AIE interfacial supramolecular polymers
2023-02-18QingyunLiZiqingHuXiaofanJi
Qingyun Li,Ziqing Hu,Xiaofan Ji
Key Laboratory of Material Chemistry for Energy Conversion and Storage,Ministry of Education,Hubei Key Laboratory of Material Chemistry and Service Failure,Hubei Engineering Research Center for Biomaterials and Medical Protective Materials,School of Chemistry and Chemical Engineering,Huazhong University of Science and Technology,Wuhan 430074,China
Keywords:Aggregation-induced emission Interfaces Metal-coordination interactions Supramolecular polymers Stimuli-responsiveness
ABSTRACT High monomer concentration is a requisite for engendering the aggregation-induced emssion (AIE) phenomenon as well as the formation of supramolecular polymers.Therefore,this is supposed to ensure the generation of AIE supramolecular polymers,wherein the monomer soluability takes effect.Nevertheless,parts of supramolecular monomers are considered as poessessing different soluability towards the same sovlent,through which the polymerzation process is thus hard to proceed.Interfacial polymerzation gets over the soluabilty restriction,providing a facile method for propelling the reaction of thesemonomers.Herein,we had prepared M1 containing tetraphenylethene (TPE) functionalized with two terpyridine derivatives,then making M1 dissolving in CHCl3 to give solutions.Cu2+ solutions were fabricated through dissolving CuCl2 into H2O.Towards mixing those solutions,AIE interfacial supramolecular polymers (AIEISPs) displaying green fluorescence were generated at the interface of two phases on the basis of metal-coordination between terpyridine and Cu2+.These AIEISPs were certificated to possess the stimuli-responsiveness,for which the excessive addition of tetrabutylammonium hydroxide would cause the structure destruction owing to the stronger bonding ability with Cu2+ than that of terpyridine.These fabricated AIEISPs had provided a new avenue to prepare AIE supramolecular polymers.
Supramolecular polymers referred to polymeric arrays consisting of monomer units,gathering up by noncovalent bonds[1,2].Stemming from the inherent dynamic nature,the incorporation of functionalized groups bestows on a diversity of material characteristics as well as functions of supramolecular polymers[3,4].Wherein,fluorophores as the special category of functional groups tend to be embedded into supramolecular polymers,furnishing them with varied fluorescence [5].This specific type of supramolecular polymers had found their wide applications in photochemistry,bioscience,medical science and so forth [6–9].According to the emission mechanism,the fluorophores are generally sorted into two types,either with aggregation-caused quenching (ACQ) effect or aggregation-induced emission (AIE)effect [10,11].Regarding fluorophores with ACQ effect,fluorescence is quenched in the case of aggregated states [12,13],whereas,it does not tally with the characteristic for the supramolecular polymer formation available only at higher concentrations.By contrast,AIE effect signified that fluorophore turned to be emissive at high concentrations,for which this phenomenon was discovered by Tang’s group in 2001 [14,15].The feature of AIE effect fitted in the concentration-dependence property of supramolecular polymer,tailoring fluorescence characteristic within it [16,17].
In view of the concentration-dependence character,the formation of supramolecular polymers states that higher monomer condition is essential [18,19].Also,the high concentration occasion ensures AIE fluorophores to be held together for producing emission [20–22].To guarantee the formation of AIE supramolecular polymers,supramolecular monomers embodying fluorophores should be assured to well dissolve in the same solution [23,24].As is known to all,the solubility of monomers is subject to their structures and the polarity of solvents [19,25].Restricted by these factors,AIE supramolecular polymers are hard to generate in the circumstance of monomers showing different solubility in the same solution,confining the development and applications.Consequently,to exploit a supramolecular polymerization pertaining for diverse solubility based supramolecular monomers becomes brook no delay.
Interfacial polymerization as a particular type offers a facile path for the reaction between monomers of distinct solubility,which has been attracted wide attention [26,27].According to the linking type between monomers,interfacial polymerization can be roughly divided into two types,covalent bond-based and non-covalent bond-based [28].For instance,Xu and coworkers synthesized polyamide nanofilms by covalent polymerization at the alkane-ionic liquids interface [29].Inspired by traditional interfacial polymerization,Zhang’s group provided a method that supramolecular polymers were fabricated at water-oil interface by thiol-maleimide click reaction [18].In addition,2D supramolecular organic frameworks,reported by Feng and coworkers,were constructed at water-toluene interface driven by CB[8]-based hostguest interactions [30].Notably,the employment of interfacial polymerization surely promoted the generation of supramolecular polymer materials with various functions,for which it occurred to us achieving the reaction of monomers possessing different solubility at the interface as well.
For the sake of fabricating AIE supramolecular polymers,a typical AIE-active dye tetraphenylethene (TPE) [31–36] was employed,for whichM1incorporating with TPE functionalized with two terpyridine (tpy) derivatives was prepared,thus being dissolved in CHCl3(Scheme 1).Additionally,CuCl2was dissolved in H2O to give Cu2+aqueous solutions.Upon mixingM1CHCl3solutions with the performed Cu2+aqueous solutions,there was an interface lying amid those two distinct phases.In this mixture,the involving molecules carried on diffusion movements,tending to be spread evenly throughout the medium.Promoted by the metalcoordination between tpy and Cu2+,M1was capable of assembling with Cu2+at the interface of mixtures to produce AIE interfacial supramolecular polymers (AIEISPs) [37].Upon increasing the time of supramolecular polymerization at the interface,a corresponding increase in the supramolecular polymer chain length is expected,which will lead to tight entanglement of supramolecular polymer chains,further reducing the average distance between the TPE groups.This reduction in inter-TPE spacing would lead to the formation of aggregate states,which is the main cause for producing interfacial supramolecular polymers with AIE effect.Through constant aggregation of AIE supramolecular polymers,this resulted in the TPE groups being concentrated,then it was apparently found out the generation of the thin film displaying green fluorescence at the interface over time [23,38].

Scheme 1. Chemical structure of M1,and the illustration of preparation process of AIE interfacial supramolecular polymers.
The synthetic routes ofM1were shown in Scheme S1 (Supporting information).1H NMR spectroscopy,13C NMR spectroscopy as well as high-resolution mass spectrometry were employed to charaterizeM1and their precursors (Figs.S1-S6 in Supporting information).So as to prepare stock solutions,M1(19.4 mg,15.0 mmol/L) were dissolved in CHCl3(1.00 mL).Additionally,Cu2+solutions were fabricated through dissolving CuCl2(2.02 mg,15.0 mmol/L) into deionized water (1.00 mL).The detailed preparation steps related to AIEISPs was presented in Fig.S7 (Supporting information).Other metal ions,such as Zn2+ions and Cd2+ions,are able to be employed to construct AIEISPs (Fig.S8 in Supporting information).
1H NMR measurements were conducted to get further insights into the interfacial assembled behaviors between Cu2+andM1[39–41].As shown in Fig.1a,Ha,Hband Hcproton signals related to tpy firstly located around 8.67−8.74 ppm,while exhibiting an upfield shift to 8.72 ppm upon the addition of Cu2+.Meanwhile,it could be observed that an upfield shift of Hdsignal ranging from 8.03 ppm to 8.01 ppm was captured after Cu2+being contributed,besides,Heproton signals shifted from 7.85 ppm to 8.01 ppm.As for Hfand Hksignals,the spectra suggested that both of these resonances displayed downfield shifts (Hf: 7.52 ppm to 7.54 ppm,Hk:6.77 ppm to 6.79 ppm) towards the addition of Cu2+(Fig.1b).Regarding the above-mentioned proton signals,the peak shape became broader (Fig.S9 in Supporting information).For other protons positioning on TPE,the peak shapes concerning Hg,Hhas well Hisignals were getting broader when Cu2+was added.Additionally,Hlsignals exhibited downfield shift roughly from 6.62 ppm to 6.64 ppm,and also Hjsignals shifted from 6.90 ppm to 6.94 ppm after mixing with Cu2+.The shift changes as well as the broadening shapes with regard to tpy protons confirmed that Cu2+was able to complex with the tpy-containedM1[39–41].Moreover,these foregoing transformations gave the direct support that polymer-like assemblies generated.
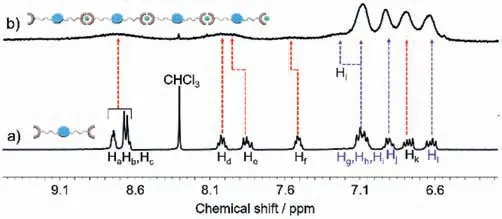
Fig.1. Partial 1H NMR spectra (400 MHz,DMSO-d6,297 K) of (a) M1 and (b)AIEISPs.
As for verifying the detailed complexation behaviors between tpy and Cu2+,the structures of AIEISPs andM1were validated by FT-IR spectra (Fig.2a and Fig.S10 in Supporting information)[37].The spectrum ofM1portrayed those two peaks appeared around 1606 cm−1and 1583 cm−1,which were assigned to C=C and C=N absorbances.After the generation of AIEISPs upon mixing Cu2+withM1,those stretching frequencies was observed to occur at 1600 cm−1.Also,a strong vibration around 1388 cm−1was noticed,for which it offered the evidence for the presence of C-H bonds in tpy structures.Then the peak vanished reflectingviathe spectrum of AIEISPs.In addition,C-H bending frequency was slightly seen at 1037 cm−1,belonging to the pyridine structures,while this peak disappeared in AIEISPs by contrast [37].These results both referred that the complexation between Cu2+and tpy still existed towards the production of AIEISPs transformed fromM1.
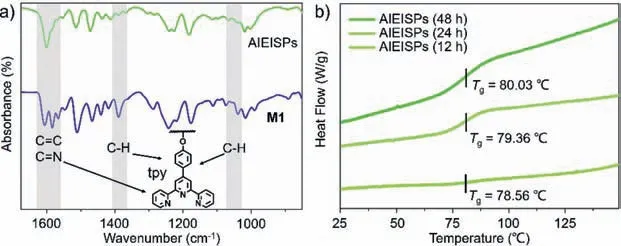
Fig.2. (a) FT-IR spectra of M1 and AIEISPs and (b) DSC thermograms of AIEISPs recorded at 12 h,24 h and 48 h.Inset: Chemical structure of tpy.
Thermodynamic properties of AIEISPs films were also evidencedviaemploying differential scanning calorimetry (DSC) and thermogravimetic analysis (TGA) tests [42].DSC measurements were carried out to explore the glass transition (Tg) of AIEISPs with time uprising (Fig.2b).At 12 h,AIEISPs showed a glass transition of 78.56◦C,upon increasing the time to 24 h,the values regardingTgquickly shifted to 79.36◦C.Then it climbed to 80.03◦C on the occasion of time reaching 48 h.The ascending trend of theTgvalues could be interpreted as the intertangled behaviors of polymeric chains,mainly attributed to the expanding process of supramolecular polymers [18].TGA curves revealed that upon increasing the time,the thermal decomposition temperature at 5% weight loss of the AIEISPs was 189◦C (12 h),193◦C (24 h) and 198◦C (48 h)respectively (Fig.S11 in Supporting information),suggesting the gradual enhanced thermal ability of AIEISPs [42].
Apart from these foregoing characterizations concerning thermodynamic properties of AIEISPs,thickness were equally the key characters to depict.Scanning electron microscopy (SEM) images were thus taken for discover the thickness characteristic of AIEISPs films (Fig.3) [43].Given these images,the thickness of AIEISPs films was found to be increased over time,gradually rising from 62.3 μm (12 h),then 105 μm (24 h),finally to 154 μm (48 h).Corroborated by these proofs,the conclusion could be drawn that the aggregation process of supramolecular polymer proceeded at the interface.

Fig.3. SEM images of AIEISPs taken at (a) 12 h,(b) 24 h and (c) 48 h.Scale bars: 50 μm.
Optical Properties acted as the symbolic property of this prepared AIEISPs,hence,UV-vis spectra at distinct time intervals were adopted (Fig.4a) [37].Notably,two absorption peaks were spotted,for one peak located at around 392 nm,while the another one was situated at 560 nm.As shown in the curves,along with increasing time,a significant enhanced absorbance at 392 nm was captured,and yet for the peak at 560 nm,the change regarding absorbance was slight.Those appearances of absorption bands were explained as the spin-allowed metal-to-ligand charge,confirming the formation of tpy-Cu2+complex [37].Meanwhile,the gradual fortified absorbance could be ascribed to that more complexes were generated,accounting for the growth of supramolecular polymers which enabled the AIEISPs become thicker.So as to study the fluorescence colors,we had taken the photos ofM1solutions as well as AIEISPs under 365 nm UV light [23].Blue color exhibited byM1solutions was able to be surveyed with naked eyes.Meanwhile,the color of AIEISPs was observed to undergo a transition from cyan to green upon the time uprising.For reconciling these observations,the fluorescent spectra were recorded (Fig.4b).It could be seen thatλmaxofM1solutions positioned at 425 nm,while it transformed to AIEISPs (for 12 h) withλmaxshifting to 483 nm.As time went by,the spectra suggested that the readily discernible shift ofλmaxwith the scope of 483 nm to 491 nm occurred at 24 h.Subsequently,around 48 h,we found thatλmaxlocated at 493 nm.As was stated above,these spectra results agreed with the obtained fluorescent colors seen by eyes.Furthermore,the sharp shift ofλmaxfrom solutions to AIEISPs resulted from the interfacial assembled behaviors of supramolecular polymers,whereas regarding the changes from 483 nm to 493 nm,the aggregation of supramolecular polymers took the key role to this phenomenon,leading to the TPE being concentrated [23].
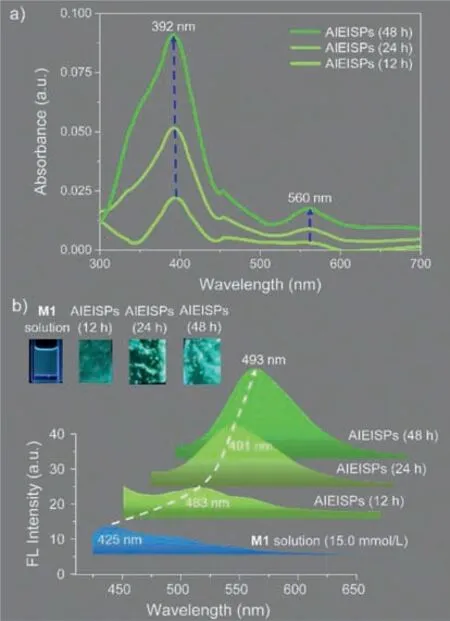
Fig.4. (a) UV-vis spectra of AIEISPs recorded at different time intervals corresponding to 12 h,24 h and 48 h.(b) Fluorescent photos and spectra of M1 solutions as well as AIEISPs films at 12 h,24 h and 48 h.λex=365 nm,silt: 15/3.
Stimuli-responsiveness is considered as the inherent characteristic of supramolecular self-assembly,thus in order to quest this,excessive tetrabutylammonium hydroxide (TBAOH,10.0 equiv.) was added into the performed AIEISPs.It was envisioned that OH−ions as the strong competitors participated into the reaction between Cu2+and tpy,showing stronger capability to interact with Cu2+than that of tpy (Fig.5a) [44].This hypothesis was further confirmedviaconducting the1H NMR spectra of AIEISPs before/after mixing with OH−.It was seen that the signals varied from broad peaks to narrow ones upon mixing with OH−(Figs.5b and c).The initial peak positioning on 8.72 ppm split into two peaks locating at 8.74 ppm (Ha) and 8.67 ppm (Hband Hc) respectively,besides,Hd,He,Hfsignals appeared again corresponding to the shifts of 8.03 ppm,7.86 ppm and 7.52 ppm.At the same time,the analogous changes regarding Hg−Hlsignals were noticed (Fig.S12 in Supporting information).Both of these transformations demonstrated that the structure of supramolecular polymers was destroyed,caused by the complexation between Cu2+and OH−[44].For further proving this effect on AIEISPs,photos under UV light/daylight were recorded (Fig.5d).It could be found that the original AIEISPs were damaged to change into solutions after mixing with OH−,to boot,fluorescence could be no more seen under UV light.On the basis of these results,it can be concluded that the specific anion-responsiveness of AIEISPs was verified.KOH,as a typical inorganic base,could achieve similar stimulus-responsiveness.(Fig.S13 in Supporting information).
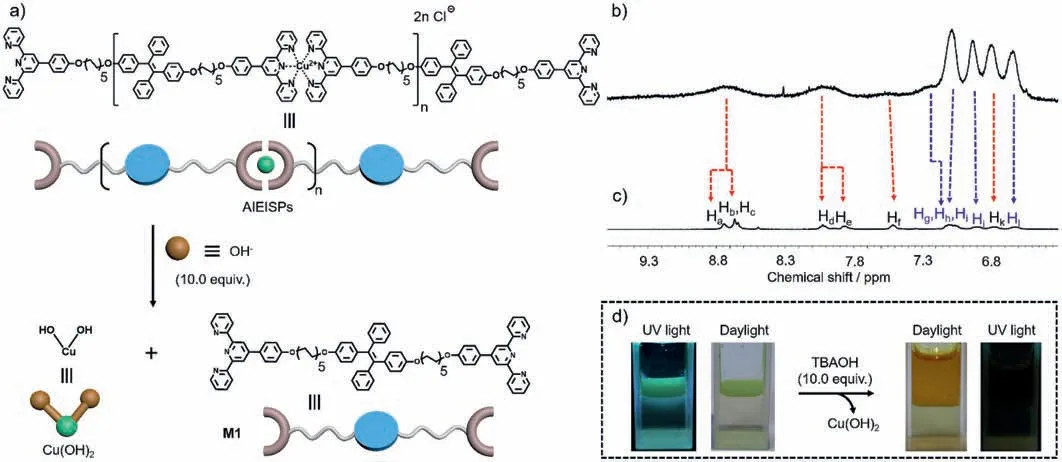
Fig.5. (a) Cartoon representation of AIEISPs treated with OH−.Partial 1H NMR spectra (400 MHz,DMSO-d6,297 K) of AIEISPs before (b) and after (c) the addition of TBAOH.(d) photos of AIEISPs upon mixing with TBAOH recorded under UV light/daylight.
In summary,we had preparedM1consisting of TPE luminophore that functionalized with tpy on both sides,then being dissolved in CHCl3.Meanwhile,Cu2+solution was given through dissolving CuCl2in H2O.Upon mixing those solutions,AIEISPs were thus produced amid phases driven by metal-coordination between tpy and Cu2+.The generation of AIEISPs was verifiedviaconducting series of tests including the1H NMR spectra,FT-IR,DSC as well as SEM.Characterized by fluorescent spectra,it could be found out that the fluorescence color of AIEISPs underwent a transition from cyan to green along with the time uprising as a result of the constant aggregation of supramolecular polymers.The stimuli-responsiveness of AIEISPs towards OH−was also confirmed,whereby upon the addition of OH−,the initial metal-coordination was destructed,leading to the disintegration behaviors of films.This kind of interfacial AIEISPs realized a novel approach to prepare AIE supramolecular polymers.We envision that it may open up new vistas for advancing the fields of supramolecular polymers,aggregate science,polymer science and so forth.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Xiaofan Ji acknowledges funding from the National Natural Science Foundation of China (No.22001087).Xiaofan Ji also appreciates the support from the Huazhong University of Science and Technology,where he is being supported by Fundamental Research Funds for the Central Universities (No.2020kfyXJJS013).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2023.108741.
杂志排行
Chinese Chemical Letters的其它文章
- Spin switching in corrole radical complex
- Benzothiadiazole-based materials for organic solar cells
- Mono-functionalized pillar[n]arenes: Syntheses,host–guest properties and applications✰
- Recent advances in two-step energy transfer light-harvesting systems driven by non-covalent self-assembly✩
- From oxygenated monomers to well-defined low-carbon polymers
- Doping-induced charge transfer in conductive polymers
