of means was undertaken by Tukey’s test with a significance level of=0.05.
3 Results
3.1 Variations of N Contents in Soils as Affected by N Enrichment
Dissimilar variations of TN, NH4+-N and NO3−-Ncontents in soils were observed among N import treatments (Figs.2a–c). For each treatment, significantly higher TN and NO3−-Ncontents occurred in topsoil during the growing seasoncompared to the subsurface soil (<0.05). The contents of TN, NH4+-N and NO3−-N in topsoil of different treatments reached the maximum during June and July and the values in the LN, MN and HN treatments were significantly higher than that in the NN treatment (<0.05). It was worth noting that, at the end of the growing season, the TN, NH4+-N and NO3−-N contents in soils of different treatments increased with varying degrees and the increase magnitudes were particularly evident in the MN and HN treatments.Generally, N additionsincreased the average contents of TN, NH4+-N and NO3−-N in different soil layers and the increasing trend was especially obvious in topsoil. Compared with the NN treatment, the mean contents of TN in topsoil in the LN, MN and HN treatments during the growing season increased by 10.85%, 30.14% and 43.98%, the average contents of NH4+-N increased by 8.56%, 6.96% and 14.34%, and the mean contents of NO3−-N increased by 35.73%, 45.99% and 46.66%, respectively (Figs.2d–f).
3.2 Variations of N Contents in Plants as Affected by N Enrichment
3.2.1 Temporal variations of TN content
Similar variations of TN contentsin roots or stems were found among N input treatmentsduring the growing season (Fig.3). For each treatment, the TN contents in roots and stems were high at the initial stage, after which both the values decreased and reached the minimums on 27 October. By comparison, the variations of TN contents in leaves in the growing season were more complex.The TN contents in leaves declined significantly before 25 June and increased greatly from 25 June to 5 October except for the MN treatment, after which the values decreased greatly again. With a few exceptions, the variations of TN contents in standing litters were similar to those in leaves during the growing season. Although the N input did not alter the variation patterns of TN contents in the tissues, the mean values in roots and stems generally decreased while those in the leaves and standing litters generally increased with increasing the N additions (Fig.3b).

Fig.2 Temporal variations of TN, NH4+-N and NO3−-N contents (a, b and c) and their means (d, e and f) in soils as affected by different nitrogen input treatments. NN, no N input treatment; LN, low N input treatment; MN, medium N input treatment; HN, high N input treatment. Values with different capital letters are significantly different at the level of P<0.05.

Fig.3 Temporal variations of TN contents (a) and their means (b) in tissues of Suaeda salsa as affected by different nitrogen input treatments. NN, no N input treatment; LN, low N input treatment; MN, medium N input treatment; and HN, high N input treatment. Values with different capital letters are significantly different at the level of P<0.05.
3.2.2 Transfer and accumulation of N
Dissimilar variations of,andratios in plants during the growing season were observed among N addition treatments (Table 1). Theratios in the four treatments showed slight fluctuations before 17 October, after which the values increased greatly. At the end of the growing season, theratios in the MN treatment were significantly higher than those in the other treatments (<0.05). Theandratios were generally high at the initial stage, after which both the values showed a decreasing trend before 17 October. At the late stage, bothandratios increased greatly and the increased magnitudes were particularly evident in the MN treat ments. During the growing season, theratios in plants of different treatments were mostly than 1, while theandratios almost were less than 1. TheNin tissues of plants in the four treatments differed among sampling periods (Table 2). TheNin roots or stems reached the maximum at the initial stage, while those in leaves or standing litters achieved the highest value on 5 October. With a few exceptions, theNin the LN, MN and HN treatments were much lower than those in the NN treatment.
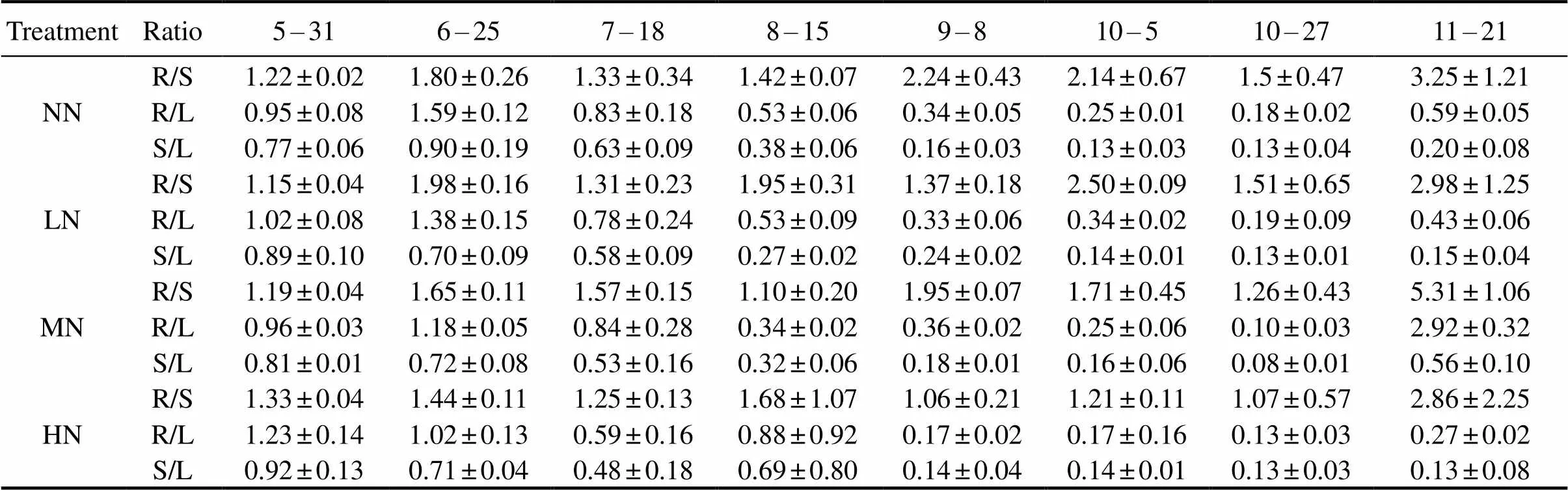
Table 1 Nitrogen concentration quotients for roots/stems (R/S), roots/leaves (R/L), and stems/leaves (S/L) in Suaeda salsa in the growing season as affected by different nitrogen input treatments
Notes: NN, no N input treatment; LN, low N input treatment; MN, medium N input treatment; HN, high N input treatment.

Table 2 Accumulation factors of nitrogen (AFN) in tissues of Suaeda salsa in the growing season as affected by different nitrogen input treatments
Notes: NN, no N input treatment; LN, low N input treatment; MN, medium N input treatment; HN, high N input treatment. NA, not available.
3.3 Variations of N Stocks in Plant-Soil Systems as Affected by N Enrichment
In the four N input treatments, aboveground living body was the main N stocks of plant subsystem, accounting for 79.90% (NN), 77.32% (LN), 74.49% (MN) and 79.04% (HN) of the total N stocks in plant subsystems, respectively (Table 3). The N stocks in plant subsystems were very low, accounting for only 4.12% (NN), 4.26% (LN), 4.14% (MN) and 4.80% (HN) of the total N stocks in plant-soil systems, respectively. Over all sampling periods, the N stocks in soils of the four treatments were the main bodies of total N stocks in plant-soil systems (> 95%). With increasing N additions, the mean N stocks in plant-soil systems of the four treatments generally show- ed HN>MN>LN>NN (<0.05).
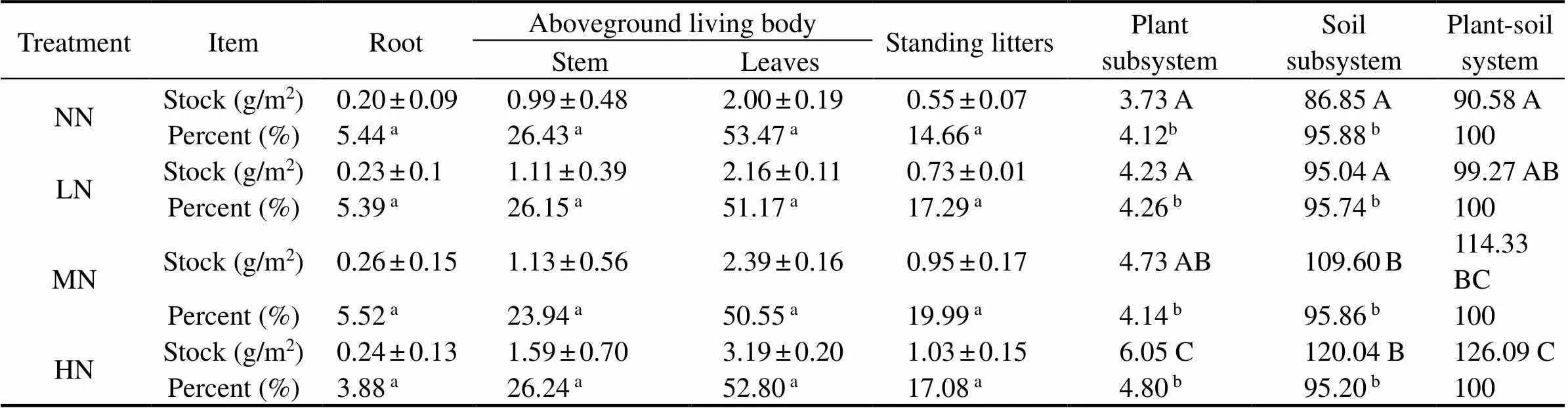
Table 3 Stocks of nitrogen in plant-soil systems of Suaeda salsa marsh as affected by different nitrogen input treatments
Notes: NN, no N input treatment; LN, low N input treatment; MN, medium N input treatment; HN, high N input treatment.aPercent of plant subsystem;bPercent of plant-soil system. Different letters (A, B and C) within the same column indicate significant differences at<0.05.
4 Discussion
4.1 Effect of N Enrichment on Variations of N Contents in Soils
This paper found that N additions generally increased the TN contents in different soil layers and the increasing trend was particularly evident in topsoil (Figs.2a, d). One possible reason could be ascribed to the decomposition oflitters in the ground surface of different experimental plots. Previous studies have shown that the nutrient released from decomposing litters was one of the most important nutrient sources for marsh soils (Song., 2018; Koceja., 2021). The decomposition of detritus generally could be accelerated by N addition, which, to some extent, stimulated the release of nutrients (.., C and N). Vivanco and Austin (2011) found that N addition increased the production of lignin-degrading enzyme which not only promoted the mass loss of recalcitrant C compounds but also stimulated the N release from decomposing litters (). Hou. (2020) implied that N addition might shift the microbial community more to the bacterial channel of carbon and nutrient cycling followed by more rapid decomposition and nutrient releasing from,,andlitters. Actually, our previous studies have confirmed that N addition indeed stimulated the decomposition oflitters in ground surface (Hu and Sun, 2021), which might result in the higher TN contents in topsoil in the LN, MN and HN treatments(Fig.2d). This paper also indicated that, in the four treatments, the TN contents in topsoil in summer were much higher than those in other sampling periods (Fig.2a), and this could be explained by the higher precipitation and temperature at this period. The better hydrothermal conditions in summer might be more favorable for the decomposition of detritus by accelerating the leaching of dissolved matter and elevating the activities of microorganisms (Hobbie., 2012; Bell., 2018). Moreover, the activities of microorganisms could also be stimulated by exogenous N addition, which in turn boosted the decomposition of detritus (Bragazza., 2012; Song., 2018; Koceja., 2021). All these might induce the higher TN contents in topsoil in summer in the four treatments.
This paper indicated that although both NH4+-N and NO3−-N contents in topsoil or subsurface soil increased with increasing N additions, the increase magnitudes were particularly evident in topsoil (Figs.2e, f). Previous studies have implied that NH4+-N and NO3−-N could be directly absorbed and utilized by marsh plants and their contents in soils were greatly influenced by plant growth rhythm (Tylova-Munzarova., 2005; Craft., 2007; Luce., 2016; Tegeder and Masclaux-Daubresse, 2018; Guan., 2019). At the initial growth stage, both NH4+-N and NO3−- N contents in soils in the LN, MN and HN treatments increased greatly. By comparison, the contents of NH4+-N in the NN treatment declined greatly while those of NO3−- N fluctuated slightly (Figs.2b, c). In order to maintain the growth of seedlings at the initial stage, large amounts of mineral N might be absorbed from soils, which induced the decline of NH4+-N contents in the NN treatment. However, the import of exogenous N in the LN, MN and HN treatments not only could relieve the deficiency of mineral N in soils but also might stimulate the mineralization of soil organic N (Sirulnik., 2007; Luce., 2016; Hu., 2020), which ultimately resulted in the increase of mineral N in the N addition treatments. Moreover, theratios in the four treatments were all less than 1 at the initial stage (Table 1), indicating that most N nutrient were transferred from stems to leaves. The result could partly verify the close relationships between the mineral N in soils and the N absorption by plants.
At the vigorous growth stage, both NH4+-N and NO3−- N contents in topsoil in the four treatments reached the maximums and the values in the LN, MN and HN treatments were significantly higher than that in the NN treatment (Figs.2b, c). As mentioned above, the better hy- drothermal conditions in summer and the N additions not only could stimulate the decomposition oflitters in ground surface (Gerdol., 2007; Vivanco and Austin, 2011; Bell., 2018; Hoyos-Santillan., 2018; Hu and Sun, 2021) but also might accelerate the mineralization of organic N in topsoil (Sirulnik., 2007; Luce., 2016; Hu., 2020). Just for these reasons, the higher contents of NH4+-N and NO3−-N in topsoil were generally observed in the N addition treatments. It was worth noting that the NO3−-N contents in topsoil in the MN and HN treatments were much lower than those in the LN treatments (Fig.2c) and there were two probable causes. First, the higher N input amounts in the MN and HN treatments might inhibit the process of nitrification, which probably resulted in the reduction of NO3−-N in soils. Liu. (2015) found that the lower N import increased the numbers of nitrate bacteria in soils while higher N input induced the nitrification to be inhibited by free NH4+-N or other substances. Hao. (2016) also showed that the continuous N input significantly de- creased net N nitrification rate and the adverse effects of N addition on soil acidity and consequently on microbial biomass, communities and enzyme activity could explain the observed decline in net N nitrification rate. Moreover, the denitrification process in the MN and HN treatments might be accelerated by the higher N input amounts, which induced the mineral N in soils to be lost in the forms of N2or N2O (Munoz-Hiñcapié., 2002; Szu- kics., 2009; Lu., 2011; Song., 2013). Munoz-Hiñcapié. (2002) found that the emissions of N2O were boosted by both NH4+-N and NO3−-N additions. Particularly, the maximum flux following NH4+-N addition was 2785 times greater than the control plots. Song. (2013) also found that, after 5 years of N addition (NH4NO3), the emissions of N2O from marsh in the Sanjiang Plain increased by 396% in the high N import treatment. Second, it might be related to the absorption and utilization of mineral N by plants in different N addition treatments. As shown in Table 1, bothandratios in plants in the four treatments were less than 1 and the values generally decreased in summer, implying that the absorption for mineral N by plants might be increased at this period. Compared with the LN treatment, the higher aboveground and belowground biomasses in the MN and HN treatments (Fig.4) probably induced higher absorption amounts for mineral N, which could partly verify the lower NO3−-N contents in topsoil in the two treatments.
At the late growth stage, both NH4+-N and NO3−-N contents in topsoil in the N addition treatments increased with varying degrees, but the increase magnitudes were particularly evident in the MN and HN treatments (Fig.2b, c). For one thing, it might be related to the transfer and accumulation of N in plants. Previous studies have implied that the roots ofwere substantially distributed in 0–15cm depth and the nutrients transferred between soil and plant mostly occurred at this depth (Mou, 2010; Sun., 2013). At the end of the growing season, bothandratios in the N import treatments (particularly in the MN and HN treatments) increased greatly (Table 1), indicating that large amounts of N might be transferred from aboveground parts to the roots. Similar results were reported by Fan. (2004) and Hammelehle. (2018) who found that, at the late growth stage, the N nutrient transferred from aboveground parts to the roots generally increased with increasing exogenous N additions.With the death of roots, the N nutrient transferred from aboveground parts might be gradually released into soils through decomposition, which resulted in higher TN, NH4+-N and NO3−-N contents in soils at this period (Figs.2a–c). For another, it was found that the litter productions in the four treatments (especially in the MN and HN treatments) increased greatly at the end of the growing season and the values in the N addition treat- ments were much higher than that in the NN treatment (Fig.4). Thus, the higher TN, NH4+-N and NO3−-N contents in topsoil in the N addition treatments might also rest with the decomposition oflitters in ground surface.
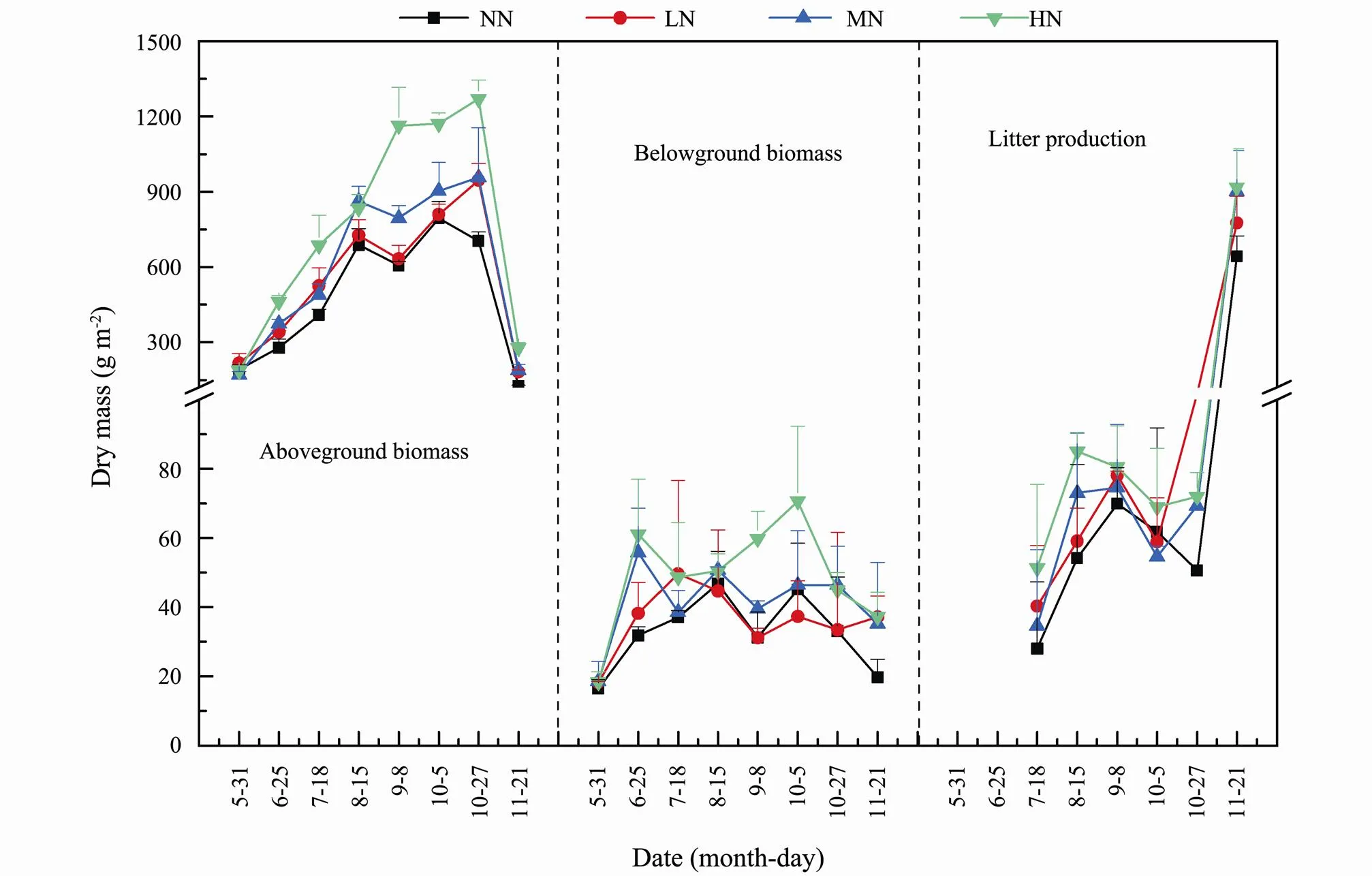
Fig.4 Temporal variations of aboveground and belowground biomasses and litter productions of Suaeda salsa as affected by different nitrogen input treatments. NN, no N input treatment; LN, low N input treatment; MN, medium N input treatment; HN, high N input treatment.
4.2 Effect of N Enrichment on Accumulation and Transference of N in Plants
This paper showed that the contents of TN in leaves in the four treatments were much higher than those in other tissues (Fig.3), which could be better explained by the lower ratios ofand(Table 1). Similar results were also reported by some previous studies (Mou., 2011; Sun., 2012). Actually, the N nutrient preferentially allocated to leaves might be more favorable for the biosynthesis of chlorophyll and the metabolism of carbohydrates in photosynthesis process (Busch., 2018; Tegeder and Masclaux-Daubresse, 2018; Liang., 2020). This paper indicated that although N input did not alter the temporal variation patterns of TN contents in organs, the accumulation and transference of N differed among tissues in distinct stages. At the initial growth stage, large amounts of mineral N might be absorbed by roots to meet the growth ofseedlings, which resulted in the higher TN contents in roots. The explanation could be better verified byandratios since both values were mostly than 1 at the initial stage. Moreover, both TN contents and AFin roots, stems or leaves at this period showed decreasing trend (Fig.3a, Table 2), which could be ascribed to the ‘dilution effect’ (Zhang and Liao, 2009) caused by the increase of biomass (Fig.4). During the vigorous growth stage, the TN contents in roots and stems declined continuously while those in leaves increased rapidly and achieved the maximums during September and October. These implied that the N nutrients at this period were largely allocated to the leaves, which was favorable for the metabolism of carbohydrates in photosynthesis process. The result could be confirmed byandratios since both values were less than 1 and declined continuously before 5 October (Table 1). It was worth noting that the maximum of TN content in leaves (+fruits) in the MN treatment was observed on 8 September while those in the other treatments occurred on 5 October, indicating that, compared with other treatments, the growth time ofseeds in the MN treatment was very likely to be advanced by approximately one month. One possible reason was related to the great impact of N input on carbon allocation in. Previousstudies have shown that appropriate amount of N addition could accelerate the metabolism of N and raise the ability of protein synthesis (Tegeder and Masclaux-Daubresse, 2018; Gao,., 2020), whereas higher or excessive N addition generally inhibited the accumulation of protein in plants (Zhong., 2019; Tognetti., 2021). Similar results were drawn by Ferrise. (2010) and Zangani. (2021) who found that there was significantly linear relationship between N import level and protein synthesis, and appropriate amount of N addition was favorable for the grain filling of seeds. Actually, the growth ofseeds (including some premature seeds) in the MN treatments was indeed observed in September duringfield sampling, which was earlier than that in the other treatments. At the late growth stage, the accumulation and transference of N in tissues of plants in the four treatments varied greatly (Fig.3). The TN contents andAFin leaves decreased suddenly while those in roots increased significantly. It should be noted that the above variation was particularly evident in the MN treatment, which could be partly explained by the highest ratios ofandat this period (Table 1).
This paper implied that, with increasing N import, both the N stocks in soil and plant showed increasing trend and the values in N addition treatments increased by 9.43%– 38.22% and 13.40%–62.20%, respectively (Table 3). However, the N loading increased by 50%–200% simultaneously. These indicated that although exogenous N could supplement N nutrient formarsh, the N supply level and the absorption amount by plants was in- consistent. The reason might be dependent on the N-limited status inmarsh and the different responses of plant to N additions. Previous studies have reported that themarsh in the Yellow River Estuary was limited by N (Sun and Mou, 2016; Jia., 2018). Thus, under N-limited condition, the eco-physiological traits ofmight be altered greatly with increasing N additions. Compared with the NN treatment, N additions (LN, MN and HN) prolonged the period of peak growth ofand its maximum aboveground biomass was delayed by approximately 20days (Fig.4). Not only that, the maximum of TN content in leaves in the MN treatment was advanced by approximately one month compared to the other treatments (Fig.3a). In addition, the accumulation and transference of N inwas also greatly altered in the MN treatment, which has been discussed as mentioned above. All these implied that, compared with other treatments, thein the MN treatments was very likely to have special response to N enrichment. Similar results were reported by some previous research. Xie. (2004) pointed out that, as the nutrients (N and P) in environment increased,could duly regulate the growth rhythm (.., delaying growth period) and the allocation of nutrient in tissues to optimize resource acquisition. Sun and Liu (2008) found that, compare with no N import treatment, N additions induced the maximum biomasses of stems and leaves ofin the Sanjiang Plain to be delayed by approximately 15–30d. Based on the above analyses, it was concluded that, as N loading reached MN level in future, the growth rhythm ofand the accumulation and transference of N in its tissues would be altered significantly, which might produce great influence on the stability and health ofmarsh ecosystem.
5 Conclusions
This paper explored the potential impacts of exogenous N enrichment on distribution and transfer of N in plant-soil system ofmarsh in the Yellow River Estuary. Results have demonstrated that: 1) N additions generally increased TN, NH4+-N and NO3−-N contents in different soil layers and the increasing trend was particularly evident in topsoil; 2) although exogenous N import did not alter the temporal variation patterns of TN contents in organs, the N transfer and accumulation differed among tissues in different treatments; 3) with increasing N import, both the N stocks in soil and plant showed increasing trend; and 4) as N loading reached MN level in future, the growth rhythm ofand the accumulation and transfer of N in its tissues would be altered significantly. Next step, to verify the theoretical significance of the present findings and to quantify the N transference in plant-soil system, the long-term N enrichment experiment should be conducted and the N isotope technique should be applied.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (Nos. 41371104, 41971128) and the Award Program for Min River Scholar in Fujian Province (No. Min [2015]31).
Alldred, M., Liberti, A., and Baines, S. B., 2017. Impact of salinity and nutrients on salt marsh stability., 8 (11): e02010.
Bai, J. H., Wang, Q. G., Deng, W., Gao, H. F., Tao, W. D., and Xiao, R., 2012. Spatial and seasonal distribution of nitrogen in marsh soils of a typical floodplain wetland in Northeast China., 184 (3): 1253-1263.
Batjes, N. H., 1996. Total carbon and nitrogen in the soils of the world., 47 (2): 151-163.
Bell, M. C., Ritson, J. P., Verhoef, A., Brazier, R. E., Templeton, M. R., Graham, N. J. D.,., 2018. Sensitivity of peatland litter decomposition to changes in temperature and rainfall., 331: 29-37.
Boussadia, O., Steppe, K., Zgallai, H., El Hadj, S. B., Braham, M., Lemeur, R.,., 2010. Effects of nitrogen deficiency on leaf photosynthesis, carbohydrate status and biomass production in two olive cultivars ‘Meski’ and ‘Koroneiki’., 123 (3): 336-342.
Bragazza, L., Buttler, A., Habermacher, J., Brancaleoni, L., Gerdol, R., Fritze, H.,., 2012. High nitrogen deposition alters the decomposition of bog plant litter and reduces carbon accumulation., 18: 1163-1172.
Busch, F. A., Sage, R. F., and Farquhar, G. D., 2018. Plants increase CO2uptake by assimilating nitrogenthe photorespiratory pathway., 4 (1): 46-54.
Chen, B. B., and Sun, Z. G., 2020. Effects of nitrogen enrichment on variations of sulfur in plant-soil system ofin coastal marsh of the Yellow River Estuary, China., 109: 105797.
Chen, M., Chang, L., Zhang, J., Guo, F., Vymazal, J., He, Q.,., 2020. Global nitrogen input on wetland ecosystem: The driving mechanism of soil labile carbon and nitrogen on greenhouse gas emissions., 4: 100063.
China Oceanic Information Network, 2011. Ocean Environmental Quality Communique of China (2010). http://www. nmdis.org.cn/.
China Oceanic Information Network, 2019. Ocean Environmen- tal Quality Communique of China (2014-2018). http://www. nmdis.org.cn/.
Cott, G. M., Caplan, J. S., and Mozdzer, T. J., 2018. Nitrogen uptake kinetics and salt marsh plant responses to global chan- ge., 8 (1): 5393.
Craft, C., Krull, K., and Graham, S., 2007. Ecological indicators of nutrient enrichment, freshwater wetlands, Midwestern Uni- ted States (U.S.)., 7 (4): 733-750.
Craig, H., Antwis, R. E., Cordero, I., Ashworth, D., Robinson, C. H., Osborne, T. Z.,., 2021. Nitrogen addition alters com- position, diversity, and functioning of microbial communities in mangrove soils: An incubation experiment., 153: 108076.
Dahmani-Muller, H., van Oort, F., Gélie, B., and Balabane, M., 2000. Strategies of heavy metal uptake by three plant species growing near a metal smelter., 109 (2): 231-238.
Davis, M. P., David, M. B., Voigt, T. B., and Mitchell, C. A., 2015. Effect of nitrogen addition on×yield, nitrogen losses, and soil organic matter across five sites., 7 (6): 1222-1231.
Deegan, L. A., Johnson, D. S., Warren, R. S., Peterson, B. J., Fleeger, J. W., Fagherazzi, S.,., 2012. Coastal eutrophication as a driver of salt marsh loss., 490 (7420): 388-295.
Duan, L., Hao, J. M., and Xie, S. D., 2002. Estimating critical loads of sulfur and nitrogen for Chinese soils by steady state method., 23: 7-12.
Fan, Z. Q., Wang, Z. Q., and Wu, C., 2004. Effect of different nitrogen supply onseedling’s biomass, N partitioning and their seasonal variation., 15 (9): 1497-1501.
Ferrise, R., Triossi, A., Stratonovitch, P., Bindi, M., and Martre, P., 2010. Sowing date and nitrogen fertilisation effects on dry matter and nitrogen dynamics for durum wheat: An experimental and simulation study., 117 (2-3): 245-257.
Gao, S., Wang, J. F., Knops, J. M. H., and Wang, J., 2020. Nitrogen addition increases sexual reproduction and improves seedling growth in the perennial rhizomatous grass., 20 (1): 106.
Gerdol, R., Petraglia, A., Bragazza, L., and Brancaleoni, L., 2007. Nitrogen deposition interacts with climate in affecting production and decomposition rates inmosses., 13 (8): 1810-1821.
Gu, F. T., 1999. Research in exploiting the green series of edibles–., 5: 43- 48.
Guan, B., Xie, B. H., Yang, S. S., Hou, A. X., Chen, M., and Han, G. X., 2019. Effects of five years’ nitrogen deposition on soil properties and plant growth in a salinized reed wetland of the Yellow River Delta., 136: 160- 166.
Hammelehle, A., Oberson, A., Lüscher, A., Mäder, P., and Mayer, J., 2018. Above- and belowground nitrogen distribution of a red clover-perennial ryegrass sward along a soil nutrient availability gradient established by organic and conventional cropping systems., 425 (1-2): 507-525.
Hao, C., Gurmesa, G. A., Wei, Z., Zhu, X. M., Zheng, M. H., Mao, Q. G.,., 2016. Nitrogen saturation in humid tro- pical forests after 6 years of nitrogen and phosphorus addition: Hypothesis testing., 30 (2): 305-313.
Hershey, N. R., Nandan, S. B., Vasu, K. N., and Tait, D. R., 2021. Anthropogenic nutrient loads and season variability drive high atmospheric N2O fluxes in a fragmented mangrove system., 11 (1): 6930.
Hester, E. R., Harpenslager, S. F., and van Diggelen, J. M. H., 2018. Linking nitrogen load to the structure and function of wetland soil and rhizosphere microbial communities., 3 (1): e00214-17.
Hobbie, S. E., Eddy, W. C., Buyarski, C. R., Adair, E. C., Ogdahl, M. L., and Weisenhorn, P., 2012. Response of decomposing litter and its microbial community to multiple forms of nitrogen enrichment., 82 (3): 389-405.
Hou, S. L., Hättenschwiler, S., Yang, J. J., Sistla, S., Wei, H. W., Zhang, Z. W.,., 2020. Increasing rates of long-term nitrogen deposition consistently increased litter decomposition in a semi-arid grassland., 229 (1): 296-307.
Howarth, R. W., 2008. Coastal nitrogen pollution: A review of sources and trends globally and regionally., 8 (1): 14-20.
Hoyos-Santillan, J., Lomax, B. H., and Turner, B. L., 2018. Nutrient limitation or home field advantage: Does microbial community adaptation overcome nutrient limitation of litter decomposition in a tropical peatland?, 106 (4): 1558-1569.
Hu, X. Y., and Sun, Z. G., 2020. Effects of temperature and nitrogen input on nitrogen mineralization of soils in the newly created marshes of Yellow River Estuary., 40 (24): 8882-8891.
Hu, X. Y., and Sun, Z. G., 2021. Effects of exogenous nitrogen import on variations of nutrient in decomposing litters ofin coastal marsh of the Yellow River Estuary, China.,28: 33165-33180.
Isbell, F., Reich, P. B., Tilman, D., Hobbie, S. E., Polasky, S., and Binder, S., 2013. Nutrient enrichment, biodiversity loss, and consequent declines in ecosystem productivity.,110 (29): 11911-11916.
Iversen, C. M., Bridgham, S. D., and Kellogg, L. E., 2010. Scaling plant nitrogen use and uptake efficiencies in response to nutrient addition in peatlands., 91 (3): 693-707.
Jeppesen, E., Kronvang, B., and Olesen, J. E., 2011. Climate change effects on nitrogen loading from cultivated catchments in Europe: Implications for nitrogen retention, ecological state of lakes and adaptation., 663 (1): 1-21.
Jia, J., Bai, J. H., Wang, W., Zhang, G. L., Wang, X., Zhao, Q. Q.,., 2018. Changes of biogenic elements inandfrom salt marshes in Yellow River Delta, China., 28 (3): 411- 419.
Jiang, H. H., Sun, Z. G., Wang, L. L., Sun, W. L., Sun, W. G., and Song, H. L., 2012. A preliminary study on methane production potential of soil in tidal wetlands of the Yellow River Estuary and its responses to organic matter and nitrogen import., 10 (4): 451-458.
Koceja, M. E., Bledsoe, R. B., Goodwillie, C., and Peralta, A. L., 2021. Distinct microbial communities alter litter decompo- sition rates in a fertilized coastal plain wetland., 12 (6): e03619.
Li, Y. S., and Redmann, R. E., 1992. Nitrogen budget ofin Canadian Mixed Prairie., 128 (1): 61-71.
Liang, X. Y., Zhang, T., Lu, X. K., Ellsworth, D. S., BassiriRad, H., You, C. M.,., 2020. Global response patterns of plant photosynthesis to nitrogen addition: A meta-analysis., 26: 3585-3600.
Liu, C. Y., Jiao, R. Z., and Dong, Y. H., 2015. Response of the N-cycling associated soil microorganism to simulated N deposition in a plantation of., 4 (4): 96-102.
Lu, M., Yang, Y. H., Luo, Y. Q., Fang, C. M., and Zhou, X. H., 2011. Responses of ecosystem nitrogen cycle to nitrogen addition: A meta-analysis., 189 (4): 1040-1050.
Lu, X., Vitousek, P. M., Mao, Q., Gilliam, F. S., Luo, Y., Zhou, G.,., 2018. Plant acclimation to long-term high nitrogen deposition in an N-rich tropical forest., 115: 5187-5192.
Luce, M. S., Whalen, J. K., and Ziadi, N., 2016. Net nitrogen mineralization enhanced with the addition of nitrogen-rich particulate organic matter.,262: 112-118.
Magill, A. H., Aber, J. D., Berntson, G. M., McDowell, W. H., Nadelhoffer, K. J., Melillo, J. M.,., 2000. Long-term nitrogen additions and nitrogen saturation in two temperate forests., 3 (3): 238-253.
Martina, J. P., Currie, W. S., Goldberg, D. E., and Elgersma, K. J., 2016. Nitrogen loading leads to increased carbon accretion in both invaded and uninvaded coastal wetlands., 7 (9): e01459.
Moffett, K., Nardin, W., Silvestri, S., Wang, C., and Temmerman, S., 2015. Multiple stable states and catastrophic shifts in coastal wetlands: Progress, challenges, and opportunities in validating theory using remote sensing and other methods., 7 (8): 10184-10226.
Mou, X. J., Sun, Z. G., Wang, L. L., and Wang, C. Y., 2011. Nitrogen cycle of a typicalmarsh ecosystem in the tidal marsh of the Yellow River Estuary., 23 (6): 958-967.
Mu, X. H., and Chen, Y. L., 2021. The physiological response of photosynthesis to nitrogen deficiency., 158: 76-82.
Munoz-Hiñcapié, M., Morell, J. M., and Corredor, J. E., 2002. Increase of nitrous oxide flux to the atmosphere upon nitrogen addition to red mangroves sediments., 44 (10): 992-996.
Pandey, A., Suter, H., He, J. Z., Hu, H. W., and Chen, D. L., 2018. Nitrogen addition decreases dissimilatory nitrate reduction to ammonium in rice paddies.,84 (17): e00870-18.
Pareja-Sánchez, E., Cantero-Martínez, C., Álvaro-Fuentes, J., and Plaza-Bonilla, D., 2020. Impact of tillage and N fertiliza- tion rate on soil N2O emissions in irrigated maize in a Mediterranean agroecosystem.,287: 106687.
Renzi, J. J., He, Q., and Silliman, B. R., 2019. Harnessing positive species interactions to enhance coastal wetland restoration., 7: 131.
Silgram, M., and Chambers, B. J., 2002. Effects of long-term straw management and fertilizer nitrogen additions on soil nitrogen supply and crop yields at two sites in eastern England., 139 (2): 115-127.
Sirulnik, A. G., Allen, E. B., Meixner, T., and Allen, M. F., 2007. Impacts of anthropogenic N additions on nitrogen mineralization from plant litter in exotic annual grasslands., 39 (1): 24-32.
Song, C. C., Wang, L. L., Tian, H. Q., Liu, D. Y., Lu, C. Q., Xu, X. F.,., 2013. Effect of continued nitrogen enrichment on greenhouse gas emissions from a wetland ecosystem in the Sanjiang Plain, Northeast China: A 5 year nitrogen addition experiment., 118 (2): 741-751.
Song, Y. Y., Song, C. C., Ren, J. S., Tan, W. W., Jin, S. F., and Jiang, L., 2018. Influence of nitrogen additions on litter decomposition, nutrient dynamics, and enzymatic activity of two plant species in a peatland in Northeast China., 625: 640-646.
Steinmuller, H. E., Dittmer, K. M., White, J. R., and Chambers, L. G., 2018. Understanding the fate of soil organic matter in submerging coastal wetland soils: A microcosm approach., 337: 1267-1277.
Sun, W. L., Sun, Z. G., Tian, L. P., and Hu, X. Y., 2017. Variation and prediction of different marsh landscapes in intertidal zone of the Yellow River Delta., 37: 215-225.
Sun, Z. G., and Liu, J. S., 2008. Distribution and fate of anthropogenic nitrogen in thewetland ecosystem of Sanjiang Plain, Northeast China., 50 (4): 402-414.
Sun, Z. G., Mou, X. J., Song, H. L., and Jiang, H. H., 2013. Sulfur biological cycle of the differentmarshes in the intertidal zone of the Yellow River Estuary, China., 53: 153-164.
Sun, Z. G., and Mou, X. J., 2016. Effects of sediment burial disturbance on macro and microelement dynamics in decomposing litter ofin the coastal marsh of the Yellow River Estuary, China., 23 (6): 5189-5202.
Sun, Z. G., Mou, X. J., Sun, J. K., Song, H. L., Yu, X., Wang, L. L.,., 2012. Nitrogen biological cycle characteristics of seepweed () wetland in intertidal zone of Huang- he (Yellow) River Estuary., 22 (1): 15-28.
Szukics, U., Hackl, E., Zechmeister-Boltenstern, S., and Sessi- tsch, A., 2009. Contrasting response of two forest soils to nitrogen input: Rapidly altered NO and N2O emissions andabundance., 45 (8): 855-863.
Tegeder, M., and Masclaux-Daubresse, C., 2018. Source and sink mechanisms of nitrogen transport and use., 217 (1): 35-53.
Ti, C. P., and Yan, X. Y., 2010. Estimation of atmospheric nitrogen wet deposition in China mainland from based on N emission data., 29 (8): 1606-1611.
Tian, D. S., and Niu, S. L., 2015. A global analysis of soil acidification caused by nitrogen addition., 10 (2): 024019.
Tilman, D., Isbell, F., and Cowles, J. M., 2014. Biodiversity and ecosystem functioning.,45: 471-493.
Tognetti, P. M., Prober, S. M., Báez, S., Chaneton, E. J., Firn, J., Risch, A. C.,., 2021. Negative effects of nitrogen over- ride positive effects of phosphorus on grassland legumes worldwide.,118 (28): e2023718118.
Tomassen, H. B. M., Smolders, A. J. P., Limpens, J., Lamers, L. P. M., and Roelofs, J. G. M., 2004. Expansion of invasive species on ombrotrophic bogs: Desiccation or high N deposition?, 41 (1): 139-150.
Tylova-Munzarova, E., Lorenzen, B., Brix, H., and Votrubova, O., 2005. The effects of NH4+and NO3−on growth, resource allocation and nitrogen uptake kinetics ofand., 81 (4): 326-342.
Uribelarrea, M., Crafts-Brandner, S. J., and Below, F. E., 2009. Physiological N response of field-grown maize hybrids (L.) with divergent yield potential and grain protein concentration., 316 (1-2): 151-160.
Vivanco, L., Irvine, I. C., and Martiny, J. B. H., 2015. Nonlinear responses in salt marsh functioning to increased nitrogen addition., 96 (4): 936-947.
Vivanco, L., and Austin, A. T., 2011. Nitrogen addition stimu- lates forest litter decomposition and disrupts species interactions in Patagonia, Argentina., 17 (5): 1963-1974.
Xie, Q., 2017. The ancient tidal characteristics and the tide current evolutionary process of the Yellow River Estuary. PhD thesis. University of Tianjin, Tianjin.
Xie, Y. H., Wen, M. Z., Yu, D., and Li, Y. K., 2004. Growth and resource allocation of water hyacinth as affected by gradually increasing nutrient concentrations., 79 (3): 257-266.
Yu, J. B., Ning, K., Li, Y. Z., Du, S. Y., Han, G. X., Xing, Q. H.,., 2014. Wet and dry atmospheric depositions of inorganic nitrogen during plant growing season in the coastal zone of Yellow River Delta., 949213.
Zangani, E., Afsahi, K., Shekari, F., Mac Sweeney, E., and Mastinu, A., 2021. Nitrogen and phosphorus addition to soil improves seed yield, foliar stomatal conductance, and the photosynthetic response of rapeseed (L.)., 11 (6): 483.
Zhang, W. N., and Liao, Z. Y., 2009. Research of impacts on forestry by nitrogen deposition., 28 (3): 21-24.
Zhong, M. X., Miao, Y., Han, S. J., and Wang, D., 2019. Nitro- gen addition decreases seed germination in a temperate steppe., 9 (15): 8441-844.
(November 11, 2021; revised May 1, 2022; accepted July 13, 2022)
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2023
Corresponding author. E-mail: zhigaosun@163.com
(Edited by Ji Dechun)
杂志排行
Journal of Ocean University of China的其它文章
- The Subduction Structure Beneath the New Britain Island Arc and the Adjacent Region from Double-Difference Tomography
- Differences of Polygonal Faults with Irregularly Polygonal Geometries: A Case Study from the Changchang Sag of Qiongdongnan Basin, Northern South China Sea
- Characterization of Bacterial Communities in Aerosols over Northern Chinese Marginal Seas and the Northwestern Pacific Ocean in Autumn
- Assessment and Application of Beach Quality Based on Analytic Hierarchy Process in Yangkou Beach, Qingdao
- Role of Resuspended Sediments as Sources of Dissolved Inorganic Phosphorus Along Different Dimensions in the Subei Shoal, South Yellow Sea, China
- Pharmacokinetics of Enrofloxacin and Its Metabolite in Carp (Cyprinus carpio) After a Single Oral Administration in Medicated Feed
