Active Carbon/SnO2 Nanocomposite Synthesized by Impregnation for Lithium-ion Batteries
2023-03-16LIHaiYANGJunLITangpengZHAOLeiWANGShixing
LI Hai, YANG Jun, LI Tangpeng, ZHAO Lei, WANG Shixing
(1.School of Material and Chemical Engineering, Tongren University, Tongren 554300, China; 2.Guizhou Weite High Tech Energy Technology Co., Ltd., Bijie 553300, China)
【Abstract】 Activated carbon/SnO2 (AC/SnO2) nanocomposite had been synthesized by immersing AC in ethanol solution of SnCl2·2H2O, vacuum filtration, and final sintering under Ar atmosphere. The nano-pores in AC could act as hard template as well as reaction vessel for the formation of SnO2 nanoparticles (NPs). The in-situ formed SnO2 NPs were dispersed uniformly in AC matrix. As anode material for lithium-ion batteries, AC/SnO2 delivered a reversible capacity of 752 mAh·g-1 in the first cycle and 476 mAh·g-1 after 200 cycles at a current density of 100 mA·g-1, and also exhibited good rate capability. The synthesis route reported here is facile. The content of SnO2 in AC/SnO2 can be adjusted easily by change the concentration of the SnCl2·2H2O solution.
【Keywords】 Activated carbon; SnO2 nanoparticles; Anode materials; Lithium-ion batteries
1 Introduction
Nowadays, various energy storage and conv-ersion devices, such as lithium-ion batteries (LIBs)[1-4], sodium-ion batteries (SIBs)[5], supercapacitors (SC)[6], lithium-sulfur batteries (LSBs)[7], fuel cells[8], and microorganisms[9], have been developed. Among them, LIBs are considered as the most suitable power source for portable electronic devices, mobile communication devices, and hybrid/electrical vehicles due to their high energy density, high voltage, and light weight. Graphite is usually employed as a standard anode electrode because of its good cycling performance and low cost, but its low theoretical capacity (372 mAh·g-1) is insufficient to meet the increasing demand for batteries with higher energy density. As one of the substitute candidates for graphite, SnO2has attracted much attention because of its high theo-retical capacity (782 mAh·g-1)[10-11], no toxicity, and low cost. However, the insulating nature and severe volume change of 300% during charge/disch-arge process raise some issues including electrode disintegration, capacity fading, and poor rate capability. In order to realize the practical application of SnO2based anode materials, tailoring the SnO2particle down to nano-scale dimension[12-14]and com-positing into carbonaceous materials matrices[15-17]are the currently effective strategies to address the above issues. The carbon in SnO2/C composites can not only buffer the volume changes of SnO2nanoparticles (NPs) but also increase electronic conductivity of composites due to its good conductivity.
Activated carbon (AC), a commercialized carbon material, has abundant open pores with nanometer scale. These pores can absorb some gases or liquids due to capillary action and surface tension. Inspired by this, AC/SnO2nanocomposite was synthesized by solution impregnation method in this work. And these nano-pores in AC have acted as hard template as well as reaction vessel for the formation of SnO2NPs. In the AC/SnO2nanoco-mposite, the SnO2NPs are uniformly dispersed in the AC matrix, resulting in superior properties.
2 Experiments
The AC/SnO2nanocomposite was synthesized by immersing, filtration, and sintering. Typically, 5 g AC (400 mesh) powder with an average pore diameter of ~6 nm and a high surface area was added to 13 g SnCl2·2H2O dissolved in 20 mL absolute ethanol. The suspension was then sonicated for 30 min to ensure infiltration of solution into the AC. After vacuum filtration, the impregnated AC was dried at 80 ℃ for 4 h in air. The drying step allows the tin precursor to infiltrate and fill the pores within AC by capillary. The dried impregnated AC was sintered at 600 ℃ for 2 h with a heating rate of 5 ℃·min-1under Ar atmosphere using tubular furnace. For comparison, bare SnO2NPs were prepared using the same process except that the sintering atmosphere was air.
The samples were characterized by powder X-ray diffraction (XRD, Cu Kα radiation), scanning electron microscopy (SEM, SU1510) equipped with energy disperse spectroscopy (EDS), transmission electron microscope (TEM, Tecnai G2 F20), specific surface area and porosity analyzer (TriStar II 3020), and thermal gravimetric analysis (TGA) system (TA Q500).
To evaluate the electrochemical performances, the active materials were mixed with acetylene black and polyvinylidene fluoride (PVDF) at a weight ratio of 80∶10∶10. The mixture was prepared as uniform slurry in N-methyl pyrrolidinone (NMP) and painted onto copper foils. The electrodes were dried under vacuum at 120 ℃ for 12 h. The mass loading is ~7 mg/cm2. CR2016-type coin cells were assembled in an argon-filled glovebox. Lithium-metal foil was used as the counter electrode and the reference electrode and microporous polypropylene (Celgard 2400) as the separator. The electrolyte was 1 M LiPF6in a mixture solvent of ethylene carbonate (EC) and dimethyl carbonate (DMC) (1∶1 v/v). The charge/discharge measurements of the cells were performed on a LAND battery tester between 0.01 and 3 V at room temperature.
3 Results and discussion
Fig.1 shows the XRD pattern of AC/SnO2nanocomposite. All diffraction peaks can be indexed to the standard tetragonal SnO2phase (JCPDS number 41-1445), indicating that crystalline SnO2NPs could be formed by this method. The diffraction peaks of carbon are not distinguishable in XRD pattern because AC is poorly crystalline.
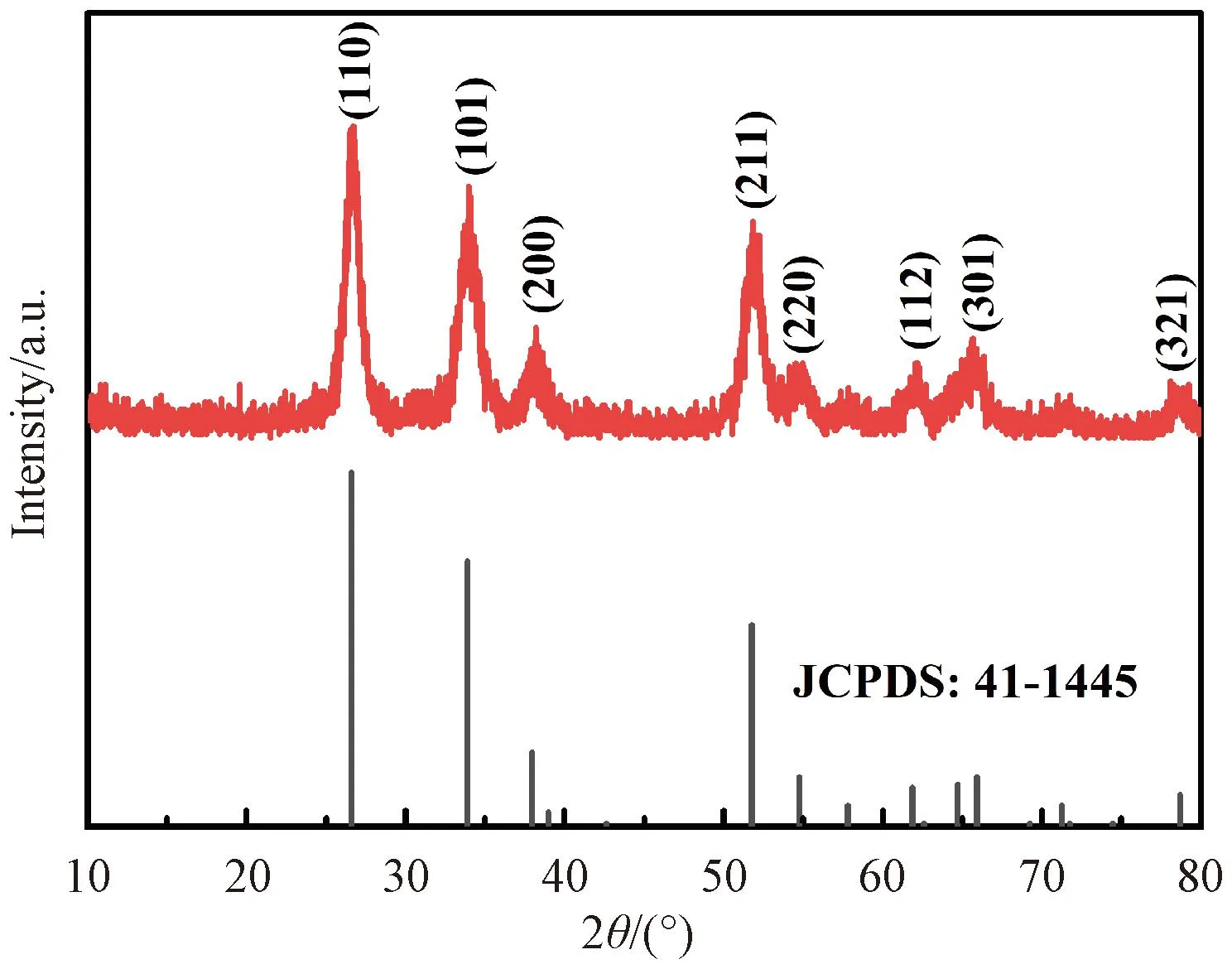
Fig.1 XRD pattern of the as-obtain AC/SnO2 nanocomposite
A clear difference between bare SnO2and AC/SnO2nanocomposite could be seen from the SEM images. The bare SnO2NPs aggregate into large particles (Fig.2(a)). The large particles can be easily pulverized owing to volume change during Li+insertion/extraction process, leading to capacity decay as an anode material for LIBs. For AC/SnO2, the SnO2NPs are uniformly distributed on the surface of AC (Fig.2(b), (c)). Elemental distribution of AC/SnO2nanocomposite was studied by EDS mapping (Fig.3). Obviously, the distribution of element O and Sn is highly corres-ponded with that of element C, suggesting SnO2NPs are dispersed uniformly in AC matrix.
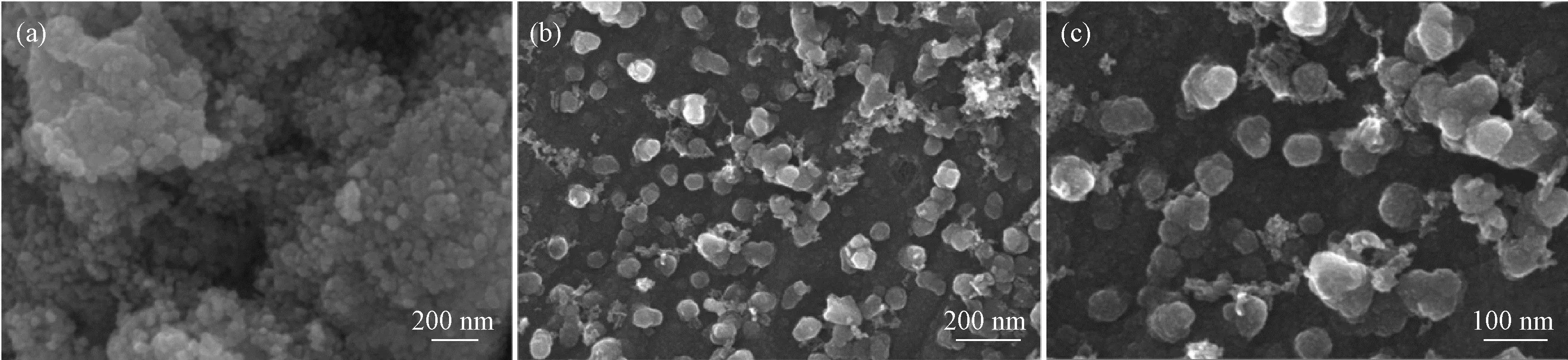
Fig.2 SEM images of (a) bare SnO2 and (b, c) AC/SnO2 nanocomposite

Fig.3 SEM image (a) and corresponding (b) C, (c) O, (d) Sn elemental mappings of AC/SnO2
TEM images were employed to further observe the microstructure of AC/SnO2nanocomposite (Fig.4). The size of SnO2NPs in the TEM image is much smaller than that in SEM (Fig.2), because the nano-pores in AC, as hard template, confine the agglomeration of SnCl2solution and result in smaller SnO2NPs in AC. The SnO2NPs inside the AC should correspond to some voids due to the volatilization of ethanol. The lattice fingers of SnO2NPs could be seen clearly in a high-resolution TEM image (Fig.4(d)). This is consistent with its XRD result in Fig.1. The effective dispersion of SnO2NPs and the existence of voids are in favor of buffering the volume change during Li+insertion/extraction process and improving the cycling of LIBs.
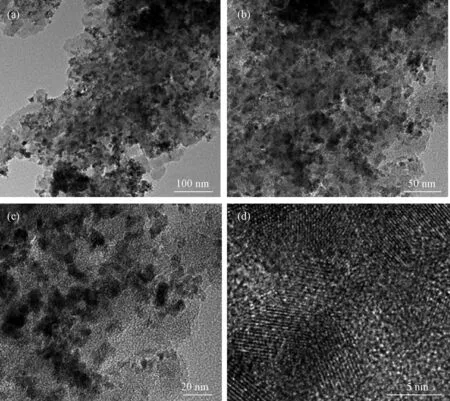
Fig.4 TEM images of AC/SnO2 nanocomposite
N2adsorption-desorption was used to evaluate the pore characteristics of pure AC and AC/SnO2(Fig.5). Pure AC shows typical IV-type isotherm and obvious hysteresis loop (P/P0=0.5-0.9), indicating the existence of abundant microporous and mesoporous and few macropores[18-19]. The AC/SnO2nanocomposite shows similar porous structure character with AC, but its adsorbed quantity is lower because of the introduction of SnO2nanoparticles. Similarly, the pore size of both pure AC and AC/SnO2nanocomposite is less than 30 nm. Brunauer-Emmett-Teller (BET) analysis indicates that the surface area of pure AC and AC/SnO2nanocomposite is 1 095 and 652 m2·g-1, respectively. And the average pore diameter of pure AC and AC/SnO2nanocomposite is 5.98 and 5.60 nm, respectively, calculated from the Barrett-Joyner-Halenda (BJH) adsorption. As anode material, the large surface area and porous structure of AC/SnO2could result in a low initial Coulombic efficiency (ICE) and good rate capability.
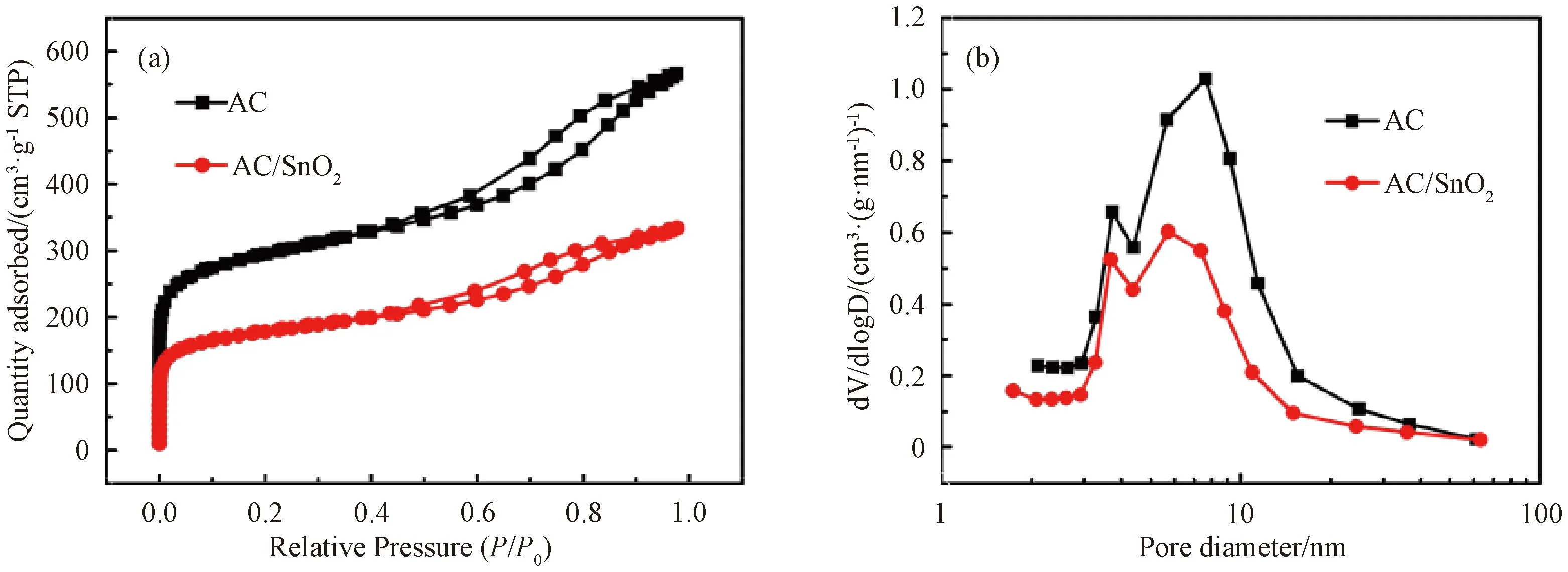
Fig.5 N2 adsorption isotherms (a) and pore-size distribution (b) of AC and AC/SnO2
Thermogravimetric analysis (TGA) was performed to determine the content of SnO2in AC/SnO2nanocomposite (Fig.6). The TGA was conducted from room temperature to 800 ℃ at a speed of 10 ℃/min in air. The oxidation reaction of AC with oxygen in air is completed at 700 ℃, and the mass percent of SnO2is 42.1%. The content of SnO2could be raised by improving SnCl2·2H2O concentration.
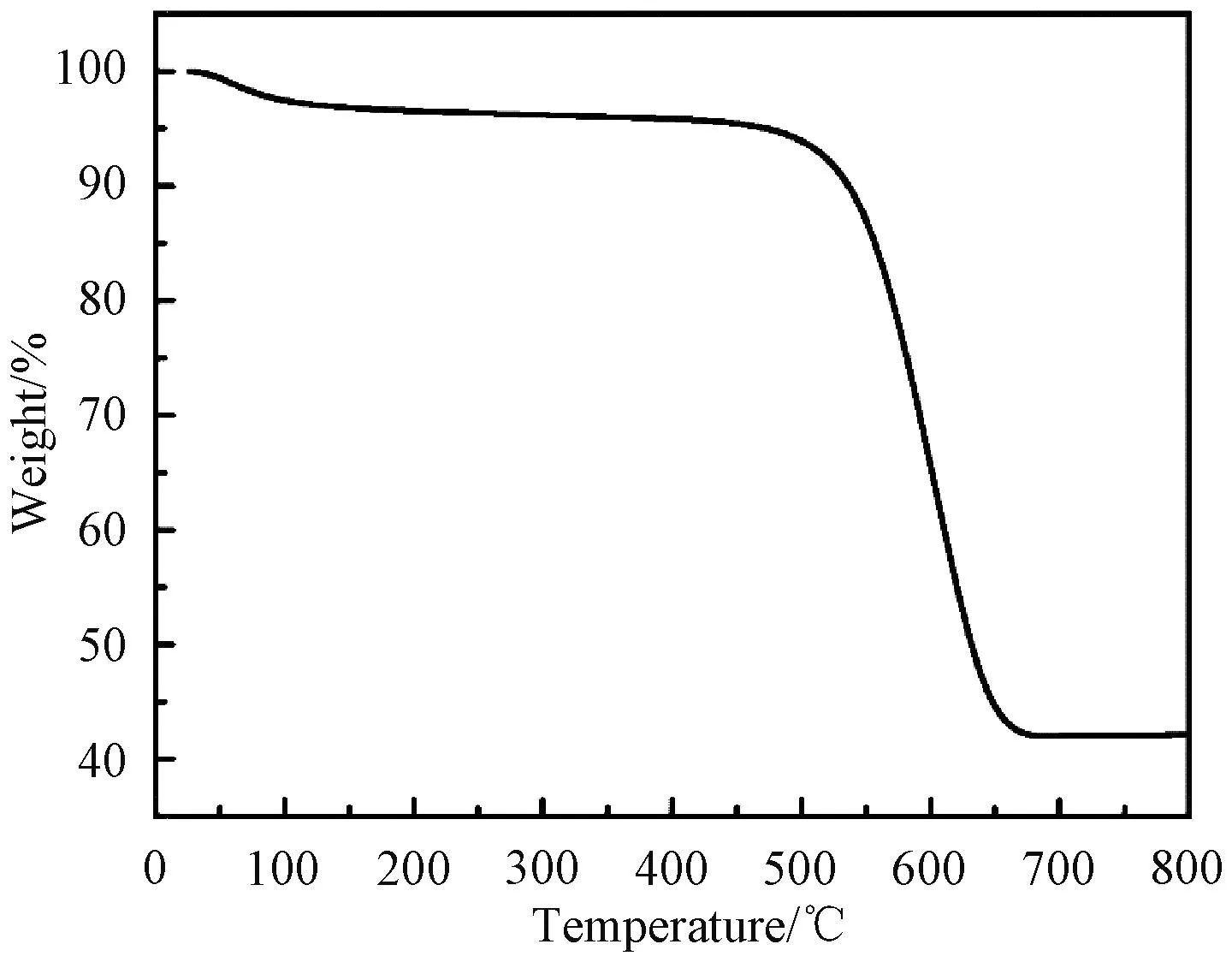
Fig.6 TGA curve of AC/SnO2 nanocomposite


Fig.7 Discharge/charge profiles (a), cycling performance (b), and rate capability (c) of AC/SnO2 nanocomposite and bare SnO2
Fig.7(b) shows the cycling performance of AC/SnO2and bare SnO2electrodes. The AC/SnO2electrode maintains a reversible capacity of 476 mAh·g-1and 309 mAh·g-1after 200 cycles under 100 mA·g-1and 200 mA·g-1, corresponding 63.3% and 61.6% of the initial reversible capacity, respectively, which is comparable to the result of composite of graphene and SnO2reported by Yaoetal[27]. Apparently, the bare SnO2shows poorer cycling performance than the AC/SnO2nanocomposite. After 100 cycles, the reversible capacity of bare SnO2under 100 mA·g-1is only 25 mAh·g-1, less than 1/14 of theoretical capacity of currently used graphite. Such good cycling performance of the AC/SnO2nanocomposite should be attributed to its morpho-logy. In the AC/SnO2, the AC matrix is as a barrier to avoid the aggregation of SnO2NPs and also a buffer to prevent the electrode material from a large volume change during Li+insertion and extraction. The barrier and buffer roles of AC could effectively repress the volume change and cracking of the electrode, leading to an enhanced cycling stability.
The rate capability of AC/SnO2and bare SnO2is shows in Fig.7(c). The AC/SnO2electrode shows a slow decrease and retains reversible capacities of 367 mAh·g-1and 268 mAh·g-1with creasing the current density to 400 mA· g-1and 800 mA·g-1, respectively. It is worth noting that the reversible capacity is completely recovered when the current density returns to 100 mA·g-1. While bare SnO2displays poor rate capability under the same current densities. The good rate capability of AC/SnO2is resulting from the conductive AC frame loading SnO2NPs and short transmission path of Li+because of the porous structure of AC.
4 Conclusions
In this work, AC/SnO2nanocomposite was successfully synthesized by immersing, filtration, and sintering in Ar atmosphere. Owing to the adsorption of nano-pores in AC, the SnO2NPs are in-situ formed in these pores. The as-synthesized AC/SnO2nanocomposite demonstrates high rever-sible capacity, superior cycling performance, and good rate capability. Such good electrochemical performance should be attributed to homogeneous distribution of SnO2NPs in AC matrix and the effective buffer from AC pores against the volume change of SnO2NPs during charge/discharge. The synthesis route reported here is facile and scalable. Moreover, the content of SnO2in AC/SnO2can be adjusted easily by change the concentration of the solution. This simple impregnation method could be extended to the synthesis of other AC/metal oxide composites.
