Productive Traits and Triploid Rate Stability in Triploidy-Induced ‘Haida No. 2’ Strain of the Pacific Oyster, Crassostrea gigas
2023-03-17LIYongguoLIQiLIUYeandXUChengxun
LI Yongguo, LI Qi, 2),, LIU Ye, and XUChengxun
Productive Traits and Triploid Rate Stability in Triploidy-Induced ‘Haida No. 2’ Strain of the Pacific Oyster,
LI Yongguo1), LI Qi1), 2),*, LIU Ye1), and XUChengxun1)
1),,,266003,2),,266237,
In order to evaluate the effects of triploidy induction on a selected strain ‘Haida No.2’ of the Pacific oyster, which is characterized with golden shell color and high growth rate, the growth, survival rate and stability of triploid rate were analyzed at different development stages in the present study.Three different conditions inhibiting the release of polar body II or polar body I were tested: (A) Cytochalasin-B (CB), 0.5mgL−1at 10min post-insemination for 15min; (B) CB, 0.5mgL−1at 15min post-insemination for 20min; and (C) CB, 0.7mgL−1, at 15min post-insemination for 20min. The triploidy induction treatments signifi- cantly reduced the D-larvae and survival rates at the larvae stage but not at the juvenile and adult stages. Triploid rate dramatically decreased at the larval stage and did not significantly change at the juvenile and adult stages. Regarding the stability of the triploid rate, there was a significant difference between the three treatment groups. Larvae from the treatment A and control groups exhibited higher growth rates in shell height than those from the other two treatment groups at day 27. Triploid juveniles and adults from the treatment A group exhibited a higher wet weight than diploids from the control group and triploids from the other treatment groups. Triploidy induction did not affect the shell color of the progeny. The results obtained in the study demonstrate that triploidy induction has the potential to be used to increase the production ofvariety ‘Haida No.2’ without modifying its golden shell color.
; selective strain; triploidy induction;survival;growth; triploid rate stability
1 Introduction
The Pacific oyster,, is one of the most important commercial mollusks worldwide becauseof its rapid growth and strong adaptability. Oyster breeding pro- grams in various countries had made mass contributions to increase yield and support the sustainability of the oysterindustry. However, spawning of the oyster during the re-productive season can reduce meat quality (Desrosiers., 1993) and render it unmarketable. This limited the supply of oysters throughout the year and further expanded pro-duction of new oyster varieties with improved traits.
Unlike diploid shellfish, triploid shellfish does not have the reproductive characteristics of massive ovulation in sum- mer, which might increase its yield (Guo, 1994; Benfey,1999). Triploid oysters can improve its marketability andmeat quality due to the decrease of gonadogenesis during the reproductive season(Nell, 2002; Qin., 2018).In addition, triploidy can potentially produce oysters with ex- cellent production performance by decreasing reproduc-tive effort (Nell, 2002; Nell and Perkins, 2005; Buestel., 2009;Jeung., 2016; Qin., 2019; Wadsworth., 2019).
Triploidy with reduction of gametogenesis is thought to have more energy for growth;however, the superiority in yield of triploid shellfish over diploid shellfish has not been demonstrated in all cases. Wadsworth. (2019) found that about 35% of the experiments with chemically induced triploids had no growth advantage than diploids. It is wide- ly believed that ploidy differences affect growth; however, the growth of triploids formed by inhibiting the release of different polar bodies is also different (Stanley., 1984; Hawkins., 1994).Retaining the first polar body (PB I) can increase gene heterozygosity compared to oysters inhi- biting the second polar body (PB II), and whether the addi- tional set of chromosomes comes from homologous chro- mosomes or non-homologous chromosomes in triploids can influence its performance(Zouros., 1988; Wang., 2002;Callam., 2016). In addition, environmental con-ditions are also an important aspect. The comparison of tri- ploid and diploid performancesis also influenced by their living environmental conditions (Cheney., 2000; Smith., 2000;Garnier-Géré., 2002;Callam., 2016). In order to increase the variety of oyster strains and exploit the commercial value of shell color, a breeding program forwas launched in 2010.After a 6-generation selec- tion, using shell color and shell height as breeding traits, a new strain of‘Haida No.2’ with golden shell co- lor and excellent growth performance has been developed (Ge., 2015). In order to develop triploid‘Hai-da No.2’, we investigated the effects of triploidy induction on the overall growth, survival and triploid rate of‘Haida No.2’ at different developmental stages in the pre- sent study.
2 Materials and Methods
2.1 Parental Animals
Two hundred adult individuals of‘Haida No.2’ (shell height>80mm) that were farmed at Sanggou Bay, China were selected as broodstock. The broodstock were transferred to hatchery for 2–3 months to promote gonadal maturation before the experiments. The broodstock were kept in a warm seawater tank (24℃±1℃) for at least 7 days prior to strip-spawning.
2.2 Gametes Preparation
The‘Haida No.2’ broodstock were randomly se-lected. Eggs were collected by 20-μm nylon mesh, and sieved through 80-μm nylon mesh to remove tissue debries and impurities. Collected eggs were suspended in filtered seawater (FSW, with a salinity of 32 practical salinity unit; and a temperature of 24℃±0.5℃) until most of the eggs were near spherical. Sperms were obtained by strip spawn- ing 10min before insemination. Sperms were sieved through50-μm nylon mesh and suspended in filtered seawater (FSW).
2.3 Fertilization and Treatment
Six females and three males were used in the experiment. All eggs were pooled before fertilization. The ratio of spermto egg was adjusted as approximately 50:1. For differenttriploid induction treatments, the fertilized eggs were equal-ly divided into four groups with different treatments: (A) Cytochalasin B (CB), 0.5mgL−1at 10min post-insemination for 15min; (B) CB, 0.5mgL−1at 15min post-insemination for 20min; and (C) CB, 0.7mgL−1, at 15min post-insemina- tion for 20min; and (D) the control group without any CB treatment. For every treatment, three replicates were set up. After the treatment, CB was thoroughly washed off the em- bryos, and the embryos were rinsed in dimethyl sulfoxide (1mLL−1) before they were transferred to 60L tanks.
2.4 Larval Rearing and Spat Grow-out
The D-larvae from every treatment group were collected at 24-h post-insemination and reared in two tanks (60L) for the measurement of triploid and survival rates, respec- tively. The initial larval density of each tank was control- led at 5 larvaemL−1. The larvae were reared according to a previously reported culture procedures (Li., 2011).When at least 50% of larvae exhibited eyespots, a string of scallop shells was placed in each tank as substrates for set- tlement. The spats were cultivated in a pond for 1 month before transferred to the oyster farm at Ailian Bay, Rong- cheng City, China.
2.5 Ploidy Analysis
The triploid rate of larvae was examined at 7, 14, 21 and 27 days by flow cytometry during the larval period. Before detection, approximately 300 larvae were collected into a 1.5mL centrifuge tube and centrifuged gently (300) to remove supernatant. Then 1mL of phosphate buffered sa- line (PBS, with the pH of 7.4) was added to the tube to sus- pend the larvae. Larvae were then disaggregated by gentle repeated pipetting with a 1.5mL syringe. Cell suspensions sieved by 50-μm nylon mesh and were fixed by cold etha- nol. After propidium iodide staining for 30min, the ploidy level of the fixed cells was analyzed by flow cytometry(Beckman CytoFLEX). Gill fragments were sampled from at least 90 individuals per treatment for flow cytometry at days 120, 330 and 450 until 30 triploid oysters were de- tected. The shell height and wet weight of each individual were measured.Each individual was labeled with a water- proof label before ploidy was detected.
2.6 Data Analysis
The survival and growth parameters of larvae were mea- sured according to Kong. (2016). The survival and growth parameters were estimated at juvenile and adult stages. From the control and three treatment groups, 90 in- dividuals were randomly selected to estimate survival rate at days 120, 330 and 450. SPSS statistical package 23.0 wasused for statistical analysis.<0.05 represented significant statistical difference.
3 Results
3.1 Triploidy, Growth and Survival of Larvae
In the flow cytometry, the larvae from the control group had only a clear peak of diploid, while diploid and triploid larvae from the treatment groups could be visibly distin- guished (Fig.1).The D-larvae rates of the control, treat- ment A, treatment B and treatment C groups were 74.53%, 25.67%, 39.70% and 40.90%, respectively (Fig.2). The D-larvae rates of the three treatment groups were significant- ly lower than that of the control group (<0.05). The D-larvae rate of treatment A group was significantly lower than those of the treatment B and treatment C groups (<0.05), but the D-larvae rate difference between the treatment B and treatment C groups was not significant.
As for growth performance, the shell height between the three treatment groups and the control group showed a significant difference at day 8 (Fig.3). At day 27, the shell height of the larvae reached 231.87μm in the control group and 229.38μm in the treatment A group, which were sig- nificantly higher than that of the treatment B group (188.25μm) and treatment C group (210.13μm) (<0.05). During the larval stage, the survival rate of all groups decreased significantly, but the survival rate of the three treatment groups was significantly lower than that of the control groupexcept at day 22. The control group showed the highest sur-vival rate (29.79%), while the lowest value was observed in the treatment B group (6.20%) at day 27.
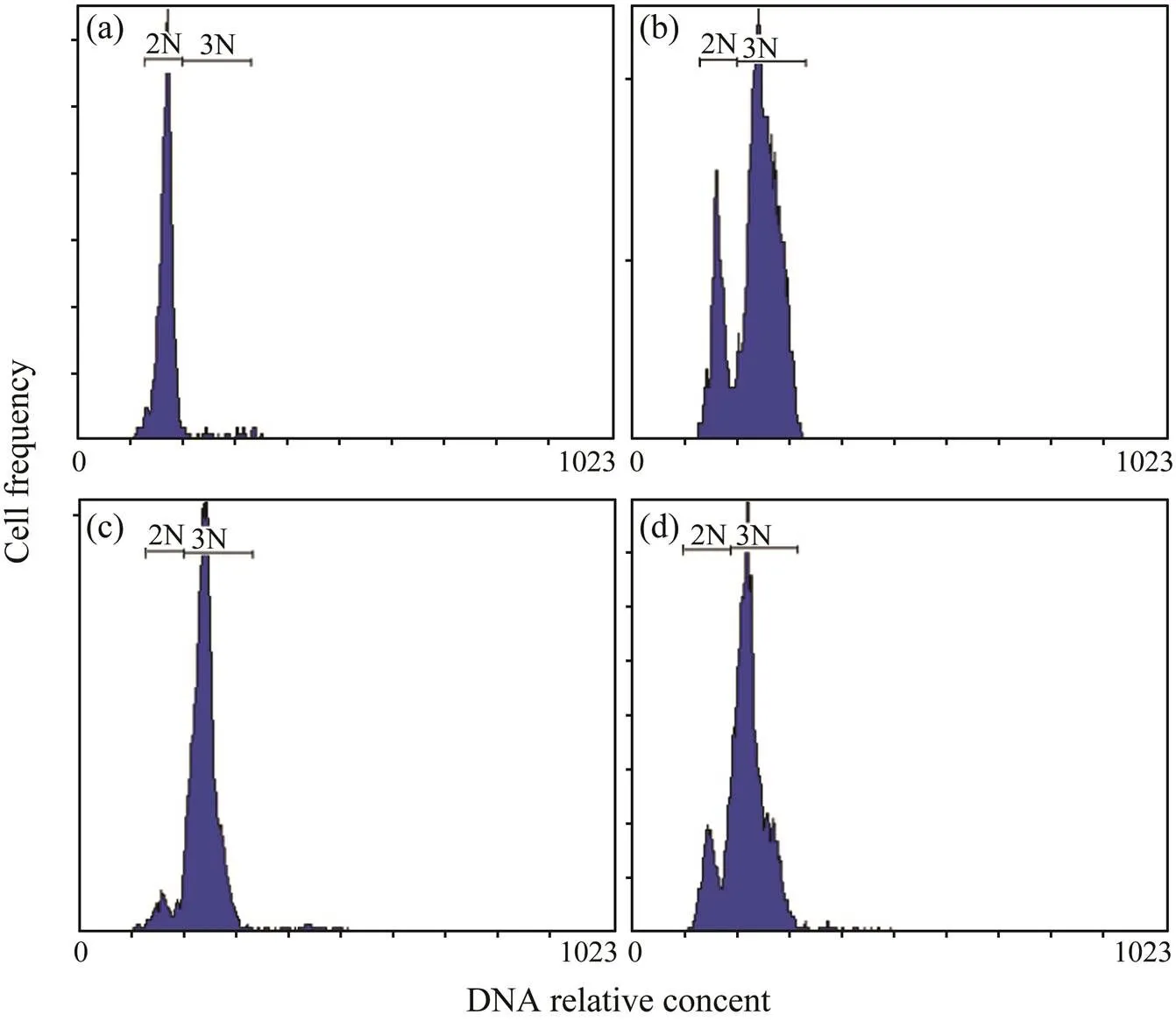
Fig.1Ploidy composition of larvae samples of control group (a), treatment A (b), treatment B (c) and treatment C (d), mea- sured by flow cytometry. 2N, diploids; 3N, triploids.
3.2 Triploid Rate from Larvae to Adult Stage
For all treatment groups, the critical period where the tri-ploid rate decreased significantly was the larval stage. Du- ring this stage, the triploid rate decreased to 31.22% in the treatment A group, 62.16% in the treatment B group and 24.54% in the treatment C group (Fig.4). Compared with treatment A and treatment C groups, the treatment B group had the higher stability of triploid rate in the larval stage. However, no significant change was observed in all treat- ment groups in juvenile and adult stages.

Fig.3 Shell height (a) and survival rate (b) of C. gigas ‘Haida No. 2’ larvae from the treatment A, the treatment B and the treatment C groups. Different lowercase letters denote significant difference (P<0.05).
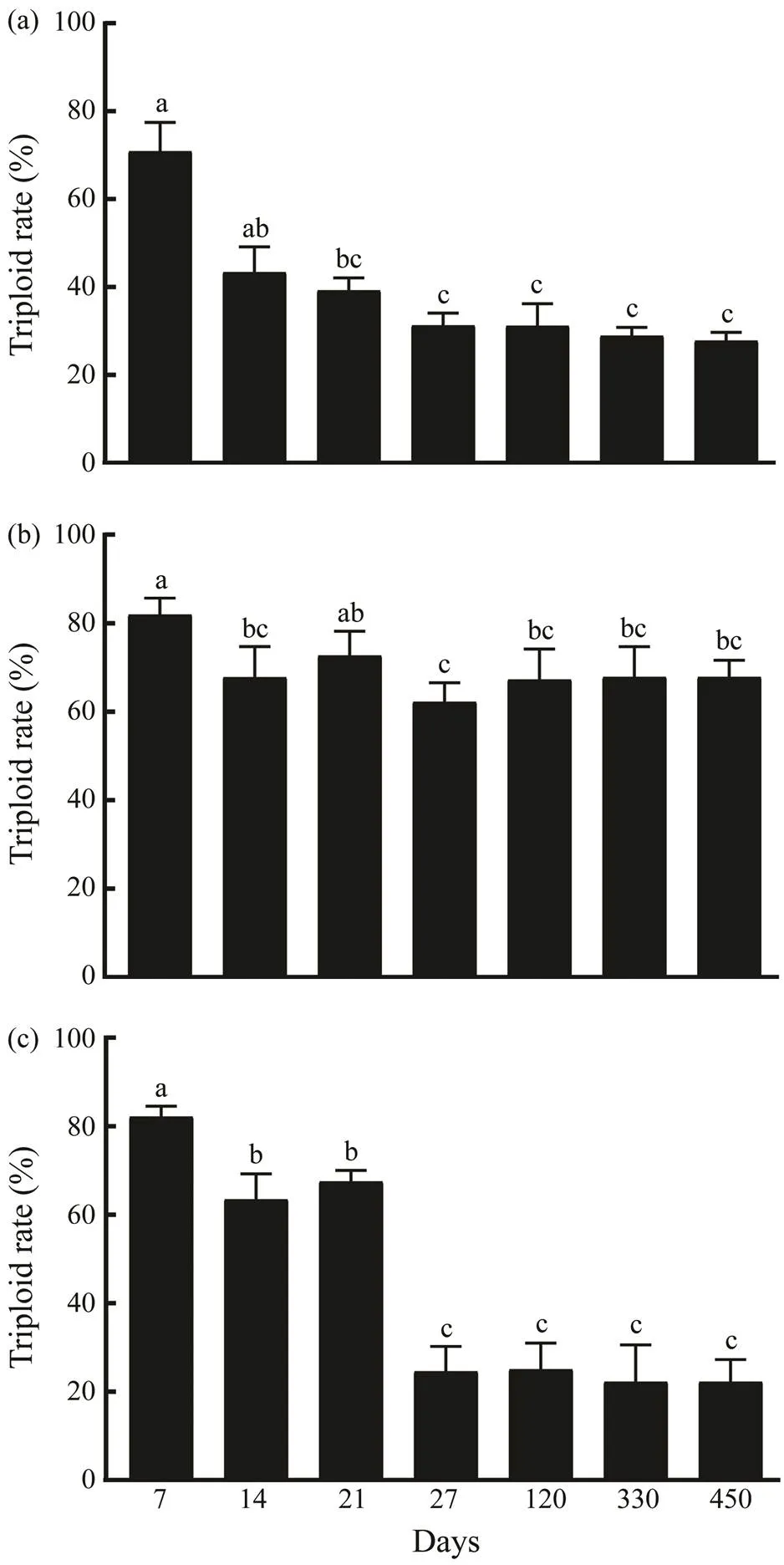
Fig.4 Variations of the triploid rate in the treatment A (a), treatment B (b) and treatment C (c) groups. Different low- ercase letters denote significant difference (P<0.05).
3.3 Growth and Survival of Juveniles and Adults
The shell height and shell length of the triploid oysters in the group A were significantly higher than those of the other two treatment groups and the control group at day 450 (<0.05) (Fig.5). The wet weight of the diploid oys- ters in the control group and triploid oysters in the threetreatment groups showed no significant difference at day 120. At day 330, the wet weight of diploid oysters from the control group was higher than that of the triploid oysters from the treatment C groups, but lower than that of the tri- ploid oysters in the treatment A group (<0.05). The wet weight at day 450 showed a similar pattern to that of day 330, with higher wet weight observed in the treatment A group compared to the other groups.
The survival rate between the three treatment groups and the control group was not different significantly at days 330 and 450. At days 330 and 450, the survival rate decreased from 88.89% to 76.67% in the control group, from 86.67% to 73.33% in the treatment A group, from 84.44% to 74.44% in the treatment B group, and from 85.56% to 75.56% in the treatment C group (Fig.6). At day 450, only treatment A group had a significant reduction in survival rate (<0.05).The shell color of the juveniles and adults from all groups was golden and aligned with their parents.
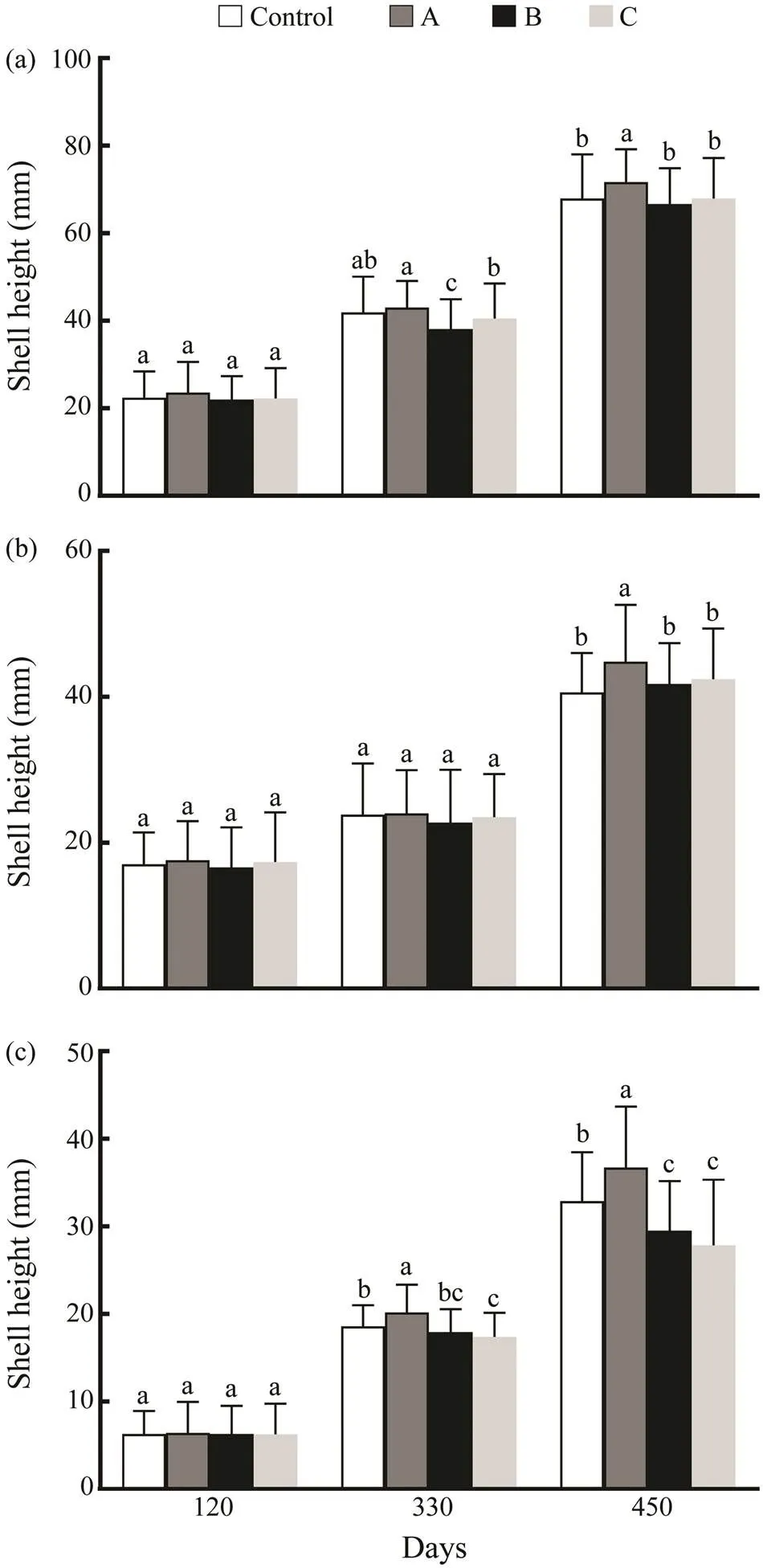
Fig.5 Growth in shell height (a), shell length (b) and wet weight (c) of triploid and diploid juveniles and adultsof C. gigas‘Haida No. 2’. A, triploids from the treatment A group; B, triploids from the treatment B group; C, triploids from the treatment C group. Different lowercase letters denote significant difference (P<0.05).
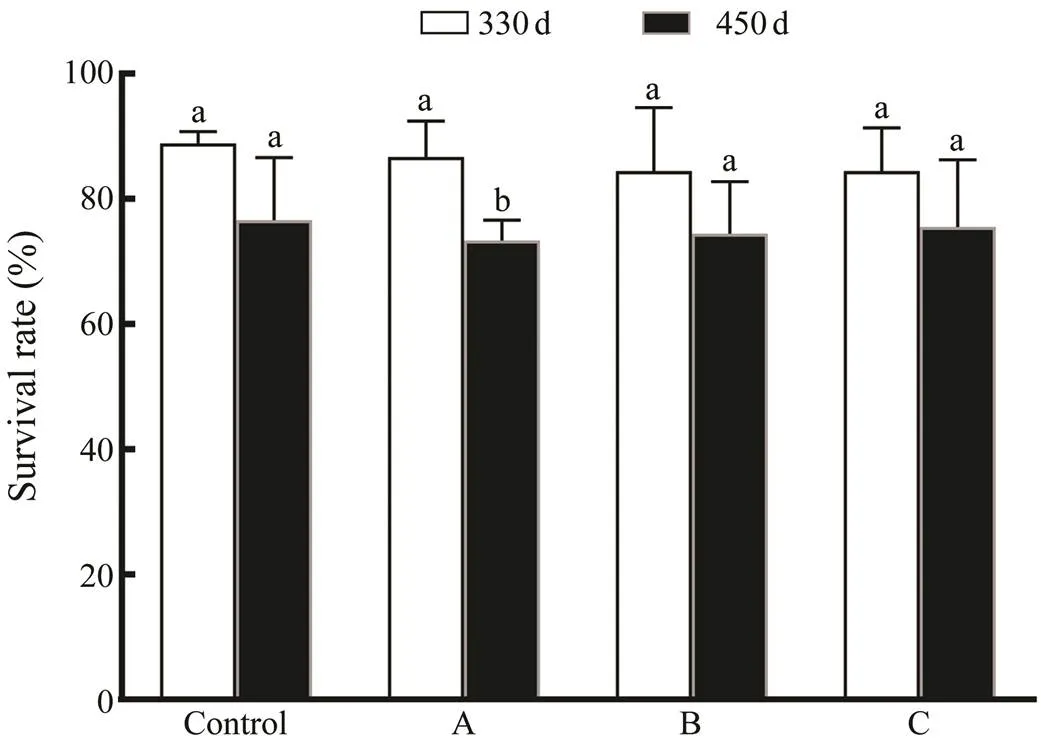
Fig.6 Survival rate of C. gigas‘Haida No. 2’ juveniles and adults from the treatment A, the treatment B and the treat- ment C groups. Different lowercase letters denote signifi- cant difference (P<0.05).
4 Discussion
Triploid oysters have been widely used in the oyster cul- ture industry for a few decades in several countries. How- ever, the effects of triploidy induction on production per- formance of selectively bred strains of the oyster are rarely reported. In order to obtain triploid oysters with outstand- ing traits, the development of triploid oysters from the se- lective bred varieties currently is necessary.
In our study, the D-larvae rate in the control group was significantly higher than those of the three treatment groups, which clearly demonstrated that triploidy induction signi- ficantly reduced the D-larvae rate of‘Haida No.2’. This is consistent with previous findings (Gerard., 1994; Gérard., 1999; Supan., 2000). The D-lar- vae rates of the treatment B and C groups were significant- ly higher than that of the treatment A group. This might be more likely attributed to the case that some of the triploid oysters in the treatment A group were derived from the re- taining PB I, as retaining PB I can cause lower survival rate compared to inhibition of PB II (Gérard., 1999). In ad- dition, blocking PB I may cause abnormalities in chromo- some segregation, resulting in a large number of aneuploidy (Guo., 1992).
During the larval stage, the survival and triploid rates in the three experiment groups decreased significantly, which was caused by treatment with high CB concentration. Simi-lar results were observed in in a previous study (Melo., 2015). Significant differences in the stability of triploid rate among the three treatment groups were also observed. The triploid rate stability of the treatment A group was lower than that of the treatment B group. One possible reason is that treatment A group has massive aneuploidy that is like- ly to cause this change of ploidy rate, considering aneuploidand triploid have similar chromosome numbers and are often difficult to distinguish by flow cytometry.Aneuploids had higher mortality than normal triploidy, which reduced the triploid rate stability of treatment A group. The differences in CB concentration contributed to the differences in thetriploid rate stability between the treatment B group and the treatment C group. The reason is that the treatment C group with high CB concentration led to a high mortality, which caused a significant decrease in the triploid rate. we observed a difference in shell height between the treatment B group and the control group, which agreed with previous studies (Desrosiers., 1993; Toro., 1995; Yang and Guo 2006). These results support that the rapid growth of trip- loid is the results of the reduction of gametogenesis. Thereis no gonadal development in the larval stage, so the trip-loids may devote almost the same amount of energy forgrowth as the diploid larvae (Allen and Downing, 1986). Another explanation is that triploid oysters fail to adapt to aberrant chromosomes.
Significant differences in survival rate of juveniles and adults between the three experiment groups and the control group were not observed. However, some studies found that the survival rate of triploid oysters was higher than diploid oysters,and the reason might be that the reduction of ga- metogenesis allowed it to maintain higher resistance ability during the summer (Gagnaire., 2006; Houssin., 2019). Therefore, the factors reducing reproductive deve- lopment could also reduce mortality. In our current study, the food available caused by excessive aquaculture was re- latively limited in Ailian Bay. Most triploid oysters are ste- rile, and the limited food availability is more beneficial to increasing the survival rate of diploid oysters by hindering reproductive development, thus the significant difference in survival rate of juveniles and adults between the three ex- periment groups and the control group was not observed. The wet weight of triploids in treatment A group was high- er than those in the treatment B and C groups. Compared with triploid oysters from the other groups, a portion of tri- ploid oysters in the treatment A group derived from the blocking PB I. Triploid oysters from the blocking PB I had a higher heterozygosity than those from the blocking PB II. One potential explanation is that the increase of gene hete- rozygosity resulted in heterosis and improved its growth (Stanley., 1984). Heterosis acts as a mechanism to im- prove the whole tissue weight in oysters (Hedgecock., 2007). Compared with the larval stage, the triploid rates of the three experiment groups showed no significant diffe- rence in the juvenile and adult stages. This might be related to the low mortality in these two stages.
5 Conclusions
The triploid rates of‘Haida No.2’ in the three treatment groups significantly decreased only in the larval stage and the stability of triploid rates under different induc-tion conditions were significantly different. The wet weight of triploid oysters from treatment A group was higher than that of diploid oysters from the control group and triploid oysters from the other two treatment groups after day 330. Meanwhile, the shell color of all triploid adults of‘Haida No.2’ was golden. Thus, the study also indicated de-veloping triploid oysters has the potential to increase the yield of‘Haida No.2’ without modifying golden shell color.
Acknowledgements
This work was supported by the grants from the China Agriculture Research System Project (No. CARS-49), and the Earmarked Fund for Agriculture Seed Improvement Project of Shandong Province (Nos. 2020LZGC016, 2021LZGC027).
Allen Jr., S. K., and Downing, S. L., 1986. Performance of triploidPacific oysters,(Thunberg). I. Survival, growth, glycogen content, and sexual maturation in yearlings., 102: 197-208.
Benfey, T. J., 1999. The physiology and behavior of triploid fishes., 7: 39-67
Buestel, D., Ropert, M., Prou, J., and Goulletquer, P., 2009. His- tory, status, and future of oyster culture in France., 28: 813-820.
Callam, B. R., Allen Jr., S. K., and Frank-Lawale, A., 2016. Ge- netic and environmental influence on triploidgrown in Chesapeake Bay: Growth., 452: 97-106.
Cheney, D. P., 2000. Summer mortality of Pacific oysters,(Thunberg): Initial finding on multiple environ- mental stressors in Puget Sound, Washington, 1998., 19: 353-359.
Desrosiers, R. R., Gérard, A., Peignon, J. M., Naciri, Y., Dufresne, L., Morasse, J.,., 1993. A novel method to produce trip- loids in bivalve molluscs by the use of 6-dimethylaminopurine., 170: 29-43.
Garnier-Géré, P. H., Naciri-Graven, Y., Bougrier, S., Magoulas, A., Héral, M., Kotoulas, G.,., 2002. Influences of triploidy, parentage and genetic diversity on growth of the Pacific oysterreared in contrasting natural environments., 11: 1499-1514.
Ge, J. L., 2015. Selective breeding and genetic analysis of shell color traits in the Pacific oyster,. PhD thesis. Ocean University of China (in Chinese with English abstract).
Gérard, A., Ledu, C., Phélipot, P., and Naciri-Graven, Y., 1999. The induction of MI and MII triploids in the Pacific oysterwith 6-DMAP or CB., 174: 229-242.
Gerard, A., Naciri, Y., Peignon, J. M., Ledu, C., and Phelipot, P., 1994. Optimization of triploid induction by the use of 6-DMAPfor the oyster(Thunberg)., 25: 709-719.
Guo, X. M., and Allen Jr., S. K., 1994. Reproductive potential and genetics of triploid Pacific oysters,(Thun- berg)., 187: 309-318.
Guo, X. M., Cooper, K., Hershberger, W. K., and Chew, K. K., 1992. Genetic consequences of blocking polar body I with cytocha- lasin B in fertilized eggs of the Pacific oyster,: I. Ploidy of resultant embryos., 183: 381-386.
Hawkins, A. J. S., Day, A. J., Gérard, A., Naciri, C., Ledu, C., Bay- ne, B. L.,., 1994. A genetic and metabolic basis for faster growth among triploids induced by blocking meiosis I but not meiosis II in the larviparous European flat oyster,L., 184: 21-40.
Houssin, M., Trancart, S., Denechere, L., Oden, E., Adeline, B., Le-poitevin, M.,., 2019. Abnormal mortality of triploid adult Pacific oysters: Is there a correlation with high gametogenesis in Normandy, France?, 505: 63-71.
Jeung, H. D., Keshavmurthy, S., Lim, H. J., Kim, S. K., and Choi, K. S., 2016. Quantification of reproductive effort of the trip- loid Pacific oyster,raised in intertidal rack and bag oyster culture system off the west coast of Korea du- ring spawning season., 464: 374-380.
Kong, L. F., Song, S. L., and Li, Q., 2017. The effect of interstrain hybridization on the production performance in the Pacific oys-ter., 472: 44-49.
Li, Q., Wang, Q. Z., Liu, S. K., and Kong, L. F., 2011. Selection re-sponse and realized heritability for growth in three stocks of thePacific oyster., 77: 643-648.
Melo, E. M. C., Gomes, C. H. A. M., Silva, F. C. D., Sühnel, S., and Melo, C. M. R. D., 2015. Chemical and physical methods of triploidy induction in(Thunberg, 1793)., 414: 889-898.
Nell, J. A., 2001. The history of oyster farming in Australia., 63: 14-25.
Nell, J. A., 2002. Farming triploid oysters., 210: 69-88.
Nell, J. A., and Perkins, B., 2005. Studies on triploid oysters in Australia: Farming potential of all-triploid Pacific oysters,(Thunberg), in Port Stephens, New South Wales, Australia., 36: 530-536.
Qin, Y. P., Zhang, Y. H., Ma, H. T., Wu, X. W., Xiao, S., Li, J.,., 2018. Comparison of the biochemical composition and nutri- tional quality between diploid and triploid Hong Kong oysters,., 9: 1674.
Qin, Y. P., Zhang, Y. H., Mo, R. G., Zhang, Y., Li, J., Zhou, Y. L.,., 2019. Influence of ploidy and environment on grow-out traits of diploid and triploid Hong Kong oystersin southern China., 507: 108-118.
Smith, I. R., Nell, J. A., and Adlard, R., 2000. The effect of grow- ing level and growing method on winter mortality,, in diploid and triploid Sydney rock oysters,., 185: 197-205.
Stanley, J. G., Hidu, H., and Allen Jr., S. K., 1984. Growth of Ame- rican oysters increased by polyploidy induced by blocking meio- sis I but not meiosis II., 37: 147-155.
Supan, J. E., Wilson, C. E., and Allen Jr., S. K., 2000. The effect of cytochalasin B dosage on the survival and ploidy of(Gmelin) larvae., 19: 125-128.
Toro, J. E., Sanhueza, M. A., Paredes, L., and Canello, F., 1995. In-duction of triploid embryos by heat shock in the Chilean nor- thern scallopLamarck, 1819., 29: 101-105.
Wadsworth, P., Wilson, A. E., and Walton, W. C., 2019. A meta-analysis of growth rate in diploid and triploid oysters., 499: 9-16.
Wang, Z. P., Guo, X. M., Allen Jr., S. K., and Wang, R. C., 2002. Heterozygosity and body size in triploid Pacific oysters,Thunberg, produced from meiosis II inhibition and tetraploids., 204: 337-348.
Yang, H. P., and Guo, X. M., 2006. Polyploid induction by heat shock-induced meiosis and mitosis inhibition in the dwarf surf-clam,Say., 252: 171-182.
Zouros, E., Romero-Dorey, M., and Mallet, A. L., 1988. Hetero- zygosity and growth in marine bivalves: Further data and pos- sible explanations., 42: 1332-1341.
(August 12, 2021; revised November 4, 2021; accepted May 10, 2022)
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2023
Correspondence author.Tel: 0086-532-82031622
E-mail: qili66@ouc.edu.cn
(Edited by Qiu Yantao)
杂志排行
Journal of Ocean University of China的其它文章
- The Subduction Structure Beneath the New Britain Island Arc and the Adjacent Region from Double-Difference Tomography
- Differences of Polygonal Faults with Irregularly Polygonal Geometries: A Case Study from the Changchang Sag of Qiongdongnan Basin, Northern South China Sea
- Characterization of Bacterial Communities in Aerosols over Northern Chinese Marginal Seas and the Northwestern Pacific Ocean in Autumn
- Assessment and Application of Beach Quality Based on Analytic Hierarchy Process in Yangkou Beach, Qingdao
- Role of Resuspended Sediments as Sources of Dissolved Inorganic Phosphorus Along Different Dimensions in the Subei Shoal, South Yellow Sea, China
- Pharmacokinetics of Enrofloxacin and Its Metabolite in Carp (Cyprinus carpio) After a Single Oral Administration in Medicated Feed
