Triplex-structure based DNA circuits with ultra-low leakage and high signal-to-noise ratio
2023-02-18HunLiuZhihoMingYunpengZhngQidongXiHoHuRuijieLiuYuhengLioYizhouLiuXioLiuXiopingZhngLongjieLiShogngWngXinjinXio
Hun Liu ,Zhiho Ming ,Yunpeng Zhng ,Qidong Xi ,Ho Hu ,Ruijie Liu ,Yuheng Lio,Yizhou Liu,Xio Liu,Xioping Zhng,Longjie Li,d,∗,Shogng Wng,∗,Xinjin Xio,e,∗
a Institute of Reproductive Health,Tongji Medical College,Huazhong University of Science and Technology,Wuhan 430030,China
b Department and Institute of Urology,Tongji Hospital,Tongji Medical College,Huazhong University of Science and Technology,Wuhan 430030,China
c Department and Institute of Urology,Union Hospital,Tongji Medical College,Huazhong University of Science and Technology,Wuhan 430030,China
d School of Life Science and Technology,Wuhan Polytechnic University,Wuhan 430030,China
e Department of Laboratory Medicine,Tongji hospital,Tongji Medical College,Huazhong University of Science and Technology,Wuhan 430030,China
Keywords:Leakage DNA strand displacement cascades Triplex DNA circuits Molecular programming
ABSTRACT DNA circuits are powerful tools in various applications such as logical computation,molecular diagnosis and synthetic biology.Leakage is a major problem in constructing complex DNA circuits.It directly affects the output signal and harms the circuit’s performance significantly.In the traditional DNA circuits,the gate complex is a duplex structure.There are insufficient energy barriers to prevent spontaneous detachment of strands,resulting in a leak prone.Herein,we have developed triplex-structure based DNA circuit with ultra-low leakage and high signal-to-noise ratio (SNR).The triplex structure improves the stability in the absence of input.At the same time,the driving force of the strand displacement cascades reduces the influence of the triplex structure on the desired reaction.The SNR of the DNA circuit was increased to 695,while the desired reaction rate remained 90% of the conventional translator circuit.The triplex-structure mediated leakage prevention strategy was further tested at different temperatures and in DNA translator and seesaw circuits.We also constructed modular basic logic gates with a high effi-ciency and low leakage.On this basis,we further constructed triplex-structure based tertiary DNA logic circuits,and the SNR reached 295,which,to the best of our knowledge,was among the highest of the field.We believe that our scheme provides a novel,valid,and general tool for reducing leakages,and we anticipate that it will be widely adopted in DNA nanotechnology.
DNA is a ubiquitous biomolecule that stores heritable information.Owing to the specificity and predictability of its Watson-Crick base-pairing,it is considered an ideal material for nanoengineering [1].Scientists have constructed DNA logic circuits that are similar to digital circuits based on DNA-strand displacement reactions and have developed a variety of applications related to logic circuits,such as logical computation [2,3],diagnostic applications [4–6],molecular amplifiers [7–9],and synthetic biology [10,11].One of the biggest challenges faced in the field of logical computation is leakage [12] (undesired triggering in the absence of an input).High leakage in systems contributes greatly to the logic circuit signal and affects system performance directly,such as lowering the sensitivity of biological monitoring [8,13],resulting in molecular calculation errors [7,14-16],or reducing the dynamic range of synthetic biological components [17].Moreover,as the complexity of the circuits (the number of DNA components)increases,the leakage worsens [18,19].This seriously damages the complexity,extensibility,and stability of DNA nanostructure networks,limiting their scale and making it difficult to construct highperformance DNA devices.
Leakage can be broadly divided into initial and asymptotic types[20].The initial leakage is mainly caused by inadequate DNA purification [21].Impurities in the DNA structure cause the signal to rise rapidly at the initial stage of the reaction,thereby generating nonspecific background signals.The asymptotic leakage is considered to be caused by undesired “toeless” strand displacement [18],which starts from the fraying at the end of double-stranded DNA(dsDNA),providing an instantaneous toehold for the leak reaction.
Several methods have been developed to prevent leak reactions.Efforts to control the initial leakage were predominantly targeted at optimizing the procedures for purifying DNA [22].In addition,two great advances have been made concerning the optimization of asymptotic leakage.One is at the sequence level,where leakage is reduced by changing a few bases.These methods can be applied to most DNA circuits.For example,extending 1–3 C/G bases at the end of dsDNA (called “clamp”) has been widely used to minimize leakage [7].Moreover,introducing a mismatch in the fraying region helps to minimize leakage [23].However,the efficacy of these methods is still unsatisfactory [18].The application of locked nucleic acids in DNA circuits can lead to better results,but the construction cost of a DNA circuit is extremely high [24–26].The other method is to improve the design of the novel logic gate elements at the system level and build a new low-leakage logic circuit.For example,introducing redundancy into the design can effectively minimize leakage [18].This method introduces a doublelong domain structure so that all gate elements are combined into a huge complex to generate a leakage signal.This method is known to be effective;however,constructing logic gates is difficult [18].Multi-arm junction structures can be used to create multiple highenergy barrier steps to minimize leakage.However,this dramatically minimizes the desired reaction rates.By building a set of shadow circuits with the same structure as that of the main circuit,the leakage response can be minimized even though the leakage mechanism was unknown.This method did not require modification of the original DNA circuit;however,the leakage prevention effect was limited [27].This significantly increases the complexity of the circuits.The conventional sequence-level methods for designing DNA circuits have faced limitations due to the traditional duplex structure,which makes it challenging to effectively regulate and optimizeΔGfor both the desired reaction and leak reaction.System-level methods offer an alternative solution,but they require changes to the migration domain of DNA strand displacement cascades,resulting in increased energy barriers for the desired reaction and complicating the reaction process,reducing its rate.These structural changes also make it difficult to apply traditional DNA logic circuit construction methods and to construct complex circuits.Therefore,there is a need for a method that can minimize leak reactions while maintaining a high desired reaction rate and a simple design.The triplex structure design offers a unique solution as it avoids changes to the migration domain,allowing for more careful regulation and optimization ofΔGwhile retaining the ability to build high-performance logic circuits.
In this study,we demonstrated a new systematic leakage prevention method for the asymptotic leakage mechanism.This method inhibits the leakage caused by DNA respiration by constructing a triplex DNA structure thermodynamically and kinetically.The SNR of the DNA logic circuit was increased by a factor of 50,while the reaction rate was not significantly affected.This method is simple and it exhibits good performance for basic logic gates and complex logic circuits.
To prepare the triplex gate strands (including translator gate,AND gate,and OR gate),we mixed the TOP,BOT and SEAL strands at 1:1:1 ration in 1×ThermoPol reaction buffer (NEW ENGLAND BioLabs).The gate strand mixtures were then thermally annealed using PCR machine,following a thermal profile of initial heating to 85°C for 5 min,then to 55°C for 3 min,next cooling to 25°C with the rate 0.1°C/s.Formulated gate strand solutions were then stored in 4 °C until use.The preparation process of duplex gate strands was basically the same as that of triplex gate strands except the SEAL strands.After the gate strands got prepared,the input strands and 5 μL of 10× ThermoPol Buffer were added to a 200 μL PCR tube,the gate strands and brought up to a total volume of 50 μL by deionized water.All the samples were mixed and prepared at room temperature.The solutions were immediately put into the Q1000 Real-Time PCR System (LongGene,China) for fluorescence measurement.Additional experimental details could be found in the Supporting information,including materials,analysis of the fluorescence data and the calculation of free energy.
First,we constructed a translator gate based on the DNA triplex,which can translate the input strands to the output strands.Fig.1 demonstrated both principles of the traditional and triplex translator.The translator gate consisted of two fuel complexes (F1and F2) and a reporter duplex (RP).Fig.1a showed the intended pathway of the desired and the leak reactions in the traditional translator system.The traditional fuel complex consists of two strands:the TOP strand and the BOT strand,while the triplex fuel complex has an extra SEAL strand.Pairs that hybridized to the SEAL strands had similar numbers of TOP and BOT strands,enabling the triplex to exist stably.Between the hybridized region of the SEAL strands,we add a free region to avoid the dissociation of the triplex structure due to excessive rigidity.A stable triplex prevents leakage.The intended pathway of the desired reaction in the triplex translator system was shown in Fig.1b.The process comprised three steps.(i) The input strands reacted with the first triplex through toehold-mediated strand displacement,thereby releasing the TOP strands of an independent sequence to the input.Meanwhile,the hybridization region of the SEAL strands and TOP strands was not sufficient for the SEAL-TOP duplexes to exist stably.Therefore,the desired reaction pathway is less affected.(ii) The first TOP strands then reacted as input to the downstream fuel triplex (or reporter)to trigger a consequent cascade reaction.Each strand of the reporter duplex was labelled by a fluorophore and a quencher.(iii)The final output hybridizes with the reporter duplex to separate the fluorophore-quencher pair.The output was monitored by fluorescence intensity.Fig.1b also demonstrated the leak pathway of the triplex translator system.The leakage was thought to be caused by the instantaneous dissociation ofδx2∗in F1,which can hybridize with the downstream unbounded toehold regionδx2to induce an undesired toehold-mediated strand displacement.However,the SEAL strands sealed the end of F1and increased the energy barrier for the instantaneous dissociation ofδx2∗,thereby inhibiting leakage kinetically.Here,we randomly design 24 groups of traditional and triplex translator cascades to perform a thermodynamic analysis.We calculated the Gibbs free energy change of both desired reaction (ΔGdesired) and the leak reaction (ΔGleak) in the traditional and triplex translator systems.In all designs,ΔGdesiredwere all less than 0,which means the desired reaction can proceed normally.Most of theΔGleakin the traditional translator system was less than 0,meanwhile,all of theΔGleakin the triplex system were greater than 0,demonstrating that the SEAL strands successfully suppressed the occurrence of the leak reaction in thermodynamics.The results are shown in Figs.1c and d.Moreover,the SEAL strands created greater steric hindrance,thus kinetically impeding leakage.
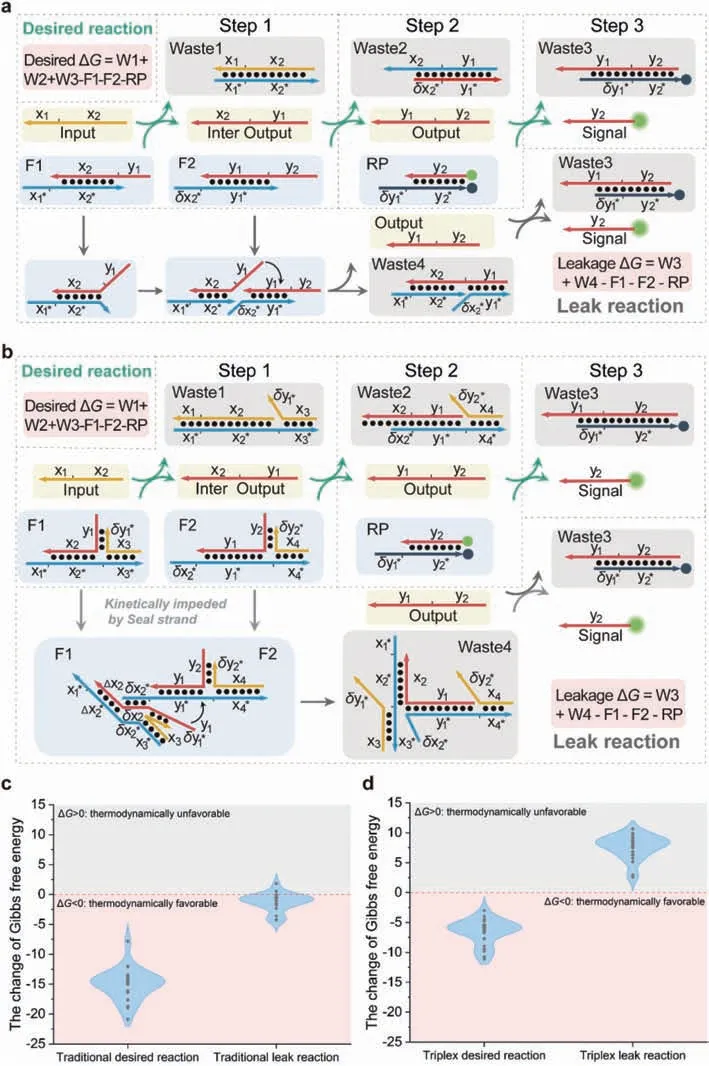
Fig.1. Comparison of traditional and triplex translator systems.The traditional translator fuel complex (TOP strand: F1-x2y1,F2-y1y2;BOT strand: F1-x1∗x2∗x3∗,F2-δx2∗y1∗x4∗)and the triplex fuel complex (SEAL strand: F1-δy1∗x3,F2-δy2∗x4;TOP and BOT strands are as same as traditional) are shown.The symbol ∗denotes the complementary domain of the original domain,and the symbol δ denotes a subsection of the original domain.(a,b) The intended pathway of the desired and leak reaction are illustrated for both systems.(c,d) The change of Gibbs free energy ΔG in the traditional and triplex translator systems.ΔGdesired in both two systems are less than 0.Most of the ΔGleak in the traditional system are less than 0,and all of the ΔGleak in the triplex system are greater than 0.The details of calculation process and sequence information are included in the Tables S3–S6 in Supporting information.
One of the most commonly used method is to add a “clamp”domain.This method has strong universality and could be well compatible with other methods,but that leakage prevention effect was limited.Herein,we compared two improvement directions to get a better effect: extending the “clamp” domain or add an extra SEAL strand.The mechanism for leakage prevention of clamp and the improvement directions of the traditional “clamp” are shown in Fig.2a.For a fair comparison,we used clamps throughout our design,including triplex or duplex,to demonstrate the leakage inhibition beyond the clamp-only methods.Figs.2b and c showed the kinetic behavior of the traditional translator system with clamp domains of different lengths with and without input strands.When the length of the clamp domain is extended to 7 nt,the reaction rate is too slow,resulting in the reaction remaining in a linear increase phase for 5 h.The results for triplex design are shown in Fig.2d.In the absence of input strands,the fluorescence signal of the triplex-based translator gate was significantly lower than that of the traditional duplex.The SNR reached 600,which was approximately 50 times that of the traditional duplex.Details of the leakage signal changes were presented in Fig.S1 (Supporting information).Meanwhile,the reaction rate did not decrease significantly.Fig.2e demonstrated the quantitative results.We designed a total of three different sets of triplex translator systems with different sequences,all of which achieved good leakage prevention effects and similar SNR values.The results are shown in Fig.S2 (Supporting information).

Fig.2. Comparison of the effect of “clamp” and the triplex structure.(a) Mechanism and improvement directions of traditional "clamp" for leakage prevention.(b,c) Kinetics of the traditional translator system with clamp domains of different lengths with and without input strands.n and n’referred to the desired and leak reactions respectively when the clamp length was n.(d) Long-time running of the triplex translator system.In 10-hour timeframe,the SNR in the triplex translator system reaches 600,50 times that of the traditional structure.(e) The SNR of the traditional translator system with clamp domains of different lengths and the triplex translator system.The SNR of the translator with the extended clamp domain can be up to almost 2 times that of the translator with the original clamp domain,while the SNR of the triplex domain is 50 times better.The inputs are added no more than 3 min before measurement at 25°C.
Extending the clamp domain at the end of the duplex only improved the SNR by 1.5 times at most.Hence,comparing with the extension of the clamp domain at the end of the duplex,the triplex design could greatly enhance the steric hindrance,thereby dynamically inhibiting the occurrence of leakage and achieving a better leakage prevention effect.
The aforementioned findings revealed that the design of the triplex not only inhibited the leak reaction but also changed the reaction rate slightly,thereby demonstrating the feasibility of this scheme.However,the triplex structure design resulted in a decrease in the signal plateau value.The reason is that the strong binding force of the triplex complex made it difficult for the input strands to completely release the TOP strands,resulting in some reduction of the downstream output.To address this,we conducted a series of experiments.The directions of optimization are shown in Fig.3a.

Fig.3. (a) Abstraction of the directions of optimization.(b) Signal-noise-ratio (SNR) improvements of the triplex translator system with SEAL strands of different lengths(7+8,7+9,7+10,8+8,8+9,8+10,9+8,9+9 and 9+10).The SNR is chosen as the reference indicator for optimizing the length of the SEAL strands.Therefore,8+10 was selected as the length of the SEAL strand used in the follow-up experiment.(c,d) Kinetics of the triplex translator system with toehold domain of different lengths(5 nt,6 nt,7 nt,8 nt).When the length of the toehold domain is 8 nt,the signal-to-noise ratio is the highest,reaching 695.(e) Five-layer triplex linear translator cascade.In the timeframe of 8 h,the SNR in the triplex translator system reaches 40 which is 12 times that of the traditional structure.The experiment was performed at 25°C.
The first challenge we faced during optimization was to determine the length of the seal strands.If the SEAL strand is too long,the unduly stable triplex fails to react with the input strands,resulting in the loss of the main signal.Meanwhile,if the SEAL strand is too short,the triplex will not form and will not act as a leakage-inhibited device.The length of the SEAL strand was defined as a+b,where a and b are the numbers of base pairs in the binding region of the SEAL strand to the TOP and BOT strands,respectively.Nine seal strands have lengths of 7+8,7+9,7+10,8+8,8+9,8+10,9+8,9+9 and 9+10,respectively.Next,we applied these SEAL strands to the triplex-based translator gate and detected fluorescent signals with and without the input.We compared the SNR of the triplex structure with that of the traditional structure and the results are demonstrated in Fig.3b.When the length of the SEAL strand was 8+10,it not only achieved less leakage,but also avoided the loss of the main signal.The raw data for the bar graph analysis were presented as line charts in Fig.S3(Supporting information).Furthermore,we did comparative experiments on free region in triplex structures.Fig.S4 (Supporting information) demonstrated the positive effect of free region on structural stability.The length of the toehold region also affected the signal plateau value.We designed and synthesized triplex-based translator gates with different lengths in the toehold region.The SEAL strands were 8+10 in length.We detected fluorescent signals with and without input.The findings are shown in Figs.3c and d.When the length of the toehold region was extended to 8 nt,the loss of the desired signal was almost negligible.Meanwhile,the SNR of the DNA circuit was increased to 695.
After resolving the problem of signal plateau-value decrease,we constructed 5-layer translator cascade based on DNA triplexes and duplexes,respectively.Experimental results in Fig.3e showed the desired triggering and leakage of the two designs.The leak reaction of the cascade based on the triplex is greatly suppressed,improving the SNR by 12 times and the desired triggering presented minimal loss,demonstrating the validity of our design in the DNA circuit.Moreover,it also demonstrated that the triplex design can be applied in complex DNA logic circuits.
Since the effect of the triplex structure against leakage is maintained by the stability of the structure,we explored various factors that can affect the binding rate of the triplex structure for further investigation.First,we explored the most important influencing factor,temperature.As the temperature increases,the stability of DNA binding decreases,leading to an increased probability of the generation of instantaneous toehold domains,which lead to greater leakage and interfered with the normal functioning of the DNA circuit.Fig.S5 (Supporting information) demonstrates the leakage prevention effects of the triplex design at various temperatures.The constructed translator gates were designed to operate at 25°C.When the temperature attained 30°C,the triplexbased translator gate worked with low leakage,increasing the SNR by five times compared with the traditional translator.When the temperature increased to 37°C,the triplex-based translator gate worked with visible leakage,while the SNR doubled compared to the traditional translator.These results indicated the efficiency of the triplex design in reducing leakage at different temperatures.However,it needs to be explored at further different temperatures.In addition to the temperature,the experimental system is also influenced by the ionic environment.Therefore,in order to investigate the effects of different ion concentrations on the DNA circuits,we adjusted the concentration of Mg2+to 2,4 and 8 times the original concentration and the concentration of K+to 2,3 and 4 times the original concentration,respectively.The results are shown in Figs.S6 and S7 (Supporting information).With increasing concentrations of both magnesium and potassium ions,the extent of the desired reaction increases continuously,while the leak reaction was not greatly affected.
The base mutations or mismatches can also destabilize the structure and thus affect the normal response of the switch.Therefore,we designed a series of experimental controls to explore the impact of mismatches on the triplex structure design by adding 1,2,4 and 6 mismatched base pairs in the migration domain,and designed an experimental control group containing 2 mismatched base pairs in the Seal-Bot combined domain.The mismatched base pairs are all on the bot strands.The details are demonstrated in Fig.S8 (Supporting information).When the mismatches were in the migration domain,the system could remain stable in the presence of 2 mismatched base pairs,while when there were 4 mismatched base pairs,the system was too unstable to maintain the normal structure and function.When the base mutations or mismatches were in the Seal-Bot hybridization domain.the triplex structure behaved more stable and can tolerate more base mutations.
Furthermore,we applied a triplex structure to the seesaw circuit.The principle and experimental results are shown in Fig.S9(Supporting information).The introduction of the triplex structure increased the SNR of the seesaw circuit by 25 times.The results showed that the triplex structure is general and can be applied to different types of circuits.
Herein,we applied the triplex structure in translator cascades in different experimental environments and in different types of DNA circuits.The results demonstrated that the triplex design is valid,stable,and general.Furthermore,we have attempted to construct complex logic circuits based on a triplex structure.
First,we constructed fundamental logic gates based on a triplex structure.As shown in Fig.4a,the structure of the OR gate was the same as that of the triplex-based translator gate mentioned above.Based on a triplex-based translator gate,we extended the length of SEAL strands to achieve the logical function of the AND gate.The hybridization region between SEAL and BOT strands was 5 nt longer and the triplex structure was stable,making it fail to react with Input 1.Toehold region y2∗with the same length as x1∗was added at the 3'-end of the SEAL strand.Input 1 and Input 2 were both necessary to react with the triplex,releasing the TOP strand as the output.However,neither Input 1 nor Input 2 could release the TOP strand alone.The intended pathway of the AND gate was shown in Fig.4b.The OR and AND gates were tested for all possible inputs.The experimental results were shown in Figs.4c and d.Correct results were obtained in all cases with low leakage,confirming that the triplex design worked well in single fundamental logic gates.
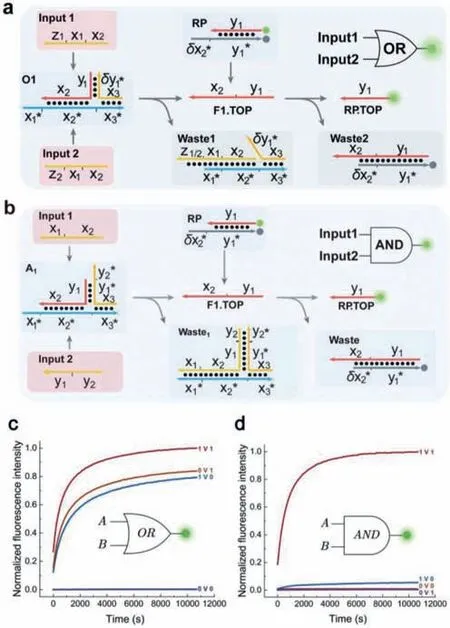
Fig.4. (a) Implementation of a one-input triplex circuit that computes OR.(b) Implementation of a two-input triplex circuit that computes AND.(c,d) Kinetics of the triplex circuits that computes AND/OR with and without input strands.The leakage signal was not measurable in the absence of input.The experiment was performed at 25°C.
To demonstrate the validity of our method,we constructed tertiary cascade OR-AND-OR.As shown in Fig.5a,it produced correct results for all the representative input combinations,and there was no obvious leak reaction.We also constructed AND-OR-AND cascade and obtained similar results (Fig.S10 in Supporting information).Finally,medium-sized circuits containing three-layer and four-gate circuits were tested.The results were shown in Fig.5b.The correct results were also obtained for all representative input combinations,and the SNR reached 295 (a/d).These results demonstrate that the proposed scheme is suitable for use in complex logic circuits.
In this paper,we demonstrated a novel DNA logic circuit architecture based on a triplex structure that could effectively reduce the leak reaction.Compared with the duplex,the triplexbased DNA logic circuit maintained the triggering reaction rate and reduced the leakage signal.The results demonstrated that it can be applied at different temperatures and in different types of DNA circuits.The triplex structure had the additional advantage of the ability to work with other existing leakage prevention strategies,such as using clamp domains,introducing a mismatch in the fraying region,using locked nucleic acids,and others.The main limitation of the triplex structure is the reduction of the main signal.This could be a problem if there was little leakage of the circuit.
We also constructed a modular basic logic gate with a high efficiency and low leakage,which is convenient for building complex circuits.On this basis,we demonstrated a medium-sized circuit without an obvious leak reaction.Overall,we believe that our scheme provides a novel,valid,and general tool for reducing leakages.We anticipate that it will be widely adopted in DNA circuits.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No.81871732),the National Key Research and Development Program of China (No.2021YFC2701402),the Open Research Fund of State Key Laboratory of Bioelectronics,southeast University (No.Sklb2021-k06),the Open Foundation of NHC Key Laboratory of Birth Defect for Research and Prevention (Hunan Provincial Maternal and Child Health Care Hospital)(No.KF2020007),the Open Foundation of Translational Medicine National Science and Technology Infrastructure (Shanghai) (No.TMSK-2021-141),and the Open Fund from Key Laboratory of Cellular Physiology (Shanxi Medical University),Ministry of Education,China (No.CPOF202103).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2023.108555.
杂志排行
Chinese Chemical Letters的其它文章
- Spin switching in corrole radical complex
- Benzothiadiazole-based materials for organic solar cells
- Mono-functionalized pillar[n]arenes: Syntheses,host–guest properties and applications✰
- Recent advances in two-step energy transfer light-harvesting systems driven by non-covalent self-assembly✩
- From oxygenated monomers to well-defined low-carbon polymers
- Doping-induced charge transfer in conductive polymers
